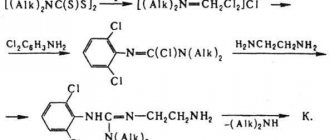
Composition per tablet:
| Active ingredient, mg: | ||
| captopril (based on 100% substance) | 25,0 | 50,0 |
| Excipients, mg: | ||
| corn starch | 7,98 | 15,96 |
| microcrystalline cellulose | 6,97 | 13,94 |
| povidone K-17 | 1,975 | 3,95 |
| talc | 1,00 | 2,00 |
| magnesium stearate | 1,00 | 2,00 |
| lactose monohydrate to obtain a tablet weighing | 100,00 | 200,00 |
"Captopril": main characteristics
The drug is a light-colored tablet with a characteristic odor. It is also available in the form of capsules of a similar composition. The active ingredient is captopril (1 tablet contains 25 mg). The therapeutic effect is based on inhibition of the action of the ACE enzyme.
Available in cardboard packaging of 10 tablets. Store at room temperature (no more than 25 degrees) and moderate humidity. The shelf life is 4 years from the date of issue. Dispensed from pharmacies only with a prescription.
Description:
Round biconvex tablets of white or almost white color with a score and a characteristic odor. Light marbling is allowed.
Pharmacotherapeutic group:
angiotensin-converting enzyme inhibitor (ACE inhibitor).
ATX code :
C09AA01
pharmachologic effect
Pharmacodynamics:
The drug Captopril-STI is an angiotensin-converting enzyme (ACE) inhibitor. Suppresses the formation of angiotensin II and eliminates its vasoconstrictive effect on arterial and venous vessels.
Reduces total peripheral vascular resistance, reduces afterload, and lowers blood pressure. Reduces preload, reduces pressure in the right atrium and pulmonary circulation. Reduces the release of aldosterone in the adrenal glands. The maximum hypotensive effect is observed within 60-90 minutes after oral administration. The degree of reduction in blood pressure is the same when the patient is in the “standing” and “lying” positions.
The effectiveness and safety of captopril in children have not been established. The literature describes limited experience with the use of captopril in children. Children, especially neonates, may be more susceptible to developing hemodynamic side effects. There have been cases of excessive, prolonged and unpredictable increases in blood pressure, as well as associated complications, including oliguria and seizures.
Pharmacokinetics:
When taken orally, the bioavailability of captopril is 60-70%. Simultaneous food intake slows down the absorption of the drug by 30-40%. The connection with blood plasma proteins is 25-30%. The half-life is 2-3 hours. The drug is excreted from the body primarily by the kidneys, up to 50% unchanged.
At what pressure will the drug be effective?
Captopril for blood pressure has an effect on ACE (angiotensin-converting enzyme), which affects the tone of blood vessels, narrowing them and causing an increase in blood pressure. As a result, the capillary walls maintain normal tone, do not contract and do not slow down blood circulation. With constant use of the medicine, you can reduce blood pressure to normal levels and maintain it within acceptable limits.
Captopril quickly reduces blood pressure, improving blood supply to the heart, relaxing its capillaries and reducing the load on the myocardium. At the same time, the heart muscle retains its full ability to pump a sufficient amount of blood into the circulatory system. Patients who take Captopril after a heart attack are able to withstand physical stress and emotional stress because their heart muscle becomes more resilient.
It is important to reduce blood pressure gradually
At what pressure should you take the tablets so that they have the maximum therapeutic effect? Captopril is usually prescribed to patients diagnosed with the first and second stages of hypertension, when blood pressure levels rise to 179/109. This condition does not require additional therapy and can be easily treated with the prescribed dose of tablets. Severe hypertension, in which the pressure rises above 180/110, requires the use of the drug together with diuretics, since in such situations Captopril does not reduce the pressure to normal levels alone.
Contraindications:
Hypersensitivity to captopril or other ACE inhibitors; angioedema (history of ACE inhibitor therapy or hereditary); severe dysfunction of the liver and/or kidneys; hyperkalemia; bilateral renal artery stenosis, stenosis of the artery of a single kidney with progressive azotemia, condition after kidney transplantation, stenosis of the aortic mouth and similar changes that impede the outflow of blood from the left ventricle, pregnancy, lactation, age under 18 years (efficacy and safety have not been established).
Rare hereditary galactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome.
Concomitant use with aliskiren and drugs containing aliskiren is contraindicated in patients with diabetes mellitus and/or moderate or severe renal impairment (glomerular filtration rate (GFR) less than 60 ml/min/1.73 m2 body surface area.
Concomitant use with angiotensin II receptor antagonists (ARI) in patients with diabetic nephropathy is contraindicated.
Concomitant use with neutral endopeptidase inhibitors (for example, with drugs containing sacubitril) due to the high risk of developing angioedema.
What does Capril help with: indications for use
The drug is indicated for use in the presence of chronic and acute diseases of the cardiovascular system:
- mild to moderate high blood pressure;
- insufficient heart function (impaired functioning of the left ventricle);
- myocardial infarction (taken during the first 24 hours, as well as as long-term prophylaxis);
- diabetic nephropathy type 1.
The effect of "Capril" is due to the fact that the active substance reduces the synthesis of angiotensin II, which leads to severe vasoconstriction. Due to this, the potassium content in the blood plasma increases. In this case, the drug reduces blood pressure without affecting the pulse.
Carefully:
Severe autoimmune connective tissue diseases (including systemic lupus erythematosus, scleroderma), suppression of bone marrow hematopoiesis (risk of developing neutropenia and agranulocytosis), cerebral ischemia, diabetes mellitus (increased risk of developing hyperkalemia), patients on hemodialysis, diet with sodium restriction, primary hyperaldosteronism, coronary heart disease, conditions accompanied by a decrease in circulating blood volume (including vomiting, diarrhea), old age (dose adjustment required).
Use during pregnancy and breastfeeding:
The use of Captopril-STI is contraindicated during pregnancy.
The drug Captopril-STI should not be used in the first trimester of pregnancy. There have been no adequate controlled studies of the use of ACE inhibitors in pregnant women. The limited available data on the effects of the drug in the first trimester of pregnancy indicate that the use of ACE inhibitors does not lead to fetal malformations associated with fetotoxicity. Epidemiological data demonstrating a risk of teratogenicity after exposure to ACE inhibitors in the first trimester of pregnancy have not been convincing, but some increased risk cannot be excluded. If the use of an ACE inhibitor is considered necessary, patients planning pregnancy should be switched to alternative antihypertensive therapy that has an established safety profile for use during pregnancy.
It is known that long-term exposure of the fetus to ACE inhibitors in the second and third trimesters of pregnancy can lead to disruption of its development (decreased renal function, oligohydramnios, delayed ossification of the skull bones) and the development of complications in the newborn (such as renal failure, arterial hypotension, hyperkalemia) . If the patient received Captopril-STI during the second and third trimesters of pregnancy, it is recommended to conduct an ultrasound examination to assess the condition of the skull bones and fetal kidney function.
The use of ACE inhibitors during pregnancy can cause developmental disorders (including arterial hypotension, neonatal hypoplasia of the skull, anuria, reversible or irreversible renal failure) and fetal death. If pregnancy is established, the use of Capotopril-STI should be discontinued as soon as possible.
Approximately 1% of a given dose of captopril is found in breast milk. Due to the risk of serious adverse reactions in the child, breastfeeding should be stopped or therapy with Captopril-STI should be discontinued in the mother during the period of breastfeeding.
Drug interactions Captopril
With simultaneous use of Captopril with diuretics, vasodilators and β-adrenergic receptor blockers, the hypotensive effect of Captopril is enhanced. The effectiveness of captopril may be reduced when taken simultaneously with indomethacin and other NSAIDs; with probenecid - it is possible to slow down the excretion of Captopril in the urine. When used in combination with potassium-sparing diuretics, potassium preparations, dietary supplements containing potassium, and potassium-containing salt substitutes, hyperkalemia may develop. Captopril increases the concentration and slows down the elimination of lithium compounds from the body, which can manifest as symptoms of lithium intoxication. The use of Captopril in patients taking immunosuppressants (for example, azathioprine, cyclophosphamide) increases the risk of developing hematological disorders.
Directions for use and dosage:
Orally an hour before meals. The dosage regimen is set individually.
To ensure the dosage regimens indicated below, if it is necessary to use captopril at a dose of 6.25 mg, captopril preparations from other manufacturers should be prescribed in the dosage form of “25 mg tablets” with a cross mark or “12.5 mg tablets” with a score line.
For arterial hypertension, the drug is prescribed at an initial dose of 12.5 mg 2 times a day. If necessary, the dose is gradually increased (with an interval of 2-4 weeks) until the optimal effect is achieved. For mild to moderate arterial hypertension, the usual maintenance dose is 25 mg 2 times a day; the maximum dose is 50 mg 2 times a day. For severe arterial hypertension, the initial dose is 12.5 mg 2 times a day. The dose is gradually increased to a maximum daily dose of 150 mg (50 mg 3 times a day).
For the treatment of chronic heart failure, Captopril-STI is prescribed in cases where the use of diuretics does not provide an adequate effect. The initial daily dose is 6.25 mg 3 times a day. In the future, if necessary, the dose is gradually increased (at intervals of at least 2 weeks). The average maintenance dose is 25 mg 2-3 times a day, and the maximum is 150 mg per day.
In cases of left ventricular dysfunction after myocardial infarction in patients in a clinically stable condition, the use of Captopril-STI can be started as early as 3 days after myocardial infarction. The initial dose is 6.25 mg per day, then the daily dose can be increased to 37.5-75 mg in 2-3 doses (depending on the tolerability of the drug) up to a maximum of 150 mg per day.
For diabetic nephropathy, Captopril-STI is prescribed in a dose of 75-100 mg, divided into 2-3 doses. For insulin-dependent diabetes with microalbuminuria (albumin release 30-300 mg per day), the dose of the drug is 50 mg 2 times a day. With a total protein clearance of more than 500 mg per day, the drug is effective at a dose of 25 mg 3 times a day.
For patients with impaired renal function with moderate renal impairment (creatinine clearance (CC) of at least 30 ml/min/1.73 m2), Captopril-STI can be prescribed at a dose of 75-100 mg/day. For more severe renal dysfunction (creatinine clearance less than 30 ml/min/1.73 m2), the initial dose should be no more than 12.5 mg/day; in the future, if necessary, at sufficiently long intervals, the dose of Captopril-STI is gradually increased, but a smaller daily dose of the drug is used than usual.
In old age, the dose of the drug is selected individually; it is recommended to start therapy with a dose of 6.25 mg 2 times a day and, if possible, maintain it at this level.
If necessary, loop diuretics are additionally prescribed rather than thiazide diuretics.
When should you take Captopril?
Captopril tablets for high blood pressure are taken on the recommendation of the attending physician, who also selects the required dosage and frequency of use of the drug. According to the instructions for use, the use of Captopril is justified not only for the treatment of hypertension, but also for other concomitant diseases:
- high blood pressure and hypertension, including those forms that cannot be treated with other types of medications;
- chronic hypertension, aggravated by regular surges in blood pressure, heart failure and angina pectoris;
- pathologies associated with impaired renal vascular function;
- ambulance for relief of hypertensive crisis;
- renoparenchymal hypertension due to recent or chronic glomerulonephritis;
- bronchial asthma, accompanied by a regular increase in blood pressure;
- renal vascular pathologies resulting from diabetes mellitus;
- chronic heart failure that cannot be treated with cardiac glycosides and diuretics;
- Conn's syndrome.
Many patients take Captopril for high blood pressure only when there are sudden changes and signs of a hypertensive crisis, which is fundamentally wrong. Hypertension should be treated systematically, combining medications prescribed by the doctor with moderate physical activity, diet and physiotherapy.
As a result, such patients belong to the group of people who independently increase the risk of developing strokes, myocardial infarction and shorten their own lives by several years
Side effect:
The frequency of adverse reactions is understood as: often - ≥ 1/100, <1/10, infrequently - ≥ 1/1000, <1/100, rarely - ≥ 1/10000, <1/1000, very rarely - < 1/10000.
From the cardiovascular system:
infrequently - tachycardia or arrhythmia, angina pectoris, palpitations, orthostatic arterial hypotension, peripheral edema, marked decrease in blood pressure, Raynaud's syndrome, flushes of blood to the facial skin, pallor; very rarely - cardiac arrest, cardiogenic shock.
From the respiratory system:
often – dry non-productive cough, shortness of breath; very rarely - bronchospasm, eosinophilic pneumonitis, rhinitis, pulmonary edema.
Allergic reactions:
often - itching of the skin, with or without rashes, skin rashes, alopecia;
uncommon – angioedema of the extremities, face, lips, mucous membranes, tongue, pharynx and larynx;
rarely - angioedema of the intestine;
very rarely - urticaria, Stevens-Johnson syndrome, erythema multiforme, photosensitivity, erythroderma, pemphigoid reactions, exfoliative dermatitis, allergic alveolitis, eosinophilic pneumonia.
From the central nervous system:
often – drowsiness, dizziness, insomnia;
uncommon – headache, paresthesia;
rarely - ataxia;
very rarely - confusion, depression, cerebrovascular accidents, including stroke and syncope, blurred vision.
From the hematopoietic organs:
very rarely - neutropenia, agranulocytosis, pancytopenia, lymphadenopathy, eosinophilia, thrombocytopenia, anemia (including aplastic and hemolytic forms), increased titer for antinuclear antibodies, autoimmune diseases.
From the digestive system:
often - nausea, vomiting, irritation of the gastric mucosa, abdominal pain, diarrhea, constipation, taste disturbance, dry oral mucosa, dyspepsia;
infrequently – anorexia;
rarely – stomatitis, aphthous stomatitis;
very rarely - glossitis, gastric ulcer, pancreatitis, gingival hyperplasia, impaired liver function and cholestasis (including jaundice), increased activity of liver enzymes, hepatitis (including rare cases of hepatonecrosis), hyperbilirubinemia.
From the musculoskeletal system:
very rarely - myalgia, arthralgia.
From the urinary system:
rarely - renal dysfunction (including renal failure), polyuria, oliguria, frequent urination;
very rarely - nephrotic syndrome.
From the reproductive organs:
very rarely - impotence, gynecomastia.
Other:
infrequently - chest pain, increased fatigue, feeling of general malaise, asthenia;
rarely – hyperthermia.
Laboratory indicators:
very rarely - proteinuria, eosinophilia, hyperkalemia, hyponatremia, increased levels of urea nitrogen, bilirubin and creatinine in the blood, decreased hematocrit, decreased hemoglobin, leukocytes, platelets, hypoglycemia.
Interaction with other drugs:
In patients taking diuretics, Captopril-STI may potentiate the hypotensive effect. Limiting the intake of table salt (salt-free diets) and hemodialysis also have a similar effect. Typically, an excessive decrease in blood pressure occurs within one hour after taking the first prescribed dose of Captopril-STI.
Vasodilators (for example, nitroglycerin) in combination with Captopril-STI should be used in the lowest effective doses due to the risk of excessive reduction in blood pressure.
Caution should be exercised when co-prescribing Captopril-STI (without or with a diuretic) and drugs that affect the sympathetic nervous system (for example, ganglion blockers, alpha-blockers). When using the drug Captopril-STI together with indomethacin (and possibly other non-steroidal anti-inflammatory drugs, for example, acetylsalicylic acid), a decrease in the hypotensive effect may be observed, especially in arterial hypertension accompanied by low renin activity. In patients with risk factors (elderly age, hypovolemia, concomitant use of diuretics, impaired renal function), simultaneous use of non-steroidal anti-inflammatory drugs (including cyclooxygenase-2 inhibitors) and ACE inhibitors (including captopril) may lead to deterioration of renal function, including acute renal failure. Usually, renal dysfunction in such cases is reversible. Renal function should be periodically monitored in patients taking Captopril-STI and non-steroidal anti-inflammatory drugs.
When treating with Captopril-ST, potassium-sparing diuretics (for example, triamterene, spironolactone, amiloride, eplerenone), potassium supplements, potassium supplements, salt substitutes (contain significant amounts of potassium ions) should be prescribed only for proven hypokalemia, since their use increases the risk of developing hyperkalemia .
When combined with drugs containing co-trimoxazole (trimethoprim + sulfamethoxazole), the risk of developing hyperkalemia increases.
With the simultaneous use of ACE inhibitors (especially in combination with diuretics) and lithium preparations, an increase in the lithium content in the blood serum and, consequently, the toxicity of lithium preparations is possible. Serum lithium levels should be periodically determined.
ACE inhibitors, including captopril, may potentiate the hypoglycemic effect of insulin and oral hypoglycemic agents such as sulfonylureas.
It is necessary to monitor the concentration of glucose in the blood at the beginning of therapy with Captopril-STI, and, if necessary, adjust the dose of the hypoglycemic drug.
Dual blockade of the renin-angiotensin-aldosterone system (RAAS), caused by concomitant use of ACE inhibitors and angiotensin II receptor antagonists or aliskiren and aliskiren-containing drugs, was associated with an increased incidence of side effects such as arterial hypotension, hyperkalemia, decreased renal function (including acute renal failure).
The use of Captopril-STI in patients taking allopurinol or procainamide increases the risk of developing neutropenia and/or Stevens-Johnson syndrome.
The use of Captopril-STI in patients taking immunosuppressants (for example, cyclophosphacine or azathioprine) increases the risk of developing hematological disorders.
The risk of developing angioedema increases with the combined use of ACE inhibitors with the following drugs:
- with mTOR inhibitors (mammalian Target of Rapamycin - target of rapamycin in mammalian cells), for example, temsirolimus, sirolimus, everolimus;
- with dipeptidyl peptidase type IV (DPP-IV) inhibitors (gliptins), for example, sitagliptin, saxagliptin, vildagliptin, linagliptin;
- with neutral endopeptidase inhibitors:
- racecadotril (an enkephalinase inhibitor used to treat acute diarrhea);
- sacubitril (neprilysin inhibitor), ACE inhibitors should be prescribed no earlier than 36 hours after discontinuation of drugs containing sacubitril. Prescription of drugs containing sacubitril is contraindicated in patients receiving ACE inhibitors, as well as within 36 hours after discontinuation of ACE inhibitors.
- with estramustine;
- with tissue plasminogen activators. Observational studies have shown an increased incidence of angioedema in patients taking ACE inhibitors following the use of alteplase for thrombolytic therapy of ischemic stroke.
How quickly does the drug work?
How long does it take for Captopril to work, patients with regular surges in blood pressure ask. The drug is characterized by good absorption into the blood serum, and the peak of action begins 15–60 minutes after administration. If a patient has just eaten and then taken a Captopril tablet, this can reduce the effectiveness of the drug by up to 40%. Therefore, doctors recommend taking the medication 1.5 hours after eating.
Also, many hypertensive patients are interested in whether to drink Captopril with water, or would it be more correct to put it under the tongue? There have been no special studies on this matter, so it is impossible to say that one of these methods of using tablets is correct and the other is not. You should try both options, after taking it, monitoring your blood pressure level using a tonometer and focusing on your own sensations. For some patients, resorption of Captopril under the tongue brings a greater effect than oral administration, while for others it is more common to take tablets with water.
Special instructions:
Before starting, as well as regularly during treatment with Captopril-STI, renal function should be monitored. In patients with chronic heart failure, it is used under close medical supervision.
When taking ACE inhibitors, a characteristic non-productive cough is observed, which ceases after discontinuation of ACE inhibitor therapy.
In rare cases, when taking ACE inhibitors, a syndrome is observed that begins with the appearance of cholestatic jaundice, turning into fulminant hepatonecrosis, sometimes with death. The mechanism of development of this syndrome is unknown. If a patient receiving therapy with ACE inhibitors develops jaundice or there is a marked increase in the activity of liver enzymes, treatment with ACE inhibitors should be discontinued and the patient should be monitored.
Some patients with kidney disease, especially those with severe renal artery stenosis, experience increases in serum urea nitrogen and creatinine concentrations after lowering blood pressure. This increase is usually reversible when therapy with Captopril-STI is discontinued. In these cases, it may be necessary to reduce the dose of Captopril-STI and/or discontinue the diuretic.
During long-term use of the drug Captopril-STI, approximately 20% of patients experience an increase in serum urea and creatinine concentrations by more than 20% compared to normal or baseline values.
In less than 5% of patients, especially with severe nephropathy, treatment discontinuation is required due to an increase in creatinine concentration. The use of dual blockade of the renin-angiotensin-aldosterone system (RAAS) caused by the simultaneous use of ACE inhibitors and angiotensin II receptor antagonists or aliskiren and aliskiren-containing drugs is not recommended, since it has been associated with an increased incidence of side effects such as arterial hypotension, hyperkalemia, decreased renal function (including acute renal failure). If the simultaneous use of ACE inhibitors and ARAII (double blockade of the RAAS) is necessary, then treatment should be carried out under the supervision of a physician and with constant monitoring of renal function, electrolyte levels in the blood, and blood pressure. The simultaneous use of ACE inhibitors with drugs containing aliskiren is contraindicated in patients with diabetes mellitus and/or with moderate or severe renal impairment (GFR less than 60 ml/min/1.73 m2 body surface area) and is not recommended in other patients.
Concomitant use of ACE inhibitors with angiotensin II receptor antagonists is contraindicated in patients with diabetic nephropathy and is not recommended in other patients.
The combined use of ACE inhibitors and angiotensin II receptor antagonists in patients with diabetic nephropathy is not recommended.
In patients with arterial hypertension, when using the drug Captopril-STI, severe arterial hypotension is observed only in rare cases; the likelihood of developing this condition increases with increased loss of fluid and salts (for example, after intensive treatment with diuretics), in patients with heart failure or on dialysis. The possibility of a sharp decrease in blood pressure can be minimized by first withdrawing (4-7 days before) the diuretic or increasing sodium chloride intake (about a week before starting treatment), or by prescribing Captopril-STI at the beginning of treatment in small doses (6 ,25-12.5 mg/day).
Prescribe with caution to patients on a low-salt or salt-free diet (increased risk of developing arterial hypotension) and hyperkalemia. An excessive decrease in blood pressure may occur in patients during major surgical operations, as well as when using general anesthesia agents that have a hypotensive effect. In such cases, to correct low blood pressure, measures are taken to increase the volume of circulating blood.
Excessive reduction in blood pressure due to antihypertensive medications may increase the risk of myocardial infarction or stroke in patients with coronary heart disease or cerebrovascular disease. If arterial hypotension develops, the patient should take a horizontal position with legs elevated. Intravenous administration of 0.9% sodium chloride solution may be required.
Caution should be exercised when taking ACE inhibitors in patients with mitral/aortic stenosis/hypertrophic obstructive cardiomyopathy; in case of cardiogenic shock and hemodynamically significant obstruction, use is not recommended.
Neutropenia/agranulocytosis, thrombocytopenia and anemia have been reported in patients taking ACE inhibitors. Neutropenia is rare in patients with normal renal function and no other abnormalities. In case of renal failure, simultaneous administration of Captopril-STI and allopurinol led to neutropenia.
Captopril-STI should be used very carefully in patients with autoimmune connective tissue diseases, those taking immunosuppressants, allopurinol and procainamide, especially in the presence of pre-existing renal dysfunction. Due to the fact that the majority of fatal cases of neutropenia due to ACE inhibitors developed in such patients, their blood leukocyte count should be monitored before starting treatment, in the first 3 months - every 2 weeks, then every 2 months.
In all patients, the number of leukocytes in the blood should be monitored monthly in the first 3 months after starting therapy with Captopril-STI, then every 2 months. If the number of leukocytes is below 4000/μl, a repeat general blood test is indicated; below 1000/μl, the drug is stopped while monitoring the patient continues. Typically, restoration of the number of neutrophils occurs within 2 weeks after discontinuation of the drug Captopril-STI. In 13% of cases of neutropenia, death was noted. In almost all cases, death was observed in patients with connective tissue diseases, renal or heart failure, while taking immunosuppressants, or a combination of both of these factors.
When using ACE inhibitors, proteinuria may occur, mainly in patients with impaired renal function, as well as when using high doses of drugs. In most cases, proteinuria when taking Captopril-STI disappeared or its severity decreased within 6 months, regardless of whether the drug was stopped or not. Renal function tests (blood urea nitrogen and creatinine concentrations) in patients with proteinuria were almost always within normal limits. In patients with kidney disease, the protein content in the urine should be determined before starting treatment and periodically throughout the course of therapy. In some cases, against the background of the use of ACE inhibitors, incl. the drug Captopril-STI, an increase in potassium content in the blood serum is observed. The risk of developing hyperkalemia when using ACE inhibitors is increased in patients with renal failure and diabetes mellitus, as well as those taking potassium-sparing diuretics, potassium supplements, or other drugs that cause an increase in potassium levels in the blood (for example, heparin). The simultaneous use of potassium-sparing diuretics and potassium supplements should be avoided. In addition, when using ACE inhibitors simultaneously with thiazide diuretics, the risk of hypokalemia cannot be excluded, therefore, in such cases, regular monitoring of potassium levels in the blood during therapy should be carried out.
When performing hemodialysis in patients receiving ACE inhibitors, the use of high-permeability dialysis membranes (for example, AN69) should be avoided, since in such cases the risk of developing anaphylactoid reactions increases. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein removal (apheresis) with dextran sulfate. The use of either a different class of antihypertensive drugs or a different type of dialysis membrane should be considered.
In rare cases, during therapy with ACE inhibitors, life-threatening anaphylactoid reactions were observed in patients undergoing desensitization with hymenoptera venom (bees, wasps). In such patients, these reactions were prevented by temporarily stopping ACE inhibitor therapy. Particular care should be taken when desensitization is performed in such patients.
If angioedema develops, the drug is discontinued and careful medical observation is carried out until the symptoms disappear completely. Angioedema of the larynx can be fatal. If the swelling is localized on the face, special treatment is usually not required (antihistamines can be used to reduce the severity of symptoms); if the swelling spreads to the tongue, pharynx or larynx and there is a threat of developing airway obstruction, epinephrine (adrenaline) should be immediately administered subcutaneously (0.3-0.5 ml in a dilution of 1:1000). In rare cases, patients after taking ACE inhibitors experienced angioedema of the intestine, which was accompanied by abdominal pain (with or without nausea and vomiting), sometimes with normal values of C-1-esterase activity and without previous facial edema. Intestinal edema should be included in the differential diagnosis of patients with complaints of abdominal pain while taking ACE inhibitors.
In representatives of the Negroid race, cases of the development of angioedema were noted with greater frequency compared to representatives of the Caucasian race.
In patients with diabetes mellitus receiving hypoglycemic agents (oral hypoglycemic agents or insulin), glycemic levels should be carefully monitored, especially during the first month of therapy with ACE inhibitors.
ACE inhibitors are less effective in blacks than in Caucasians, which may be due to the higher prevalence of low renin activity in blacks.
During major surgery or when using hypothetical agents for general anesthesia, patients taking ACE inhibitors may experience an excessive decrease in blood pressure. In these cases, the volume of circulating blood can be increased.
When taking Captopril-STI, a false-positive reaction may occur when testing urine for acetone.