The problem should not be ignored, because the substance in question is considered a very important electrolyte present in muscle cells, working together with sodium. It is potassium that ensures proper muscle contraction, and its lack can lead to frequent cramps, decreased strength potential, and tissue dehydration. Potassium preparations are presented in quite a large number to choose from, so special attention should be paid to determining the most suitable remedy. Here you need to take into account the composition, quantity and mass fraction of components in order to achieve maximum effect. Potassium can be presented in the form of an independent preparation, or also be part of various preparations necessary for rehydration.
IMPORTANCE OF POTASSIUM FOR CVS
Let's take a closer look at the effect of potassium on heart function. The electrical activity of myocardial cells depends on transmembrane ionic gradients, as well as on time- and voltage-dependent disturbances in the conduction of ionic currents. Electrolyte abnormalities can cause or facilitate the development of clinically significant arrhythmias even in normal cardiac tissue by modulating ion conduction through specific myocardial ion channels. The Na+ current into the cell upon activation of Na+ channels forms a phase of rapid depolarization in cardiomyocytes. As depolarization increases, permeability to Na+ decreases due to inactivation of Na+ channels, but channels for incoming Ca++ currents, which are necessary for the formation of the plateau phase, open. Subsequent activation of potassium channels leads to repolarization of the cardiomyocyte membrane to the level of the PP. Potassium, especially extracellular potassium, is the most important factor determining membrane PP. The electrophysiological effects of potassium depend not only on its extracellular concentration, but also on the direction (hypo- or hyperkalemia) and the rate of its change. Potassium ion channels are of primary importance for the regulation of the transmembrane potassium gradient. Potassium channels are transmembrane proteins that selectively allow potassium ions to pass through: potassium moves under the influence of an electrochemical gradient at a rate of 106 to 108 ions per second [1]. There are voltage-gated potassium channels and numerous channels that open for potassium ions or are blocked by various substances - ligands of the corresponding receptors. Such channels maintain the background conductivity of membranes for potassium ions and form the PP of excitable and non-excitable cells*. Hypokalemia (<3 mmol/L) reduces membrane permeability to potassium [24]. Thus, the conductance for the incoming (anomalous) potassium rectifying current is proportional to the square root of the extracellular potassium concentration [20, 21]. The dependence of the activation of the delayed (outgoing) rectifying current on the extracellular potassium concentration helps to understand why the AP duration is shorter at high potassium concentrations and longer at low potassium concentrations [31]. But the effects of potassium on the membrane PP are also modulated by the simultaneously created Ca++ concentrations. Their interaction is such that increased Ca++ levels reduce the depolarizing effect caused by increased potassium levels. In turn, low Ca++ levels attenuate the depolarization caused by hypokalemia.
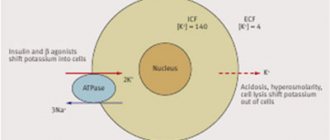
Factors stimulating the transmembrane movement of potassium:
- from the cell to the extracellular space:
– acidosis; – stimulation of α-adrenergic receptors; – digitalis preparations;
- from the extracellular space into the cell:
– alkalosis; – stimulation of β2-adrenergic receptors; – insulin.
Damage to membrane phospholipids in the processes of lipid peroxidation (LPO) also leads to disruption of the barrier function of the membrane and increased potassium loss by the cell. Activation of LPO occurs in pathological conditions such as dystrophy, inflammation, myocardial ischemia, etc.
ELECTROPHYSIOLOGICAL EFFECTS OF HYPOKALEMIA
Hypokalemia leads to an increase (more electronegative value) of the membrane PP and, at least during electrical diastole, reduces membrane excitability due to an increase in the difference between the PP and the threshold potential. It is assumed that extracellular potassium is necessary for the opening of delayed rectifying current channels [31]. Low extracellular potassium levels reduce the delayed (outward) potassium current, leading to increased AP duration and slower repolarization. The most important here is the disruption of the AP configuration, especially the slowing down of the “slope” of the 3rd phase of repolarization. An AP with a long “tail” is formed, which leads to an increase in the relative refractory period (RPP) and a decrease in the difference between the AP and the threshold potential in the final phase of the AP. Therefore, the myocardium exhibits increased excitability and an associated tendency to ectopic activity during a significant portion of the AP. Conduction slows as depolarization begins in incompletely repolarized fibers. Moreover, hypokalemia lengthens the plateau phase in Purkinje fibers, but shortens it in ventricular fibers [10]. The repolarization phase (“tail”) of AP in the conduction system is prolonged more than in the ventricles, which increases the spread of repolarization. Hypokalemia accelerates diastolic depolarization in Purkinje fibers, thereby increasing automaticity. In total, the electrophysiological effects of hypokalemia are manifested in a decrease in conduction velocity, shortening of the effective refractory period (ERP), prolongation of the ORP, increased automaticity and early afterdepolarization.
ECG manifestations of hypokalemia:
- due to changes in repolarization:
– decrease in amplitude and expansion of the T wave; – noticeable wave U; – reduction of the ST segment; – fusion of T and U waves (with severe hypokalemia);
- due to conduction disorders:
– increase in the duration of the QRS complex; – atrioventricular block; – increase in amplitude and expansion of the P wave; – slight increase in the P–R interval; - cardiac arrest.
When the U wave exceeds the T wave in amplitude, the plasma potassium level is <3 mmol/L (see figure).
Symptoms of deficiency and excess in the body
With a lack of potassium, a person quickly gets tired and constantly feels tired. Children may experience causeless tearfulness. Macronutrient deficiency also manifests itself as the following symptoms:
- hair loss;
- dryness and flaking of the skin;
- hypertension;
- muscle weakness;
- frequent urge to urinate;
- heart rhythm disturbances;
- limb cramps, especially often observed at night;
- drowsiness.
Excess potassium intake or accumulation in the body caused by various diseases can lead to hyperkalemia. This pathology can be identified by the following symptoms:
- rapid heartbeat, in severe cases the work of the heart muscle stops;
- numbness of the limbs;
- muscle weakness;
- dizziness;
- temporary paralysis;
- digestive problems, stomach pain.
To avoid negative consequences, when taking high doses of potassium, it is recommended to periodically monitor the content of this macroelement in the blood using timely tests.
ARRHYTHMOGENIC POTENTIAL AND CLINICAL CONSEQUENCES OF HYPOKALEMIA
The increase in excitability caused by hypokalemia is clinically manifested by the development of supraventricular and ventricular extrasystole. Decreased potassium and magnesium levels have been shown to correlate with an increased incidence of premature ventricular complexes [30]. Hypokalemia contributes to the development of the reentry phenomenon through slowing conduction against the background of prolongation of the ORP, which leads to an increase in the spread of refractoriness. The suppressive effect of hypokalemia on the operation of the Na+-K+ pump leads to excessive accumulation of Ca++ in the cell, which contributes to the development of delayed afterdepolarization through a transient inward current. In the experiment, hypokalemia increased the tendency to ventricular fibrillation in both normal and ischemic myocardium [12]. A connection has been established between hypokalemia and ventricular fibrillation in patients with acute myocardial infarction [7, 18]. Hypokalemia increases the binding of cardiac glycosides to Na+-K+-adenosine triphosphatase (ATPase), reduces the rate of elimination of digoxin and potentiates the toxic effects of digitalis preparations. When using potassium preparations, it is necessary to remember one important phenomenon - the Zwaardemaker-Libbrecht effect, which develops as a result of a rapid increase in the level of extracellular potassium from low to high and manifests itself as a short-term stop of pacemaker cells, a decrease in the duration of AP and hyperpolarization [13] . This phenomenon highlights the fact that the rate of intravenous potassium administration is more important in terms of arrhythmogenic effects than the absolute amount of potassium administered and the final level of extracellular potassium.
MAGNESIUM
Ionized magnesium is in 2nd place after potassium in terms of total content in the cell. The magnesium content in blood plasma is 0.8–1.5 mmol/l; in muscle tissue its content is 10 times higher than in plasma. Thanks to this depot of magnesium in the muscles, its level in the blood can remain stable for a long time even with significant losses. The significance of disturbances in magnesium metabolism is still debated due to the difficulties of measuring it and the common association of these disturbances with other electrolyte disorders [14, 27]. Magnesium is involved in the functioning of muscles and the nervous system, in the regulation of heart rate, cholesterol, lipid, phosphorus and calcium metabolism. It serves as an important cofactor for numerous enzymatic reactions involved in nucleotide and carbohydrate metabolism, protein synthesis and other processes also necessary for normal cardiovascular physiology. Magnesium prevents an increase in blood pressure, enhances inhibition processes in the central nervous system, causing sedative, tranquilizing effects and preventing the manifestations of convulsive activity. Being a natural antagonist of Ca++, magnesium reduces blood clotting and vascular tone [2, 4]. The daily requirement of an adult for magnesium is from 300 to 500 mg. Magnesium deficiency occurs frequently, but its electrophysiological consequences for the myocardium elude scientists even with the most careful study. It is known that the use of magnesium preparations (magnesium) in pharmacological doses is useful for the treatment of torsades de pointes. Toxic effects of magnesium are rare (with the exception of patients with impaired renal function). With magnesium deficiency, nervousness, irritability, and sleep disturbances develop against the background of muscle weakness, increased fatigue and paresthesia. With chronic magnesium deficiency, skeletal deformations are possible: scoliosis, funnel chest, flat feet.
Properties of drugs
Potassium preparations have numerous properties that are valuable for the body of an ordinary person and an athlete. They are as follows: • stabilization of heart function, reduction of myocardial excitability and conductivity; • strengthening blood vessels and increasing their permeability; • stabilization of blood pressure in vessels; • increasing the elasticity of bone tissue; • increased muscle contractility; • stimulation of enzyme functionality; • normalization of the processes of transmission of nerve impulses; • restoration of proper kidney function; • maintaining healthy skin; • normalization of water balance in the body; • effective cleansing of the body from accumulated toxins; • ensuring proper oxygen supply to the brain and all organs and body systems; • neutralization of the negative effects of sodium on the body when it is in excess; • increasing the body's endurance, facilitating easier perception of intense physical activity; • improved appetite; • normalization of liver function; • reducing the period of recovery of the body after various diseases. Another important point: potassium has a very useful assistant, such as sodium. Together, these components have the following effect: sodium retains fluid in the body, potassium pumps it into muscle cells. Due to this, a beautiful muscle relief is formed and active muscle growth is stimulated. That is why potassium supplements are widely used among professional athletes. They are used no less frequently than carbohydrates and proteins, which are also very popular and are available in the form of numerous sports supplements. Due to its numerous beneficial properties, potassium preparations have become widespread and are also often used in complex therapy of various pathologies. But it is important to take the medications correctly in order to achieve maximum effect and eliminate the development of side effects.
ELECTROPHYSIOLOGICAL EFFECTS AND ECG MANIFESTATIONS OF HYPOMAGNESIEMIA
At very low extracellular calcium concentrations, magnesium affects the transmembrane current or currents that modulate the duration of the plateau phase of AP in the ventricles. It has been established that at normal calcium concentrations, magnesium deficiency has a negligible effect on the PP of the papillary muscle of the heart of dogs [11]. However, when the calcium concentration decreases to 1/10 of normal, complete removal of magnesium from the perfusion solution prolongs the plateau phase of AP, which was already prolonged due to low calcium concentration, from normal values (100–150 ms) to 1000 ms or more. Magnesium blocks calcium channels, shifts the inactivation curve of fast sodium channels in the direction of hyperpolarization, modulates the effects of hyperkalemia, and modulates potassium currents. In a study in healthy patients, the following ECG effects of intravenous magnesium administration were noted: significant prolongation of the P–R interval; lengthening of the conduction interval from the atria to the His bundle; increase in sinoatrial conduction time; Elongation of the ERP in the atrioventricular node [16]. Hypermagnesemia reduces atrioventricular and intraventricular conduction. Neither hypermagnesemia nor hypomagnesemia causes any specific ECG changes. Intravenous administration of magnesium sulfate to patients with a prolonged QT interval and torsade de pointes (TdP) may reverse ventricular tachycardia if baseline magnesium levels are normal or low. O. Takanaka et al. [28] studied the effects of magnesia and lidocaine on AP duration and barium-induced early afterdepolarization in canine Purkinje fibers. Their data confirm that hypomagnesemia can have an arrhythmogenic effect when combined with hypokalemia and bradycardia; under these conditions, the administration of magnesium can suppress trigger activity, mainly directly preventing the development of trigger APs. There were no specific electrophysiological effects or arrhythmias associated with isolated magnesium deficiency. However, magnesium may influence the development of cardiac arrhythmias through direct effects or by modulating the effects of potassium or acting as a calcium channel blocker. It is known that magnesium deficiency has a negative effect on the normal functioning of membrane ATPase, slowing down the transfer of sodium from the cell and potassium into the cell. This disrupts the transmembrane equilibrium of potassium and can lead to changes in membrane PP, changes in potassium transmembrane conductance, and disturbances in the repolarization phase [9]. There is evidence that dietary magnesium intake may have a moderate inverse correlation with the risk of developing coronary heart disease, particularly in men [5].
Release forms, dosage and rules of administration
There are several forms of potassium available on the market:
- Pills . They can cause irritation of the gastrointestinal mucosa, so taking them for a long time is not recommended.
- Capsules . They are absorbed faster than tablets, but if taken continuously, they can also be harmful to the sensitive lining of the intestines and stomach.
- Solutions . Safe for the gastrointestinal tract, quickly begin to act. Before use, they need to be diluted with a small amount of water or juice.
- Powder , soluble tablets. They do not have a negative effect on the digestive tract, but may be inconvenient to use. The tablets must be dissolved in a glass of water, and the powder must be swallowed during meals, having previously measured the required dose.
Taking potassium tablets
Instructions for using potassium may differ from one manufacturer to another. As a rule, for preventive purposes, you need to take 1 tablet or capsule per day with a low dosage of 99 mg, unless otherwise directed by your doctor.
Why is the dosage of only 99 mg in all supplements, which is only 2% of the daily value? There are two reasons:
- Research has found that oral medications containing potassium chloride, which in terms of net weight exceeds 99 mg, are dangerous to health (damage the walls of the small intestine).
- European and American regulatory organizations, based on scientific research, require manufacturers to label food products warning that they contain more than 99 mg of potassium, which is hazardous to health. All manufacturers have chosen safe dosage tactics. Otherwise, on each package there were such inscriptions that would repel all buyers.
To avoid stomach upset, you should take potassium supplements with food. Tablets and capsules should be swallowed without chewing. You cannot combine potassium vitamins with sparing diuretics (Amiloride, Triampur, etc.)
USE OF POTASSIUM AND MAGNESIUM PREPARATIONS IN CLINICAL PRACTICE
Briefly summarizing the above, it must be said that potassium and magnesium play an important role in the regulation of the functions of the cardiovascular system and central nervous system, ensure the normal course of numerous metabolic biochemical processes in almost all tissues and organs, regulate neuromuscular transmission, have a positive effect on phosphorus-calcium metabolism and support electrolyte balance in the body. Therefore, preparations of these macroelements can and should be used for a very wide range of indications - both in the treatment of various pathological conditions and for prophylactic purposes, primarily to prevent hypokalemia and hypomagnesemia in patients taking diuretics (thiazide and, especially, loop diuretics ) and (or) cardiac glycosides (digitalis preparations). In practical medicine, preparations containing one of these elements are used - potassium (potassium chloride, potassium orotate) or magnesium (Magne B6, Magnerot, magnesium sulfate), as well as complex preparations containing both cations (potassium aspartate + magnesium aspartate: Panangin, asparkam , potassium and magnesium aspartate BerlinChemie). In this series, one of the most effective and balanced in composition preparations of potassium and magnesium aspartate, solid experience of successful use of which has been accumulated by domestic and world medical practice, is Panangin. The combined administration of potassium and magnesium salts provides the advantage of a potentiating effect of their interaction. This combination is all the more justified since the metabolism of potassium and magnesium is closely related and clinically significant hypomagnesemia usually develops against the background of hypokalemia or complicates existing hypokalemia. Aspartic acid, which is part of Panangin, being a natural amino acid, easily penetrates the cell and facilitates the entry of potassium and magnesium into it. It is also useful because it is part of many proteins, plays an important role in the metabolism of nitrogenous substances, participates in transamination reactions and in the formation of pyrimidine bases of nucleic acids. Despite the real achievements of medical science in recognizing the etiopathogenesis of CVD, their prevalence is steadily growing, they remain the leading cause of disability and mortality in the population both in Russia and throughout the world. Therefore, the most relevant indications for the use of Panangin remain diseases such as chronic heart failure, ischemic heart disease, including myocardial infarction, cardiac arrhythmias, especially associated with taking digitalis drugs, and their combinations. Panangin is the drug of choice in the prevention and replenishment of potassium and magnesium deficiency and in other clinical situations. In the future, the indications for the use of Panangin will expand. Information is accumulating on the benefits of using potassium and magnesium for the treatment and prevention of cerebrovascular accidents and hypertension. Thus, replacing regular table salt with salt fortified with potassium and magnesium in individuals with normal or moderately elevated blood pressure led to a significant decrease in sodium intake and a decrease in systolic blood pressure [22]. A study by A. Ascherio et al., which included more than 43 thousand men aged 40 to 75 years, found a significantly lower relative risk of stroke (0.62) in people with high potassium intake (average - 4. 3 g/day) than in individuals with low consumption (on average 2.4 g/day) [6]. There was also an inverse correlation between magnesium intake (but not calcium intake) and the relative risk of stroke. All of these correlations were more pronounced in men with arterial hypertension than in normotensive individuals. The authors concluded that potassium supplementation may be more widely used in patients with hypertension. However, this information cannot justify the indiscriminate prescription of potassium supplements to everyone, since their uncontrolled use can be dangerous. Magnesium intake from drinking water has been reported to statistically significantly reduce the risk of death from cerebrovascular disease [32]. One prospective study showed that an increase in potassium intake by 10 mmol/day was accompanied by a significant reduction in the risk of death from stroke by 40% [15]. This effect was independent of other risk factors or variables, including magnesium intake. A recent meta-analysis of prospective studies found a statistically significant inverse correlation between potassium intake and the risk of stroke, especially ischemic stroke [17]. For every 1,000 mg increase in potassium intake, the risk of stroke decreased by 11%. Almost simultaneously with this work, another meta-analysis was published in the Journal of the American College of Cardiology, which reported a 21% reduction in the risk of stroke when taking a potassium supplement in a daily dose of 1.42 g and a significant reduction in the risk of coronary events and cardiovascular complications in general [8]. Potassium and magnesium preparations are also used in surgical anesthesiology. R. Soave et al. report that the use of magnesium in anesthesiological practice not only provides an antinociceptive effect due to the blockade of NMDA receptors and associated channels [33], but is also necessary to correct magnesium deficiency, which in its pure form was observed in 7–11% of hospitalized patients, and in combination with other electrolyte disturbances, especially hypokalemia and hypophosphatemia, in more than 40% of patients [25]. Replenishment of magnesium deficiency in this case, according to the observations of the authors, is necessary to prevent an increase in morbidity and mortality in the postoperative period. The administration of potassium and magnesium aspartate is especially important during operations on the heart and large vessels, since a significant decrease in the level of potassium and magnesium is also observed during extracorporeal circulation [3]. If potassium and magnesium aspartate were not pre-administered, hypomagnesemia developed in 50% of patients. Thus, potassium and magnesium preparations, including Panangin, are increasingly used in everyday clinical practice.
Literature 1. Vislobokov A.I., Ignatov Yu.D., Melnikov K.N. Pharmacological regulation of ion channels of neuronal membranes / St. Petersburg: Publishing house of St. Petersburg State Medical University. – 2006; 288 p. 2. Lesiovskaya E.E., Bakhtina S.M., Boyko I.N. Vitamins, macro- and microelements / St. Petersburg: publishing house of St. Petersburg KhFA. – 2004; 140 pp. 3. Trekova N.A., Andrianova M.Yu., Tolstova I.A., et al. The use of a solution of potassium and magnesium aspartate to maintain the balance of potassium and magnesium during cardiac surgery under artificial circulation // Anesthesia. and resuscitator. – 2008; 5:17–21. 4. Shestakova S.A., Dolgodvorov A.S., Kubynin A.N. Disturbances of water-electrolyte metabolism and their pharmacological correction / St. Petersburg: Publishing house of St. Petersburg State Medical University. – 2005; 91 p. 5. Al-Delaimy W., Rimm E., Willett W. et al. Magnesium intake and risk of coronary heart disease among men // J. Am. Coll. Nutr. – 2004; 23 (1): 63–9. 6. Ascherio A., Rimm E., Hernan M. et al. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men // Circulation. – 1998; 98:1198–204. 7. Cooper W., Kuan P., Reuben S., Vanderburg M. Cardiac arrhythmias following acute myocardial infarction: Assosiation with serum potassium level and prior diuretic therapy // Eur. Heart J. - 1984; 5:464–9. 8. D'Elia L., Barba G., Cappuccio F., Strazzullo P. Potassium intake stroke and cardiovascular disease. A meta-analysis of prospective studies // J. Am. Coll. Cardiol. – 2011; 57:1210–19. 9. Dykcner T., Wester P. Relation between potassium, magnesium and cardiac arrhythmias // Acta Med. Scand. Suppl. – 1981; 647:163–9. 10. Gettes L., Surawicz B. Effects of low and high concentrations of potassium on the simultaneously recorded Purkinje and ventricular action potentials of the perfused pig moderator band // Circ. Res. – 1968; 23: 717–29. 11. Hoffman B., Suckling E. Effect of several cation on transmembrane potentials of cardiac muscle // Am. J. Physiol. – 1956; 186:317–24. 12. Hohnloser S., Verrier R., Lown B., Reader E. Effect of hypokalemia on susceptibility to ventricular fibrillation in the normal and ischemic canine heart // Am. Heart J. - 1986; 112:32–5. 13. Ito S., Surawicz B. Transient, “paradoxical” effects of increasing extracellular K+ -concentration on transmembrane potential in canine cardiac Purkinje fibers // Circ. Res. – 1977; 41: 799–807. 14. Keller P., Aronson R. The role of magnesium in cardiac arrhythmias // Prog. Cardiovasc. Dis. – 1990; 32:433–48. 15. Khaw K., Barrett-Connor E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study // N. Engl. J. Med. – 1987; 316:235–40. 16. Kulick D., Hong R., Ryzen E. Electrophysiologic effects of intravenous magnesium in patients with normal conduction systems and no clinical evidence of significant cardiac disease // Am. Heart J. - 1988; 115:367–73. 17. Larsson S., Orsini N., Wolk A. Dietary potassium intake and risk of stroke. A dose-response meta-analysis of prospective studies // Stroke. – 2011; 42 (10): 2746–50. 18. Nordrehaug J., Johanessen K., Von der Lippe G. Serum potassium concentration as a risk factor of ventricular arrhythmias early in acute myocardial infarction // Circulation. – 1985; 71:645–49. 19. Paice B., Paterson K., Onyanga-Omara F. et al. Record linkage study of hypokaliemia in hospitalized patients // Postgrad. Med. J. – 1986; 62:187–91. 20. Pelzer D., Trautwein W. Currents through ionic channels in multicellular cardiac tissue and single heart cells // Experentia. – 1987; 43: 1153–62. 21. Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea pig heart // J. Physiol. – 1984; 347:641–57. 22. Sarkkinen E., Kastarinen M., Niskanen T. et al. Feasibility and antihypertensive effect of replacing regular salt with mineral salt – rich in magnesium and potassium – in subjects with mildly elevated blood pressure // Nutr. J.– 2011; 10: 88. 23. Schulman M., Narins R. Hypokalemia and cardiovascular disease // Am. J. Cardiol. – 1990; 65:4–9. 24. Sheu S, Korth M, Lathrop DA et al. Intra- and extra-cellular K+ and Na+ activities and resting membrane potential in sheep cardiac Purkinje strands // Circ. Res. – 1980; 47: 692–700. 25. Soave PM, Conti G., Costa R., Arcangeli A. Magnesium and anesthesia // Curr. Drug Targets. – 2009; 10 (8): 734–43. 26. Surawicz B. Relation between electrocardiogram and electrolites // Am. Heart J. – 1967; 73:814–43. 27. Surawicz B., Lepeschkin E., Herrlich H. Low and high magnesium concentrations at various calcium levels: Effect on the monophasic action potential, electrocardiogram and contractility of isolated rabbit hearts // Circ. Res. – 1961; 9: 811–8. 28. Takanaka C., Ogunyankin K., Sarma J., Singh B. Antiarrhythmic and arrhythmogenic actions of varying levels of extracellular magnesium: Possible cellular basis for the differences in the efficacy of magnesium and lidocaine in torsades de pointes // J. Cardiovasc . Pharmacol. Ther. – 1997; 2: 125–34. 29. Thompson R. Gobb L., Hypokalemia after resuscitation out-of-hospital ventricular fibrillation // JAMA. – 1982; 248:2860–3. 30. Tsuji H., Venditti F., Evans J., et al. The associations of levels of serum potassium and magnesium with ventricular premature complexes (the Framingham Heart Study) // Am. J. Cardiol. (US). – 1994; 74:232–5. 31. Yang T., Roden D. Extracellular potassium modulation of drug block of Ikr // Circulation. – 1996; 93:407–11. 32. Yang C. Calcium and magnesium in drinking water and risk of death from cerebrovascular disease // Stroke. – 1998; 29:411–4. 33. Zhu Y., Auerbach A. K+-occupancy of the n-methyl-d-aspartate receptor channel probed by Mg2+-block // J. Gen. Physiol. – 2001; 117(3): 287–98.
What foods contain potassium?
Potassium in food
Table of potassium content in food:
| The product's name | Potassium content per 100 g | Percentage of daily requirement |
| Dried white mushrooms | 3937 mg | 157% |
| Dried peach | 2043 mg | 82% |
| Dried apricots | 1781 mg | 71% |
| Dried apricots | 1717 mg | 69% |
| Soybean (grain) | 1607 mg | 64% |
| Wheat bran | 1260 mg | 50% |
| Low-fat dry milk | 1224 mg | 49% |
| Powdered milk 25% | 1200 mg | 48% |
| Beans (grain) | 1100 mg | 44% |
| Pistachios | 1025 mg | 41% |
| Powdered milk 15% | 1010 mg | 40% |
| Mash | 1000 mg | 40% |
| Sea kale | 970 mg | 39% |
| Chickpeas | 968 mg | 39% |
| Dried pear | 872 mg | 35% |
| Prunes | 864 mg | 35% |
| Raisin | 830 mg | 33% |
| Parsley (greens) | 800 mg | 32% |
| Spinach (greens) | 774 mg | 31% |
| Almond | 748 mg | 30% |
| Peas (shelled) | 731 mg | 29% |
| Dry cream 42% | 726 mg | 29% |
| Dried figs | 710 mg | 28% |
| Dried acorns | 709 mg | 28% |
| Lentils (grain) | 672 mg | 27% |
| Peanut | 658 mg | 26% |
| Sunflower seeds (seeds) | 647 mg | 26% |
| Watercress (greens) | 606 mg | 24% |
| Pine nut | 597 mg | 24% |
| Dried apples | 580 mg | 23% |
| Horseradish (root) | 579 mg | 23% |
| Buckwheat flour | 577 mg | 23% |
| Potato | 568 mg | 23% |
| Oat bran | 566 mg | 23% |
| Cashew | 553 mg | 22% |
| Champignon mushrooms | 530 mg | 21% |
| Parsnip (root) | 529 mg | 21% |
| Cilantro (greens) | 521 mg | 21% |
| Sorrel (greens) | 500 mg | 20% |
| Sesame | 497 mg | 20% |
| Avocado | 485 mg | 19% |
| Walnut | 474 mg | 19% |
| Porcini mushrooms | 468 mg | 19% |
| Milk chocolate | 462 mg | 18% |
| Barley (grain) | 453 mg | 18% |
| Chanterelles | 450 mg | 18% |
| Halibut | 450 mg | 18% |
| Egg powder | 448 mg | 18% |
| Hazelnut | 445 mg | 18% |
Potassium is included in many products, but it must be taken into account that during heat treatment a significant part of this substance is destroyed (source)