What should you remember when determining the concentration of troponin in the blood? D.S.Ben…
Home \ Publications \ What should you remember when determining the concentration of troponin in the blood? D.S.Benevolensky
What should you remember when determining the concentration of troponin in the blood? D.S. Benevolensky , product specialist at
Radiometer Medical ApS (Denmark) Clinical need for determining the concentration of troponin in the blood. Determining the concentration of cardiac troponins I or T in the patient’s blood is the cornerstone of modern laboratory diagnosis of myocardial infarction. According to the recommendations of both the European Society of Cardiology [1] and the National Academy of Clinical Biochemistry (USA) [2], determination of cardiac troponin concentrations should be carried out in all patients with suspected acute coronary syndrome. Similar recommendations have been adopted by the All-Russian Scientific Society of Cardiologists. Order of the Ministry of Health and Social Development of the Russian Federation dated August 19, 2009 No. 599n “On approval of the procedure for providing planned and emergency medical care to the population of the Russian Federation for diseases of the circulatory system of a cardiological profile” prescribes to all medical institutions where emergency care is provided to patients with cardiac -vascular diseases, determine the level of troponins “in an emergency (urgent) manner and at any time of the day.” This attention to troponins, and more specifically to the cardiac isoforms of troponins I and T, is determined by their unique biochemical structure, which makes it possible to create test systems with the highest clinical sensitivity and specificity for myocardial damage. In 2007, by decision of the most authoritative American, European and international cardiological associations, the diagnosis of myocardial infarction was directly related to an increase in troponin levels in the blood [3]. According to this document, “the term myocardial infarction should be used when there is evidence of myocardial necrosis in the presence of a clinical picture consistent with myocardial ischemia.” Moreover, data on myocardial necrosis is understood as “detection of an increase and/or decrease in the level of cardiac biomarkers (preferably troponin) with at least one value exceeding the upper reference limit,” and at least one of the following is considered sufficient evidence of ischemia: • The presence of symptoms ischemia; • ECG changes indicating the appearance of ischemia (the appearance of ST-T changes or the appearance of a left bundle branch block); • Development of a pathological Q wave on the ECG; • Radiation findings indicating loss of viable myocardium or the appearance of local wall motion abnormalities. In fact, there is only one option for making a diagnosis without determining the level of troponins: “sudden, unexpected cardiac death, including cardiac arrest, often with symptoms of myocardial ischemia, and accompanied by presumably new ST elevation, or new LBBB, and/or detection of a new thrombus at coronary angiography and/ or autopsy, but death that occurred before blood samples were collected or before cardiac biomarkers appeared in the blood.” To diagnose acute myocardial infarction, it is extremely important to detect a rise and/or fall in troponin levels, that is, a “wave” of concentration that occurs after the appearance of areas of necrosis, and at least at one point this concentration must be above the upper reference limit (URL) during the first 24 hours after a painful attack [2]. The upper reference limit is established based on a study of a group of at least 120 healthy people with no history of heart disease. For troponins I and T, the threshold value for detecting cardiac damage is recognized as the 99th percentile of the distribution of the results obtained, that is, in 99% of healthy people the level of the corresponding analyte in plasma is below this value. Ideally, each laboratory should establish its own VRP for each analyzer, appropriate to the local population. However, given the complexity of conducting such a study, it is permissible to rely on the figures provided by equipment manufacturers. According to the recommendation of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), the analytical sensitivity of the method should be approximately 5 times lower than the clinically significant threshold level [4]. In addition, the total error, determined by the coefficient of variation (CV), on the GRP should not exceed 10%, otherwise the test will give many false positive and false negative results. Out-of-laboratory diagnostic devices (POCT analyzers) must meet the same criteria [4]. Using the 99th percentile of the distribution of troponin concentrations in healthy subjects as the GRP provides excellent clinical sensitivity and specificity for acute myocardial infarction. Radiometer's AQT 90 FLEX non-laboratory emergency diagnostic analyzer The concentration of 0.023 μg/l, corresponding to the 99th percentile, was taken as the threshold value that distinguishes normal from pathology.
| Table 1. Clinical sensitivity and specificity for acute myocardial infarction at the level of GRP (99th percentile) | |||
| Upon admission (0–2 hours) | 6–9 hours after admission | ||
| Sensitivity | Specificity | Sensitivity | Specificity |
| 65% | 91% | 91% | 88% |
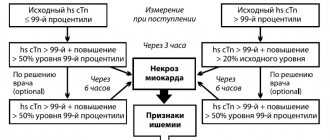
A fairly accurate quantitative determination of troponin levels allows not only to confirm myocardial necrosis, but also to determine the degree of risk of serious complications and death in order to select the most adequate treatment method. Figure 1 shows a simplified algorithm for diagnosing acute coronary syndrome, adopted by the European Society of Cardiology.
| Admission | ® | Chest pain | ||
| Working diagnosis | ® | Suspicion of acute coronary syndrome | ||
| ECG | ® | Persistent ST segment elevation | ST/T disorders | Normal or indeterminate ECG changes |
| ↓ ↘ | ↙ ↓ | |||
| Biochemistry | ® | Troponin ↑ | Troponin negative twice | |
| Risk stratification | ® | High risk | Low risk | |
| Diagnosis | ® | MI (+) ST | MI (–) ST | Unstable angina |
| Treatment | ® | Reperfusion | Invasive | Non-invasive |
Figure 1. Examination algorithm for acute coronary syndrome [1]. All current clinical and laboratory guidelines do not distinguish between troponin I and T from the point of view of diagnosing myocardial infarction.
Difficulties in determining the concentration of troponins in the blood. In the blood of healthy people, the concentration of these proteins is extremely low. The concentration of troponin I at the level of VRP is approximately 3,000,000,000 less than the total concentration of proteins in plasma, 5000 and 300 times less than the corresponding concentrations of other well-known biomarkers of necrosis - myoglobin and creatine kinase MB. The triple complex of troponins I, T and C, entering the blood from necrotic cardiomyocytes, breaks down into free troponin T and a double complex of troponins I and C. These are the main forms of troponins in the blood. However, already inside cardiomyocytes, the proteolytic cleavage of troponins I and T[5] begins. In the blood, proteolysis continues, which leads to the gradual cleavage of the terminal fragments of troponin I molecules (Fig. 2). In addition, troponin I undergoes phosphorylation/dephosphorylation and oxidation. Therefore, it is not troponin itself that circulates in the blood, but a constantly changing spectrum of its modified fragments. All currently available methods for determining the concentration of troponin are based on an immunochemical reaction, the so-called sandwich analysis. Typically, 1–2 types of antibodies are used to bind the antigen and 1–2 types of detector antibodies, labeled in some way. Mouse monoclonal antibodies produced against a specific epitope of cardiac troponin are used. They are able to contact only some fragments of the troponin molecule. Differences in antibody sets among different test manufacturers are the main reason for the spread in absolute values obtained by different test systems when determining troponin in the blood of patients. A special committee of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), together with the American Association of Clinical Chemistry (AACC), has made significant efforts to standardize troponin I tests. The US National Institute of Standards and Technology (NIST) has adopted a new standard reference material for the human cardiac troponin complex, SRM 2921, which should assist in the future standardization of troponin tests. With almost perfect correlation of values obtained by the best test systems, the absolute value of the concentration can differ by 5–10 times. Therefore, direct comparison of absolute values is not possible, and normal limits must be determined separately for each method. Although the clinical interpretation of the results, taking into account the relevant reference ranges, is generally consistent for all tests, this situation creates an obvious inconvenience. There are also “gray zones”, especially in the range of values close to GRP. This is partly due to the statistical nature of the data obtained, and partly due to the difficulty and inaccuracy of determining the GRP, that is, the 99th percentile of the concentration distribution in a group of healthy people. Until recently, test systems for determining troponin T were produced by only one manufacturer - RocheDiagnostics, so the results of determining its concentration by different analyzers were comparable.
| AQT 90 FLEX analyzer can detect both troponin I and troponin T in a single sample. |
It is necessary to remember the statistical nature of the values obtained as a result of the analysis, and the lower the concentration, the greater the determination error. For example, if the true concentration of troponin in the sample is 0.039 µg/l, and the CV at this point is 10%, then with ideal operation of the analyzer, according to the laws of statistics, out of 1000 determinations, 956 will fall in the range of 0.031–0.047 µg/l, but in one case the obtained the value will be below 0.027 µg/l, and in another one – above 0.051 µg/l. Possible errors when determining the concentration of troponins in the blood. If laboratory data differ significantly from the clinical picture, it is possible that this was due to an analytical error. Preanalytical errors
. The preanalytical stage is the source of approximately 60–70% of errors in the express laboratory[6]. They may be caused by incorrect identification of the patient or sample, errors in blood sample collection, use of incorrect tubes or anticoagulants, or improper sample handling and storage. A significant number of errors can be avoided by using standard blood collection devices, such as vacuum tubes. When working with serum, the cause of false-positive troponin results may be the presence of fibrin clots as a result of incomplete blood coagulation, for example in patients with coagulopathy or receiving anticoagulant therapy. Errors may also be caused by other microparticles in the sample. Some test systems are very sensitive to hemolysis.
| AQT 90 FLEX analyzer works with most manufacturers' standard heparin or EDTA vacuum tubes and does not require any handling or sample dispensing, saving time and eliminating a significant portion of preanalytical errors. The built-in scanner recognizes the barcode on the tube. The use of the 1stautomatic system allows you to link data about the patient, sample and operator and transfer it to the information system during sample collection, which eliminates identification errors. |
Analytical errors.
Incorrect operation of the analyzer is a possible cause of analytical errors. The first step is to ensure that the analyzer is calibrated to the manufacturer's specifications and that proper quality control has been carried out. A hidden reason for obtaining false-negative or false-positive troponin results may be the presence of interfering substances in the blood sample, for example: heterophile antibodies, human antibodies against animal antigens, rheumatoid factor and autoantibodies to troponin; endogenous blood components such as bilirubin and hemoglobin; immune complexes; high concentrations of alkaline phosphatase Heterophilic antibodies are antibodies formed against unknown antigens (often foreign proteins). Human antibodies against animal antigens are circulating human antibodies against animal proteins. Circulating antibodies specific to a wide range of animal immunoglobulins have been reported, such as mouse, rat, rabbit and others. A patient may develop these antibodies due to various reasons, including the use of mouse monoclonal antibodies for radiological diagnosis and treatment of cancer; contact with microbial antigens; exposure of veterinarians, farmers and food industry workers to foreign proteins; presence of pets in the house. Autoimmune diseases lead to the formation of autoantibodies such as rheumatoid factor (antibodies to the Fc fragment of immunoglobulins G). The prevalence of interfering antibodies among the population is quite high. All of these antibodies in the blood can cross-react with the binding and detector antibodies of the test system, leading to a positive result in the absence of troponin in the sample. In addition, in 5.5% of people without signs of cardiovascular disease and in 21% of patients with acute autoantibodies to troponin I were detected in coronary syndrome [7]. By interacting with the central part of the troponin molecule, they can block the binding of test system antibodies, which sometimes leads to false negative test results. Due to the wide variety of interfering antibodies, there is no specific test that could confirm or exclude the presence of interfering antibodies in the sample does not exist. Laboratory professionals should suspect the presence of interfering antibodies in a troponin test when the test result: is inconsistent with the clinical data of the patient; is not reproducible on the same or another test system; the result is nonlinear after a series of dilutions.
| The test system of the Radiometer AQT 90 FLEX contains mouse IgG and bovine γ-globulin to prevent the effect of interfering antibodies. One of the binding and detector antibodies of the test system interacts with troponin outside its central region - the target of autoantibodies to troponin, which reduces the influence of autoantibodies on the test results. A purely physical method was chosen as a method for obtaining a signal from detector antibodies: time-resolved fluorescence of europium chelate. This eliminates the influence of excess alkaline phosphatase noted with the enzymatic method. Finally, hemolysis does not affect the results of either troponin I or troponin T assays [8]. |
Post-analytical errors.
Processing information, comparing with reference ranges and transferring data to the attending physician are significantly simplified, and the risk of errors is reduced when the analyzer is connected to an information system. It is important to remember that troponins are not released into the blood immediately after necrosis occurs. Therefore, the result of troponin determination may be negative against the background of obvious signs of acute myocardial infarction if blood samples were taken in the first hours after the onset of a painful attack. At the same time, analysis of a blood sample immediately after the patient's admission to the emergency department provides important clinical information (Table 1). The most important part of the post-analytical stage is to help clinicians interpret the data obtained. This requires a thoughtful approach and the development of general rules for assessing the results obtained by different test systems, for example, on portable analyzers at the patient's bedside and in a central laboratory. Although an increase in troponin levels in the absence of acute myocardial infarction occurs quite rarely, it can mislead the doctor. If there is doubt about the reliability of the result, you should try to find another possible clinical reason for the increase in troponin levels that is not associated with myocardial infarction, re-analyze the clinical picture and prescribe additional diagnostic tests. Possible reasons for increased troponin concentrations are given in Table 2.
| Table 2. Reasons for increased troponin levels in the absence of coronary heart disease [3]. |
| Cardiac contusion or other trauma due to surgery, catheter destruction, cardiac pacing, etc. Congestive heart failure - chronic or acute Aortic dissection Aortic valve disease Hypertrophic cardiomyopathy Tachy or bradyarrhythmias or atrioventricular block "Broken heart" syndrome (Takotsubo cardiomyopathy) Rhabdomyolysis with cardiac involvement Throm pulmonary embolism, severe pulmonary hypertension Renal failure Acute neurological diseases, including stroke and subarachnoid hemorrhage Infiltrative diseases such as amyloidosis, hemochromatosis, sarcoidosis and scleroderma Inflammatory diseases such as myocarditis or myocardial damage in endo- or pericarditis Drug or toxic substance use Critical conditions, especially in combination with respiratory failure or sepsis Burns , especially if they affect >30% of the body surface Excessive exercise |
What to do if you have doubts about the correct determination of troponins in the blood? 1) First of all, you need to make sure that the analyzer is working correctly. To achieve this, calibrations and maintenance must be carried out at the frequency recommended by the manufacturer. When in doubt, measure quality control samples according to the manufacturer's recommendations. At least one control sample must meet the clinically relevant threshold concentration (CRL) of troponin. 2) Check that the blood sample was taken correctly, and repeat the sample collection and testing. If the analyzer passes all quality controls, the blood sample is taken in accordance with the manufacturer's instructions, and the analysis result still does not correspond to the clinical picture, then you need to try to find a hidden reason in the characteristics of the sample or disease: 1) Repeat the analysis of the sample after several dilutions and check linearity of results, the violation of which makes it possible to identify interfering antibodies. 2) Use commercially available heterophilic blocking agents (usually a mixture of immunoglobulins) or normal mouse (or other animal) serum as the blocking agent. 3) Check for the possibility of a negative effect of bilirubin and/or hemoglobin. 4) If possible, repeat the analysis of the same sample using a different test system to check the result. If there is no other test system, then send the sample to another laboratory for testing in a different way. 5) If sufficient sample is available, retain a portion of the sample for further testing by another laboratory or manufacturer. 6) Discuss with the attending physician the contradictions between the test result and the clinical picture. Re-evaluate the clinical picture and order additional diagnostic tests (eg, consider another cause of chest pain, repeat ECG, etc.), and try to find another possible clinical cause of elevated troponin levels that is not related to myocardial infarction.
REFERENCES [1] Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. European Heart Journal
(2007) 28, 1598–1660.
[2] National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical Characteristics and Utilization of Biochemical Markers in Acute Coronary Syndromes. Circulation
(2007) 115, e356–e375.
[3] Thygesen K, Alpert JS, White HD on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation
(2007) 116(22), 2634-53.
[4] Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: Analytical issues for biochemical markers of acute coronary syndromes. Circulation
(2007) 115(13), e352-5.
[5] Labugger R, Organ L, Collier Ch, Atar D, Van Eyk JE. Extensive Troponin I and T Modification Detected in Serum From Patients With Acute Myocardial Infarction. Circulation
(2000) 102, 1221–1226.
[6] Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clinical Chemistry
(2007) 53(7), 1338-42.
[7] Pettersson K, Eriksson S, Wittfooth S, Engström E, Nieminen M, Sinisalo J. Autoantibodies to Cardiac Troponin Associate with Higher Initial Concentrations and Longer Release of Troponin I in Acute Coronary Syndrome Patients. Clinical Chemistry
(2009) 55, 938-945. [8] Bais R. Characterization of the Radiometer AQT90 Flex Troponin I and T Assays. Abstract AACB Conference 2010.
Figure 2. Factors influencing the choice of antibodies for troponin I detection.
News all
07/06/2021 X Baltic Forum "Current problems of anesthesiology and resuscitation" June 30 - July 3, 2021
06/03/2021 The 7th Conference “Laboratory Diagnostics of Emergency Conditions 2021” took place
05/11/2021 Conference “Laboratory diagnostics of emergency conditions 2021” June 3, Novotel, St. Petersburg
01/22/2021 Seminar for managers of veterinary clinics
04/09/2020 Manufacturers and suppliers called for simplification of registration of medical devices
Introduction
Despite clinical trial results showing benefits of statin therapy in asymptomatic patients at high cardiovascular risk, debate continues about the balance between the benefits and harms of therapy for primary prevention.
Importantly, high troponin levels may reflect subclinical coronary artery disease (CAD) and may help identify high-risk individuals who are candidates for targeted preventive therapy. The purpose of the WOSCOPS analysis was to evaluate the ability of cardiac troponin I, measured at baseline and 1 year after statin treatment, to predict coronary events.
general characteristics
High-sensitivity troponin I (hs-cTnI) is a sensitive early biomarker of myocardial infarction and an independent predictor of adverse outcomes in any symptomatic and positive patient with acute coronary syndrome (ACS). hs-cTnI have better diagnostic accuracy and the potential to detect myocardial damage earlier than current cTn tests. Low concentrations of hs-cTn can completely exclude acute myocardial infarction. In patients with congestive heart failure, high serum hs-cTn concentrations are a useful prognostic predictor independent of either reduced LV ejection fraction or BNP levels, suggesting that elevated hs-cTnI concentrations sensitively reflect developing myocardial injury.
Indications for use
1. The presence of symptoms indicating myocardial ischemia, incl. without any changes on the ECG. 2. Diagnosis of myocardial infarction in patients with chronic renal failure who have symptoms of myocardial ischemia (regardless of the severity of kidney damage). 3. Diagnosis of myocardial damage in patients receiving chemotherapy. 4. Patients with heart failure with or without preserved ejection fraction to assess the risk of mortality or hospitalization. Troponin levels greater than the 99th percentile are associated with a high likelihood of poor outcome. 5. All patients with signs of acute heart failure to exclude acute myocardial infarction.