Heart Attack Symptoms
Because cardiogenic shock typically occurs in people who have had a severe heart attack, it is important to know the signs and symptoms of a heart attack. These include:
- Pressing, bursting or squeezing pain in the chest lasting more than 15 minutes;
- Pain radiating to the shoulder, arm, back or teeth and lower jaw;
- Increased frequency of attacks of chest pain;
- Prolonged pain in the upper abdomen;
- Dyspnea;
- Sweating;
- A looming feeling of fear;
- Fainting;
- Nausea and vomiting.
If you see your doctor right away when these signs or symptoms appear, you can prevent the possibility of developing cardiogenic shock. Prompt treatment of a heart attack increases the chances of survival and reduces damage to the heart. Don't ignore these symptoms if they last more than five minutes. Call an ambulance immediately. If you cannot call an ambulance, ask someone to take you to the nearest hospital.
Cardiogenic shock during myocardial infarction - symptoms
The main manifestations increase quickly. The patient turns pale, the skin becomes cold and clammy, blood pressure drops sharply, the pulse is thready, breathing is rapid. Possible loss (confusion) of consciousness.
Emergency care algorithm for cardiogenic shock:
- call an ambulance;
- Place the patient on a hard surface with his legs slightly elevated;
- free the chest from constrictive clothing;
- provide access to fresh air;
- perform resuscitation measures in case of cardiac arrest.
More detailed recommendations for providing first aid for cardiogenic shock are described on the pages of our website.
Causes
Cardiogenic shock occurs when the heart loses its ability to pump enough blood to the rest of your body. The most common cause of cardiogenic shock is damage to the left ventricle, the main pumping chamber of the heart, due to lack of oxygen due to a heart attack.
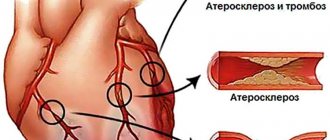
A heart attack occurs when one or more of the arteries that carry oxygenated blood to your heart (coronary arteries) becomes blocked. Sometimes, over time, a narrowing of the coronary arteries occurs due to the deposition of cholesterol on their walls. The formation of these deposits, called plaques, in the arteries throughout the body is called atherosclerosis.
During a heart attack, one of these plaques may rupture, forming a blood clot at the site of the rupture, blocking blood flow through the arteries. Without oxygenated blood entering the heart, the heart muscle weakens and cardiogenic shock develops.
In rare cases, cardiogenic shock develops when the right ventricle of the heart is damaged. From the right ventricle of the heart, blood travels to the lungs, where it is enriched with oxygen before being sent to the rest of your body. Damage to the right ventricle causes the heart to lose its ability to pump blood effectively to the lungs, causing the body to not receive enough oxygen.
Although a heart attack is the most common cause, other conditions that cause cardiogenic shock include inflammation of the heart muscle (myocarditis) or infection of the heart valves (endocarditis). Causes include drug overdose or poisoning from substances that affect the pumping function of your heart.
Cardiogenic shock: what's new? Review of the European Society of Cardiology consensus document
Despite significant advances made in the treatment of chronic heart failure, the prognosis of patients with acute heart failure and its most severe form, cardiogenic shock, still remains extremely unfavorable. Thus, in-hospital mortality in patients with cardiogenic shock ranges from 30 to 60%, and about half of these deaths occur in the first 24 hours after the patient is admitted to the hospital. All this actualizes the need to systematize knowledge and approaches to the management of patients with cardiogenic shock. In this regard, the European Society of Cardiology and the European Heart Failure Association issued a consensus document on the epidemiology, pathophysiology and management of patients with cardiogenic shock [1].
At the very beginning, the authors emphasize that a single definition of cardiogenic shock still does not exist. Its main symptom, which is also typical for other types of shock, is the presence of severe organ hypoperfusion, leading to hypoxia of organs and tissues, and the cause of this hypoperfusion is low cardiac output caused by primary cardiac pathology. Despite the fact that the final pathophysiological changes are similar in all patients with cardiogenic shock, such shock is polyetiological, and determination of the cause of shock should form the basis of its therapy.
According to current guidelines for the management of patients with heart failure [2], one of the signs of cardiogenic shock is the presence of hypotension, which is defined as a decrease in systolic blood pressure less than 90 mmHg. Art. for more than 30 minutes or the need for catecholamines to maintain systolic blood pressure at >90 mmHg. Art. The new document emphasizes that hypoperfusion is not always combined with hypotension, and therefore, when assessing patients with suspected cardiogenic shock, more attention should be paid to the clinical and laboratory signs of hypoperfusion.
In addition, for the first time, the authors proposed a classification of stages of cardiogenic shock based on the severity of patients and their response to therapy. Thus, three states are distinguished: 1)
pre-cardiogenic shock (clinical signs of peripheral hypoperfusion with normal systolic blood pressure; it is important to separate this stage from “normotensive” cardiogenic shock),
2)
cardiogenic shock and
3)
refractory cardiogenic shock (cardiogenic shock with signs of tissue hypoperfusion, despite adequate doses of two vasoactive drugs and treatment of the condition that contributed to the development of cardiogenic shock).
It is noted that a few decades ago, the leading (81%) cause of cardiogenic shock was acute coronary syndrome with the development of myocardial infarction, while now, due to the improvement of methods of treatment of myocardial infarction, most cases of cardiogenic shock are caused by a cause not related to acute coronary syndrome, for example , ischemic cardiomyopathy, non-ischemic cardiomyopathy, ventricular tachycardia, severe valve pathology, pulmonary embolism, etc. And it is the timely determination of the cause and, if possible, the start of therapy specific to it, according to the authors, that determine the success of treatment of cardiogenic shock.
Methods of examination and treatment of patients with cardiogenic shock
The methods proposed in the document for studying patients with cardiogenic shock are relatively standard. For clinical signs, it is noted that when monitoring the patient, it is especially important to monitor the level of systolic blood pressure in patients with “normotensive” shock, since its decrease may be an early marker of deterioration in the condition of such patients.
All patients with cardiogenic shock are recommended to immediately register an electrocardiogram and connect them to electrocardiographic monitoring, due to the need to identify ischemic changes if they are present or early registration of life-threatening cardiac arrhythmias.
Of course, one of the leading methods is echocardiography. It is noted that, if possible, a full (rather than a focal) study should be immediately performed with the involvement of an expert in this field. And it is echocardiography that often makes it possible to determine the cause of the development of cardiogenic shock.
The role of noninvasive hemodynamic monitoring is still uncertain, but invasive hemodynamic evaluation is recommended in all patients. However, according to the results of the relatively recently published ESCAPE study [3], the strategy of adjusting therapy based on pulmonary artery pressure in such patients is unlikely to be successful and is not recommended in this document.
Of the biochemical indicators, the assessment of lactate level is important, since it is its increase that serves as a marker of the development of tissue hypoxia and is associated with a worse prognosis. Daily assessment of complete blood count, electrolytes, creatinine, liver function assessment, coagulation profile, troponin, and arterial blood gases is also recommended.
As has been noted many times before, the management of patients with cardiogenic shock is determined by the leading cause of its development. Therefore, distinguishing shock associated with acute coronary syndrome and other causes is critical. In the first case, immediate coronary angiography with possible percutaneous coronary intervention is necessary. Which, in accordance with the results of the CULPRIT-SHOCK study [4], should be performed initially only for infarction of the responsible artery. With the development of mechanical complications of a heart attack (acute mitral regurgitation, rupture of the interventricular septum, etc.), surgical intervention is advisable, and before it is performed, it is possible to use mechanical circulatory support devices (intra-aortic balloon counterpulsation, etc.). In the case of cardiogenic shock not associated with acute coronary syndrome, the goal should also be to determine the cause of shock and eliminate it as much as possible (drainage of the pericardial cavity during cardiac tamponade, etc.).
In conclusion, I would like to note that given the low level of evidence for most recommendations, the severity and complexity of managing patients with cardiogenic shock, the so-called “heart team”, including cardiologists, specialists in intravascular interventions and surgeons, should be involved in decision-making. And treatment of such patients, if possible, is recommended to be performed in expert centers with extensive experience in managing patients with cardiogenic shock.
Sources:
1. Chioncel O, et al. Eur J Heart Fail. 2020;10.1002/ejhf.1922. doi:10.1002/ejhf.1922
2. Ponikowski P, et al. Eur J Heart Fail. 2016;18(8):891-975. doi:10.1002/ejhf.592
3. Binanay C, et al. JAMA. 2005;294(13):1625-1633. doi:10.1001/jama.294.13.1625
4. Thiele H, et al. N Engl J Med. 2017;377(25):2419-2432. doi:10.1056/NEJMoa1710261
Complications
If not treated promptly, cardiogenic shock becomes a fatal condition. Another serious complication of cardiogenic shock is organ damage.
If the heart cannot pump enough oxygenated blood to the rest of your body, damage to the liver, kidneys and other organs develops. When the liver and kidneys are damaged, cardiogenic shock is worsened because the kidneys release chemicals into the blood that support muscle function and the liver produces proteins that help blood clot. With long duration of cardiogenic shock, permanent organ damage can develop.
Diagnostic methods
Diagnosis of cardiogenic shock requires immediate action. Doctors will check for signs and symptoms of shock and then order additional tests to determine the cause of your condition. Methods for diagnosing cardiogenic shock include:
- Blood pressure measurement. People with shock often have low blood pressure. If a person in shock is taken to hospital by ambulance, blood pressure is measured before admission to hospital.
- Electrocardiogram (ECG). This is the first test to diagnose a heart attack. It is often done at the same time as a survey about symptoms. The test involves recording the electrical activity of the heart using electrodes attached to the skin. The pulses appear as "waves" displayed on a monitor or printed on paper. Because the heart muscle cannot conduct electrical impulses normally when damaged, an ECG can help determine whether you are having a heart attack or are in the process of having one.
- X-ray of the chest organs. A chest x-ray will help your doctor evaluate the size and shape of your heart and its blood vessels.
- Blood tests. Blood tests can determine if you have kidney or liver damage, detect signs of heart infection, and also determine if you are having a heart attack. Another type of blood test (arterial blood gas test) is also ordered to determine the amount of oxygen in the blood.
- Echocardiogram. This test uses sound waves to produce images of the heart. During echocardiography, sound waves are sent to the heart from a wand-shaped device (transducer) placed on the chest. Sound waves bounce off the heart and back through the chest and are processed electronically to produce a video image of the heart. An echocardiogram can help identify areas of damage to your heart and problems with the heart's pumping function.
- Catheterization of coronary arteries (angiography, coronary angiography). This test will look for narrowing and blockage of the coronary arteries. Liquid contrast dye is injected into the arteries of the heart through a long, thin tube (catheter) that is passed through an artery in the leg or arm to the arteries of the heart. As the contrast agent fills the arteries, they become visible on X-rays, revealing areas of blockage.
Additionally, during catheterization, your doctor may remove the blockage in the artery through coronary angioplasty and stenting. Angioplasty uses tiny balloons that are inserted through a blood vessel into the coronary artery to widen the blocked area. After angioplasty, a mesh tube (stent) is placed inside the artery to maintain sufficient lumen and prevent re-narrowing in the future.
Despite numerous studies and the use of the latest modern drugs, technologies and devices, cardiogenic shock (CS) remains the main and leading cause of death in patients with acute myocardial infarction (AMI) and a number of other acute heart diseases [12, 14]. This severe cardiac complication is stage IV of acute heart failure according to the classification of T. Killip (1967), which is characterized by systolic blood pressure less than 90 mm Hg, signs of systemic hypoperfusion and peripheral vasoconstriction, oliguria, cyanosis, sweating. Pulmonary rales may be heard, but may be absent. In ⅓ of patients with CABG, pulmonary edema is not diagnosed either clinically or according to instrumental methods [24, 30].
The incidence of CABG in patients with ST-segment elevation AMI (STEMI) according to various authors, studies and international registries is different and varies from 3.4 to 15% [60]. The largest studies (NRMI-2, 3, 4), which analyzed data from 1,970,000 patients in the United States with STEMI obtained in 1994-2004, demonstrated an overall rate of CABG of 8.6%, with no trend to decrease or increase over time. specified period [3].
A slightly lower incidence of CABG is observed in patients with non-ST segment elevation AMI (NSTEMI). In the GUSTO IIb study, which included 7986 patients with NSTEMI, CABG developed in 2.6% [26], the same incidence of CABG (2.5%) in NSTEMI was reported in the PURSUIT study [19]. In the more recent GRACE registry, the incidence of CABG over a 6-year period (1999–2006) decreased from 2.1 to 1.8% [13].
Mortality in CABG ranges from 50 to 90% in all age groups [12], while the majority of patients with CABG are women, elderly and senile people, patients with concomitant diabetes mellitus and arterial hypertension [14].
Unfortunately, a generally accepted optimal treatment strategy for patients with CABG has not yet been developed [29].
Myocardial revascularization—percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)—is one of the main and defining moments in the treatment of patients with AMI complicated by CABG. Revascularization can significantly reduce mortality and improve treatment outcomes in this group of patients [12, 29, 57] both in the early postoperative period and in the long term [24, 33]. According to the 2013 ACC/AHA guidelines for the treatment of STEMI, urgent revascularization through PCI or CABG for CABG is recommended for all eligible patients with CABG events, regardless of the timing of disease onset (Evidence Level IB). If it is impossible to perform myocardial revascularization by any method and in the absence of contraindications, thrombolytic therapy is recommended (level of evidence IB) [36].
The problem regarding the volume of myocardial revascularization during CABG remains unresolved. The 2012 ACC/AHA/SCAI guidelines indicate that in multivessel disease, revascularization of infarct-unrelated arteries may be necessary to maximize myocardial perfusion. As an alternative strategy, in this case, CABG is preferable [32]. Complete myocardial revascularization during CABG according to D. Mylotte et al. [33] improved 6-month survival: 43.9% (revascularization of the infarct-related artery (ISA) only) versus 20.4% ( p
<0,002).
In the GUSTO-I trial, PCI resulted in a 22% reduction in 30-day mortality. Moreover, mortality was lower both among patients in whom CABG developed before admission and in the group of patients in whom CABG occurred after admission [25]. An analysis of 2000 patients with CABG demonstrated the advantage of the myocardial revascularization strategy: 30-day mortality in the PCI and CABG group was 38%, while drug therapy was associated with 62% of deaths [4]. A recent registry [60] that analyzed in-hospital mortality among 1333 CABG patients undergoing primary PCI reported a mortality rate of 46%. However, all these studies and works were non-randomized. In addition, patients included in the revascularization group were obviously younger and, accordingly, had a lower risk of death, which influenced the treatment results [21]. An analysis of the SHOCK registry showed that mortality in older patients (76%) was higher than in younger patients (55%; p
<0.001).
However, the mortality rate of operated patients of senile and elderly age was significantly less than in patients of the same age category, but without myocardial revascularization: 48% versus 81%; p
=0.0002 [11].
According to J. Hochman et al. [24], exclusion criteria for performing PCI in CABG are stenosis of the ISA of less than 70% with TIMI III blood flow through it, as well as an angiographic picture of the lesion with a possible high risk of developing microcirculatory obstruction syndrome (no-reflow). Left main coronary artery disease, unsuitable anatomy for PCI, multiple coronary artery disease, mechanical complications of AMI, or failure of PCI are indications for CABG [24].
However, there are studies that do not demonstrate the benefits of invasive treatment for CABG. One of the small randomized trials, SMASH (The Swiss Multicenter trial of Angioplasty Shock), which included 55 patients, was stopped early: 30-day mortality in the PCI group was 69% versus 78% in patients who received medical therapy. After 12 months, mortality was 74 and 83%, respectively. The authors were unable to demonstrate the benefit of emergency PCI in improving survival outcomes after CABG [53].
The SHOCK trial randomized 302 patients to emergency revascularization (PCI or CABG) with medical stabilization (thrombolytic therapy, inotropic and vasopressor support) followed by long-term revascularization. There were no statistically significant differences in 30-day mortality between the revascularization and drug stabilization groups: 46.7% versus 56% ( p
=0.11). However, within 6 months and over the course of a year, the differences in mortality between these two groups became statistically significant in favor of patients with emergency revascularization: 53% versus 66% (
p
<0.03) [23].
Although PCI and CABG are common approaches in the treatment of coronary artery disease, each of these myocardial revascularization strategies has different objectives in patients with CABG. Stenting of the coronary arteries does not always achieve a high percentage of technical success, accompanied in some cases by the development of no-reflow syndrome, which only worsens the results of PCI in patients with CABG [22]. Thus, TIMI III blood flow in 1333 patients with CABG, according to German authors, was achieved only in 75.2% of cases [60]. According to I. Porto et al. [38], additional perioperative myocardial damage as a result of distal embolism (studied using magnetic resonance imaging) during PCI was diagnosed in 23% of patients; in another 11% of cases, the cause of perioperative myocardial infarction was the “snow-plowing” mechanism, when as a result of implantation stent, the atherosclerotic plaque is displaced with compromise of the mouths of the side branches of the ISA or collaterals [38]. In addition, most patients with CABG have three-vessel or left main coronary artery disease, making them candidates for CABG [24, 56]. Additional advantages of CABG for CABG, according to some authors, are the protection of damaged and ischemic myocardium with the help of cardioplegia, cardiopulmonary bypass to unload both ventricles of the heart and revascularization of non-infarcted areas of the heart. Chronic occlusions of the coronary arteries and mechanical complications of AMI are also successfully corrected thanks to open cardiac surgery [24]. In the SHOCK study, 47 patients with CABG underwent CABG, representing 36% of the total number of patients undergoing revascularization. Survival after CABG at 30 days (57.4%) did not differ from that in the PCI group (55.4%; p
=0.86).
At one year, survival after CABG was 46.8% versus 51.9% in patients after PCI ( p
= 0.71) [58]. According to J. Hochman et al. [24], in the future, with the development of anesthesia, the advantages of cardioplegia, the development of new and improvement of existing devices for circulatory support will significantly improve the results of cardiac surgical treatment for CABG [24].
An important point in the treatment of patients with ACS is the identification of factors whose presence increases the risk of developing CABG [37]. Clinical prognostic factors for the occurrence of CS include female gender, elderly and senile age, acute disturbance of cerebral blood supply, angina pectoris and AMI in history, low left ventricular ejection fraction (<35%), extensive nature of the infarction (according to dynamic assessment of cardiac enzymes), diseases peripheral arteries and diabetes mellitus [18, 31]. The PURSUIT study assessed risk factors for the development of CABG in patients with non-ST segment elevation ACS. ST segment depression on the initial electrocardiogram is also a risk factor for the development of CABG [19]. According to the GUSTO-I study [45], His bundle branch block in patients with AMI resulted in higher mortality and incidence of CABG. A sharp increase in the incidence of CABG (12% vs. 6%; p
<0.035) was observed in patients with elevated blood glucose concentrations on admission. According to the analysis, hyperglycemia was the strongest prognostic factor for the development of CABG [59].
A number of studies have attempted to identify various prognostic factors for death in patients with CABG. Among the independent factors influencing mortality in CABG, different authors [1, 14] indicate age, history of angina pectoris, creatinine level and hyperlipidemia. TIMI 0-I ISA blood flow after thrombolysis and TIMI blood flow less than 3 after PCI are also unfavorable prognostic factors in patients with CABG [14]. Older age, left main coronary artery disease, three-vessel coronary artery disease, and a significant period from the onset of shock to PCI according to another registry are also independent prognostic factors for death [60]. In the work of R. Andrié et al. [1] analyzed various factors, including humoral ones, that can influence the results (primarily 30-day mortality) of treatment for CABG. Among the plasma factors, the authors identified interleukin-6, procalcitonin and the N-terminal precursor of brain natriuretic peptide. The concentration of these markers in shock patients who underwent revascularization and intra-aortic balloon counterpulsation (IABP) was determined upon admission, after 24 and 72 hours. Interleukin-6 turned out to be the most significant prognostic factor of early postoperative mortality in CABG [1]. Among other factors influencing the results of treatment in CABG, a number of authors highlight interferon-γ, tumor necrosis factor α, macrophage inflammatory protein-1β, granulocyte colony-stimulating factor, etc. [41].
In the long-term period after PCI, prognostic factors for unfavorable outcome are age over 65 years, high initial levels of creatinine and glucose [5].
A number of works [14, 57] are devoted to the use of various drugs in the treatment of patients with CABG. Inotropic support (dobutamine, norepinephrine) is a necessary component in the complex treatment of CABG; in the case of catecholamine-resistant CABG, the administration of levosimendan is recommended [57].
The use of glycoprotein IIb/IIIa platelet receptor blockers, according to a number of studies, improves the results of treatment of STEMI, but its role in the complex treatment of AMI complicated by CABG remains unknown [2, 9, 16, 27]. The PURSUIT study [19] showed that shock patients treated with eptifibatide had a 50% reduction in 30-day mortality. In another study [2], in the group of patients with abciximab, 30-day mortality was 18% versus 42%, where this drug was not used. A. Chan et al. [9] compared the effectiveness of abciximab in 4 groups of patients with CABG: the stenting + abciximab group, the isolated stenting group, the balloon angioplasty + abciximab group, and the isolated balloon angioplasty group. Efficiency was assessed over 2.5 years. The mortality rate for the specified period of time was 33, 43, 61 and 68%, respectively. TIMI III blood flow according to ISA was achieved more often in patients using abciximab: 85% versus 65%; p
=0.048 [9].
Positive results from the use of abciximab (in terms of the frequency of achieving TIMI III blood flow, mortality, recurrent infarctions) were also obtained in the work of S. Giri et al. [16]. However, in a study by P. Tousek et al. [51], which compared the effectiveness of abciximab in 2 groups (usual use and selective use), the benefit of its usual use was not demonstrated for any of the 30-day outcome measures (death, reinfarction, stroke, renal failure). However, significant hemorrhagic complications occurred more than 2 times more often in patients with routine use of abciximab: 17.5% versus 7.5% ( p
= 0.31) [51]. Despite the majority of beneficial effects of IIb/IIIa receptor blockers in the treatment of CABG, no randomized trials have been conducted on this issue.
The use of IABP in CABG has also been the subject of many studies, the results of which are contradictory [10, 12, 20, 39]. Class I recommendations and level of evidence The benefits of using IABP in the complex treatment of CABG are available according to European guidelines, while the ACC/AHA recommendations indicate class IIa and level B if CABG is refractory to medical treatment [36]. The intra-aortic balloon is placed in the descending aorta, just below the origin of the left subclavian artery. The principle of operation of the device is to mechanically pump blood in both the proximal and distal directions, due to the inflation of the balloon during diastole. This leads to an increase in blood flow through the coronary arteries, myocardial perfusion and support for the pumping function of the left ventricle. Sharp deflation of the balloon during presystole reduces afterload on the left ventricle of the heart, reducing myocardial oxygen consumption. The incidence of IABP use in patients with CABG ranges from 20 to 98% [47, 50, 58]. In Germany, IABP is used in approximately ¼ of patients with CABG [61]. In the work of G. Fornaro et al. [12] presented the experience of treating 20 patients with CABG, in whom myocardial revascularization (endovascular interventions or CABG), elimination of atrial septal defects and mitral insufficiency were carried out against the background of IABP. The mortality rate was 35%: 7 patients died (4 from CABG, 1 from a hemorrhagic complication, 1 from septic shock, 1 from heart failure after CABG). According to the authors, IABP is a useful and necessary component in the complex treatment of patients with CABG, allowing to stabilize their condition and improve the results of myocardial revascularization [12]. In the IABP SHOCK Trial, patients with AMI complicated by CABG and undergoing PCI were randomized into 2 groups: with or without IABP. It was shown that the use of IABP had a moderate positive effect on the condition of patients in accordance with the APACHE II scale, improved cardiac index, reduced the systemic inflammatory response, and reduced the content of brain natriuretic peptide compared to patients in whom IABP was not used [39]. In a more recent study, the authors assessed the effect of using an IABP in 40 patients with CABG who also underwent PCI. While causing a temporary improvement in the main hemodynamic parameters in patients with CABG, IABP did not lead to statistically significant differences in these parameters between patients with IABP and those receiving only drug treatment. According to the authors [40], the effectiveness of using IABP in patients with CABG remains unknown [40].
In a study conducted by T. Sanborn et al. [43], four groups of patients with CABG were compared: 1st - without thrombolytic therapy and IABP, 2nd - only with IABP, 3rd - only with thrombolysis and 4th - with IABP and thrombolytic therapy. Thrombolysis compared with no thrombolysis reduced in-hospital mortality (54% vs. 64%; p
=0.005).
The same situation was observed in patients with CABG and IABP, in whom mortality (50%) was lower, in contrast to patients with CABG and without IABP (72%) [43]. K. Sjauw et al. [47] demonstrated experience in treating 292 patients with CABG. In the group of patients ( n
=93) in whom IABP was not used, mortality was 47%, while among patients (
n
=199) with IABP it was 28%.
Among 199 patients with IABP, two groups were also identified: 1st - with IABP before PCI and 2nd - IABP after PCI, while mortality was higher in group 1: 62% versus 40% [47]. One of the latest and largest studies (prospective, open, multicenter and randomized) assessing the effectiveness of IABP in shock patients was conducted by H. Thiele et al. [50]. The work analyzed the results of treatment of two groups: 1st ( n
= 298) - with the use of IABP and 2nd (
n
= 300) - optimal drug therapy.
Myocardial revascularization was carried out in all studied patients. At the same time, no significant differences were found between the two groups either in 30-day mortality or in the incidence of hemorrhagic and ischemic complications, sepsis or stroke. The use of IABP did not reduce 30-day mortality in CABG (39.7% vs. 43.1%; p
= 0.69) [34]. The lack of reduction in mortality and the incidence of various cardiac and vascular complications in the case of the use of IABP has been demonstrated in other studies. In turn, the feasibility of using IABP for CABG against the background of mechanical complications of AMI (rupture of the interventricular septum, chordal avulsion, etc.) is beyond doubt [7].
Recent studies have questioned the effectiveness of IABP in the treatment of CABG, and the scope of its application may be reduced to a minimum [20, 61].
A. Jacobs et al. [29] compared the results of treatment with CABG in 881 patients with STEMI ( n
=729) and STEMI (
n
=152).
The authors found that patients with CABG and NSTEMI had a significantly more severe clinical condition and aggravated medical history: these were patients of older age groups who more often had AMI, heart failure, reconstructive vascular surgery, and a history of peripheral arterial disease. Myocardial revascularization was performed in both groups in approximately the same proportion: 36.8% in NSTEMI and 41.9% in NSTEMI ( p
= 0.277). Mortality in the STEMI group was 62.5%, which was not statistically significantly different from the rate for STEMI (60.4%). Despite almost the same mortality, the authors [29] point to patients with NSTEMI and CABG as the most difficult cohort of patients requiring earlier intervention and aggressive reperfusion therapy.
In recent years, studies have appeared demonstrating the equal effectiveness and safety of radial access compared to femoral for PCI in the treatment of patients with CABG [5, 15, 42]. In addition to the comparable effectiveness of using radial access, according to T. Fujii et al. [15], it also demonstrated fewer complications. Mortality during CABG in the radial approach group did not differ from that in patients with femoral access: 28.9% versus 25.5% ( p
=0.7) [15].
Comparing both approaches in the treatment of patients with CABG, I. Bernat et al. [5] showed that radial access is effective and safe in more than 50% of patients presenting with clinical signs of CABG. In addition, the use of radial access led, according to the authors [5], to a decrease in one-year mortality from 64% for the femoral approach to 44% for the radial approach ( p
= 0.0044).
According to O. Rodriguez-Leor et al. [42], radial access was successfully used in ⅔ of patients with CABG, demonstrating an advantage over femoral access in terms of the incidence of complications at the puncture site, the need for transfusion of blood components, and mortality (32.5% versus 64.3%; p
= 0.001) , as well as in the incidence of major cardiovascular complications (death, heart attack, stroke, severe bleeding and posthypoxic encephalopathy) - 43.8% versus 73.8% (
p
= 0.001).
A number of works have also been devoted to the use of extracorporeal membrane oxygenation (ECMO) in CABG, but most of them study the use of this method in severe respiratory disorders or post-cardiotomy CABG [6, 44, 49, 52]. The device takes venous blood, saturates it with oxygen using a membrane oxygenator and returns it to the systemic arterial circulation. The technique is most suitable in cases of severe right-left ventricular and cardiopulmonary failure, cardiac arrest. Peripheral cannulation involves access through the femoral or jugular vein with drainage of blood into the femoral artery; in the case of central cannulation (mainly in cardiac surgery), venous blood is taken from the right atrium with subsequent drainage into the ascending aorta [24]. The combined use of ECMO and IABP in patients with CABG treated with PCI increased 1-year survival to 63.4%, while isolated use of IABP was associated with only 24% 1-year survival [52]. The need to use ECMO in order to improve the results of treatment of CABG for thrombosis of the left main coronary artery is indicated by F. Hussain et al. [28]. Among 105 patients, the technical success of using ECMO was achieved in 95%, the overall mortality rate was 7.6%, while among patients younger than 75 years and without intervention on the left main coronary artery, the mortality rate was 2.6% [55]. According to F. Shawl et al. [46], ECMO is often the only alternative in the treatment of refractory CABG, allowing ⅓ of patients to be discharged from the hospital. The disadvantages of this technique are the high risk of hemorrhagic and ischemic complications due to the large diameter of the devices used, which are installed in the vessels (16-18 F) [24].
Moderate hypothermia (32-34 °C) in the complex treatment of patients with CABG is beginning to take a confident position, demonstrating effectiveness both in experimental and clinical conditions. There are invasive methods (using special heat-exchange catheters installed in the femoral vein) and non-invasive methods of hypothermia, when cold water circulates through a special heat-exchange blanket covering at least 70% of the patient's body [8, 17, 35, 48]. Moderate hypothermia improves treatment outcomes in CABG by reducing oxygen consumption and using vasopressor support, which makes it possible to recommend the use of the method in hemodynamically unstable patients [62]. The technique also improves metabolic and hemodynamic parameters in patients with CABG [54], positively affecting survival in CABG patients [48]. However, the effectiveness of hypothermia in the treatment of patients with CABG has not been sufficiently proven.
Thus, cardiogenic shock still remains a severe and in most cases fatal complication of AMI and a number of other heart diseases. Despite the development of interventional cardiology, cardiac surgery and the emergence of various circulatory support devices, cardiogenic shock and methods of its treatment require further study with additional randomized studies.
Medications
Medicines to treat cardiogenic shock are prescribed to improve blood flow through the heart and improve the pumping function of your heart.
- Aspirin. Aspirin reduces blood clotting and maintains blood flow through the narrowed artery. Take aspirin on your own while waiting for emergency services to arrive, only if your doctor tells you to do so if you are experiencing symptoms of a heart attack.
- Thrombolytics. These drugs help dissolve a blood clot that is blocking blood flow to the heart. The sooner you are given a thrombolytic drug during a heart attack, the better your chance of survival and less damage to your heart. Thrombolytics are prescribed if emergency catheterization of the heart arteries and stenting cannot be performed.
- Antiplatelet agents. Doctors in the emergency room may give you other drugs that are similar to aspirin to prevent new blood clots from forming. These include drugs such as clopidogrel (Plavix) and other platelet glycoprotein IIb/IIIa receptor blockers.
- Other blood thinning drugs. You will likely be prescribed other medications. These include heparin, to reduce the likelihood of dangerous blood clots. Heparin is given intravenously or subcutaneously for the first few days after a heart attack.
- Inotropic drugs. Dopamine or epinephrine is prescribed to improve and maintain heart function.
Surgical interventions
Surgery to treat cardiogenic shock aims to restore blood flow through the heart. They are performed in specialized cardiac centers. Such interventions include:
- Angioplasty and stenting. Typically, when blood flow through the blocked artery is restored, the signs and symptoms of cardiogenic shock improve. Emergency angioplasty works to clear blocked arteries, allowing blood to flow freely to your heart. Doctors insert a long, thin tube (catheter) that is threaded through an artery in your leg or arm to the blocked artery in your heart. This catheter is equipped with a special balloon. When installing a stent in the area of narrowing, the balloon is inflated to restore the lumen of the artery. In addition, a metal mesh stent may be inserted into the artery to keep it open for a long time and restore blood flow to the heart. Doctors install stents that slowly release drugs into the blood to keep the artery wide enough.
- Balloon pump. Depending on your condition, doctors may place a balloon pump in your heart's main artery (aorta). The balloon pump inflates and deflates to mimic your heart and keep your blood flowing.
Cardiogenic shock
This is acute heart failure. Its peculiarity is the primary damage to the hemodynamic “motor” of the body - the heart. Most often, shock causes myocardial infarction. Due to myocardial necrosis or cardiac arrhythmia, its pumping function is sharply reduced; low cardiac output syndrome occurs. The characteristic hemodynamic manifestations of this condition are high central venous pressure, low blood pressure, and compensatory peripheral vascular spasm.
Myocardial infarction is an acute disease caused by the occurrence of ischemic necrosis in the heart muscle. The most common cause of myocardial infarction is atherosclerosis of the coronary arteries. On the artery wall affected by atherosclerosis, blood clots can form, which block the lumen of the vessel and disrupt the blood supply to the muscle. Sometimes myocardial infarction can occur due to spasm of the coronary vessels (for example, during mental trauma). The cause may also be septic endocarditis, which leads to the closure of the lumen of the coronary vessels with thrombotic masses.
Depending on the size of necrosis, small or extensive myocardial infarction is distinguished. According to its distribution:
- subendocardial,
- subepicardial,
- intramural (in the lumen of the muscle),
- transmural (the entire thickness of the myocardium is affected).
During myocardial infarction, the patient experiences unbearable pain behind the sternum, which can radiate to the left shoulder blade, shoulder, shoulder girdle or other parts of the body. The patient “freezes” in place: the slightest movement increases the pain. The skin becomes pale and cold. Faces with a bluish tint, covered with sweat. As blood pressure decreases, consciousness is suppressed. The pulse is thread-like, weak in filling and tension, frequent, sometimes arrhythmic. An increase in central venous pressure is accompanied by stagnation of blood in the pulmonary circulation system, and sometimes pulmonary edema. At the same time, breathing becomes noisy, squeaky, and foamy pink sputum is released from the mouth. Diuresis decreases.
There are the following forms of cardiogenic shock (according to E.I. Chazov):
1. Reflex shock. Its clinical manifestations are pain with a decrease in vascular tone and blood pressure. This shock is relatively easy to treat.
2. True shock (described above).
3. Arrhythmogenic shock. Heart rhythm disturbances come to the fore, which, in turn, worsens coronary blood flow and contributes to the spread of the necrosis zone.
4. Areactive shock is a severe form of cardiogenic shock. It is manifested by severe hypotension that is not amenable to conservative therapy.
Emergency measures for cardiogenic shock
- Place the patient in bed in a semi-sitting position.
- Release the chest from compression by clothing (unfasten the collar), provide access to fresh air.
- Palpate the pulse, measure blood pressure, assess the state of consciousness.
- If there is noisy breathing or foamy sputum, reduce blood flow to the heart by applying venous tourniquets to the lower and upper extremities.
- Give the patient nitroglycerin under the tongue.
- As part of a medical team, a nurse provides oxygen therapy to a patient. Oxygen in a volume of 5-7 liters per minute must be passed through a solution of ethyl alcohol or antifomsilan (has antifoaming properties).
- According to indications (severe pain), the patient is given nitrous-oxygen mask anesthesia (nitrous oxide in relation to oxygen = 1: 1).
- To combat pain, narcotic analgesics are used (morphine hydrochloride 1 ml of 1% solution), neuroleptanalgesia (2 ml of 0.005% fentanyl solution and 1-2 ml of 2.5% droperidol solution intramuscularly), non-narcotic analgesics (2 ml of 50% solution analgin).
- Reduction of venous hypertension is achieved by using diuretics (furosemide 20-40 mg intravenously), venous vasodilators (nitroglycerin, sodium nitroprusside 100 mg, diluted in 200 ml of 0.9% sodium chloride solution, administered at a rate of 5-10 drops per minute under control HELL).
Specialized medical care
Drug correction of cardiac output is determined by the level of blood pressure:
a) with systolic AO <70 mm Hg. use solutions of norepinephrine 1-30 mcg/min.) or dopamine (5-20 mcg/kg/min.),
b) with systolic AO 70-100 mm Hg. - solution of dopamine (2.5 - 20 mcg / kg per minute),
c) with systolic AO > 100 mm Hg. – dobutamine (2-20 mcg/kg per minute),
d) increase in diastolic blood pressure more than 100 mm Hg. is an indication for the use of peripheral vasodilators (nitroglycerin, sodium nitroprusside).
- In some cases, an effective means of combating pulmonary edema is tracheal intubation and providing the patient with artificial ventilation of the lungs with positive end-expiratory pressure.
- Heart rhythm disturbances are treated using antiarrhythmic drugs. For bradycardia, atropine sulfate, dopamine, adrenaline, isadrine are prescribed, and cardioversion is used. Tachykadia more than 130 beats. / min. requires topical diagnosis using ECG. For atrial fibrillation and fibrillation, cardiac glycosides, beta-blockers, and anticoagulants are used. For paroxysmal supraventricular tachycardias - vagal tests and adenosine.
Ventricular tachycardia is treated with repeated administration of lidocaine (1-1.5 mg/kg every 5-10 minutes). If there is no effect, novocainamide (20-30 mg/min), ornid (5-10 mg/kg), and cardioversion are used.
Exotoxic shock occurs during acute exogenous poisoning. It falls into the category of mixed shocks.
Operations
If drug treatment and the listed surgical interventions are ineffective, the doctor will recommend surgery:
- Coronary artery bypass grafting. Bypass surgery involves sewing veins or arteries to bypass the area of blockage or narrowing of the coronary artery. This restores blood flow to the heart. The doctor will suggest this surgery after the heart has recovered from a heart attack.
- Surgeries to repair heart damage. Sometimes the cause of cardiogenic shock is a rupture of one of the chambers of the heart or damage to the heart valve. To correct these problems, the doctor will suggest surgical treatment.
Clinical picture
First aid for collapse.
First aid for cardiogenic shock.
Clinic.
First aid for cardiogenic shock.
Cardiogenic shock is the most serious complication of myocardial infarction. The immediate cause of shock is pain. Cardiogenic shock is based on a decrease in the contractility of the left ventricular myocardium due to necrosis of the heart muscle.
The patient is adynamic, experiences progressive weakness, answers questions with difficulty, and consciousness is confused. The condition is extremely serious. Facial features are pointed. The skin is pale with an ash-gray or cyanotic tint. Sometimes there may be a marbled skin pattern. Sticky cold sweat on forehead. Peripheral veins collapsed. The pulse is frequent and threadlike. Blood pressure<80 ml.Hg, pulse<30 ml.Hg. Heart sounds are muffled. Breathing is rare and noisy. Oliguria.
| actions | justification |
| Call a doctor . | To provide qualified medical care |
| Create absolute physical and mental peace, lay him horizontally, calm the patient, lower the head end, raise the foot end of the bed by 200. | Increases blood flow to the head. |
| Cover with a blanket, apply heating pads to your feet, and give you hot tea to drink. | Warm the patient. |
| Measure blood pressure, calculate pulse, respiratory rate. | Condition monitoring |
| Ventilate the room, give access to fresh air, provide moisturizing oxygen. | Reduce hypoxia of the heart muscle. |
| Take an ECG and connect it to a cardiac monitor. | Condition monitoring. |
Prepare for the doctor's arrival:
— system for intravenous infusion, syringes for intravenous, intramuscular and subcutaneous administration of drugs, tourniquet, cotton balls, 700 alcohol, EC apparatus, cardiac monitor, defibrillator, Ambu bag, pulse oximeter, defibrillator, anesthesia apparatus for nitrous-oxygen analgesia;
- medications: fentonyl, droperidol, morphine, atropine, 50% analgin solution, diphenhydramine, adrenaline, prednisolone, dopamine, dopamide, mezaton, lasix, polyglucin, rheopolyglucin, isotonic sodium chloride solution, polarizing mixture (500 ml 10% glucose, 50 ml 4 % potassium chloride solution, 8 units of insulin), Relanium, lidocaine (amp.).
Collapse is a pathological condition characterized by a drop in vascular tone and a sharp decrease in circulating blood volume.
Develops acutely, suddenly. First, severe weakness, dizziness, tinnitus, “veil” before the eyes, chilliness, cold extremities, and thirst appear.
Consciousness is preserved, but patients are inhibited, indifferent to their surroundings, answer questions in monosyllables, and almost do not react to external stimuli.
The skin and visible mucous membranes are initially pale, then bluish with a gray tint; profuse cold sticky sweat. The facial features become sharper, the gaze is dull and indifferent. The pupils are dilated. Veins collapsed. Breathing is frequent and shallow. Pulse is frequent, thread-like, blood pressure < 80 mm Hg. and may not even be determined. Heart sounds are muffled. Body temperature is reduced, oliguria.
Prevention
The best way to prevent cardiogenic shock is to prevent heart attacks. Lifestyle changes used to treat heart disease can help prevent a heart attack. These lifestyle changes include:
- Control of high blood pressure (treatment of hypertension). One of the most important steps to reduce your risk of heart attack and cardiogenic shock is to control your blood pressure. Regular exercise, managing stress, maintaining a healthy body weight, and limiting salt and alcohol intake will help control high blood pressure. In addition to recommendations for lifestyle changes, your doctor may prescribe medications to treat hypertension, such as diuretics, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers.
- To give up smoking. Quitting smoking reduces the risk of having a heart attack. Several years after quitting smoking, a smoker's risk of developing a stroke is the same as a non-smoker's.
- Maintaining normal body weight. Excess weight contributes to the risk factors for heart attack and cardiogenic shock: high blood pressure, cardiovascular disease and diabetes. Losing just 10 pounds of weight will lower your blood pressure and cholesterol levels.
- Reduce cholesterol and saturated fat in your diet. Eating less cholesterol and fat, especially saturated fat, will reduce your risk of heart disease.
- Regular exercise. Exercise reduces your risk of having a heart attack. Exercise lowers blood pressure, increases high-density lipoprotein (HDL) levels, and improves the overall health of your blood vessels and heart. They will help you lose weight, control diabetes and reduce stress. Get regular exercise such as walking, jogging, swimming or cycling for 30 minutes every day.
Help with cardiogenic shock, prevention
Medical care is to increase blood pressure to normal levels. For this purpose, dopamine or dobutamine is used. In case of ventricular fibrillation, defibrillation is performed, and in case of cardiac arrest, indirect cardiac massage is performed.
Help with cardiogenic shock in a hospital setting: oxygen therapy, prescription of vasopressors, cardiac glycosides, analgesics, prednisolone, heparin, diuretics. The dosage and treatment regimen are prescribed by the doctor in each specific case individually.
Prevention:
- healthy lifestyle, giving up bad habits;
- balanced diet;
- adequate sleep, stress management;
- moderate physical activity;
- timely treatment of any cardiac pathology.
Once a year, undergo a medical examination with a cardiologist. If you have heart disease, preventive examination is recommended every six months. In our center you can undergo a full examination of the body and receive a transcript of the results. During the consultation, the doctor will answer your questions and tell you about the symptoms of cardiogenic shock during myocardial infarction. Registration online and by phone.