Heart Attack Symptoms
Because cardiogenic shock typically occurs in people who have had a severe heart attack, it is important to know the signs and symptoms of a heart attack. These include:
- Pressing, bursting or squeezing pain in the chest lasting more than 15 minutes;
- Pain radiating to the shoulder, arm, back or teeth and lower jaw;
- Increased frequency of attacks of chest pain;
- Prolonged pain in the upper abdomen;
- Dyspnea;
- Sweating;
- A looming feeling of fear;
- Fainting;
- Nausea and vomiting.
If you see your doctor right away when these signs or symptoms appear, you can prevent the possibility of developing cardiogenic shock. Prompt treatment of a heart attack increases the chances of survival and reduces damage to the heart. Don't ignore these symptoms if they last more than five minutes. Call an ambulance immediately. If you cannot call an ambulance, ask someone to take you to the nearest hospital.
Symptoms
Symptoms of cardiogenic shock
- rapid breathing;
- dyspnea;
- sudden rapid heartbeat (tachycardia);
- foggy consciousness;
- loss of consciousness or fainting;
- weak pulse;
- pallor and increased moisture of the skin;
- cold hands and feet;
- decrease in the amount of urine excreted (oliguria).
Symptoms of myocardial infarction
Most often, cardiogenic shock develops in people who have suffered an acute myocardial infarction, and therefore it is important to know the signs and symptoms of myocardial infarction.
They include:
- pressing or squeezing pain in the center of the chest that lasts several minutes;
- the pain can radiate to the shoulder, arm, back, or even to the teeth and jaw;
- prolonged pain in the upper abdomen;
- shortness of breath;
- cold sweat;
- feeling of anxiety, depression;
- fainting;
- nausea and vomiting.
Calling your doctor immediately when these signs and symptoms appear can help reduce your chance of developing cardiogenic shock.
Causes
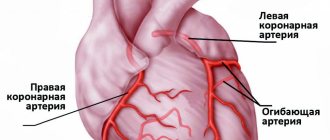
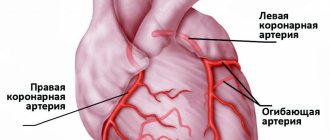
Cardiogenic shock occurs when the heart loses its ability to pump enough blood to the rest of your body. The most common cause of cardiogenic shock is damage to the left ventricle, the main pumping chamber of the heart, due to lack of oxygen due to a heart attack.
A heart attack occurs when one or more of the arteries that carry oxygenated blood to your heart (coronary arteries) becomes blocked. Sometimes, over time, a narrowing of the coronary arteries occurs due to the deposition of cholesterol on their walls. The formation of these deposits, called plaques, in the arteries throughout the body is called atherosclerosis.
During a heart attack, one of these plaques may rupture, forming a blood clot at the site of the rupture, blocking blood flow through the arteries. Without oxygenated blood entering the heart, the heart muscle weakens and cardiogenic shock develops.
In rare cases, cardiogenic shock develops when the right ventricle of the heart is damaged. From the right ventricle of the heart, blood travels to the lungs, where it is enriched with oxygen before being sent to the rest of your body. Damage to the right ventricle causes the heart to lose its ability to pump blood effectively to the lungs, causing the body to not receive enough oxygen.
Although a heart attack is the most common cause, other conditions that cause cardiogenic shock include inflammation of the heart muscle (myocarditis) or infection of the heart valves (endocarditis). Causes include drug overdose or poisoning from substances that affect the pumping function of your heart.
Publications in the media
Cardiogenic shock is shock that occurs as a result of a sudden decrease in cardiac output.
Etiology • As a rule, it occurs with extensive MI against the background of multivessel lesions of the coronary arteries. Shock develops when more than 40% of the myocardial mass is involved, and is observed in 5–20% of patients with infarction • Other causes •• Acute myocarditis •• Severe, acute aortic or mitral stenosis •• Severe, acute aortic or mitral regurgitation •• Rupture interventricular septum •• Arrhythmias.
Risk factors • Old age • Decrease in left ventricular ejection fraction below normal (according to EchoCG) • Extensive MI (according to ECG, infarct changes in 8-9 leads; large zone of akinesia according to EchoCG), • Previous MI • Diabetes.
Pathogenesis . Severe impairment of myocardial contractile function with the additional addition of factors that aggravate myocardial ischemia.
• Activation of the sympathetic nervous system due to a drop in cardiac output and a decrease in blood pressure leads to an increase in heart rate and increased myocardial contractility, which increases the heart's need for oxygen.
• Fluid retention due to decreased renal blood flow and an increase in blood volume, which increases preload on the heart, contributes to pulmonary edema and hypoxemia.
• Increased peripheral vascular resistance due to vasoconstriction, leading to increased afterload on the heart and increased myocardial oxygen demand.
• Violation of diastolic relaxation of the left ventricle of the myocardium due to impaired filling and decreased compliance, which causes an increase in pressure in the left atrium and contributes to increased blood stagnation in the lungs.
• Metabolic acidosis due to prolonged hypoperfusion of organs and tissues.
Clinical manifestations
• Arterial hypotension - systolic blood pressure less than 90 mm Hg. or by 30 mm Hg. below normal levels for 30 minutes or more. Cardiac index less than 1.8–2 l/min/m2.
• Impaired peripheral perfusion •• kidneys - oliguria •• skin - pallor, increased humidity •• CNS - workload, stupor.
• Pulmonary edema as a manifestation of left ventricular failure.
When examining the patient, cold extremities, impaired consciousness, arterial hypotension (average blood pressure below 50–60 mm Hg), tachycardia, muffled heart sounds, oliguria (less than 20 ml/min) are detected. Auscultation of the lungs may reveal moist rales.
Special research methods
• Installation of a Swan-Ganz catheter to monitor central hemodynamics, measurement of cardiac output - by echocardiography or invasively.
• Arteriovenous difference in oxygen is more than 5.5 ml%.
Diagnostics . It is necessary to exclude other causes of arterial hypotension: hypovolemia, vasovagal reactions, electrolyte disturbances (for example, hyponatremia), side effects of drugs, arrhythmias (for example, paroxysmal supraventricular and ventricular tachycardias).
TREATMENT
An emergency condition requires urgent treatment. The main goal of therapy is to increase blood pressure.
Drug therapy . The following drugs are used (preferably administered through special dispensers). With their help, blood pressure should be increased to 90 mm Hg. and higher • Dobutamine (selective b1-adrenergic agonist with a positive inotropic effect and minimal positive chronotropic effect, i.e. the effect of increasing heart rate is insignificant) at a dose of 2.5–10 mcg/kg/min • Dopamine (has a more pronounced positive chronotropic effect , i.e., it can increase heart rate and, accordingly, myocardial oxygen demand, thereby slightly aggravating myocardial ischemia) at a dose of 2–10 mcg/kg/min with a gradual increase in dose every 2–5 min to 20–50 mcg/kg/min • Norepinephrine at a dose of 2–4 mcg/min (up to 15 mcg/min), although it, along with increased myocardial contractility, significantly increases peripheral vascular resistance, which can also aggravate myocardial ischemia.
Intra-aortic balloon counterpulsation ( mechanical injection of blood into the aorta using a pump during diastole, which increases blood flow in the coronary arteries ) . Carry out in the presence of appropriate equipment and the ineffectiveness of drug treatment for cardiogenic shock.
Percutaneous transluminal coronary angioplasty - restoration of the patency of the coronary arteries with its help in the first 4-8 hours from the onset of a heart attack not only preserves the myocardium, but also interrupts the vicious circle of pathogenetic mechanisms of cardiogenic shock.
Observation . In case of cardiogenic shock, constant monitoring of blood pressure, heart rate, diuresis (indwelling urinary catheter), pulmonary capillary wedge pressure (balloon catheter in the pulmonary artery), as well as monitoring of cardiac output using echocardiography or radionuclide angiography is recommended.
Forecast . The mortality rate for cardiogenic shock is 50–90%.
ICD-10 • R57 . 0 Cardiogenic shock
Complications
If not treated promptly, cardiogenic shock becomes a fatal condition. Another serious complication of cardiogenic shock is organ damage.
If the heart cannot pump enough oxygenated blood to the rest of your body, damage to the liver, kidneys and other organs develops. When the liver and kidneys are damaged, cardiogenic shock is worsened because the kidneys release chemicals into the blood that support muscle function and the liver produces proteins that help blood clot. With long duration of cardiogenic shock, permanent organ damage can develop.
Cardiogenic shock in heart attack patients: what's new?
Cardiogenic shock is an acute violation of the perfusion of body tissues caused by significant damage to the myocardium and disruption of its contractile function. The main causes of cardiogenic shock include myocardial infarction (MI), myocarditis, cardiomyopathies, toxic myocardial lesions, cardiac tumors, severe heart defects, trauma, pericardial tamponade, pulmonary embolism, severe cardiac arrhythmia.
Most often, a practicing physician has to deal with cardiogenic shock in patients with acute coronary syndrome (ACS), primarily with ST-segment elevation MI. Cardiogenic shock is the leading cause of death in patients with MI.
Typically, cardiogenic shock develops in the first hours after the onset of the first symptoms of MI and much less often in a later period. The risk of developing this formidable complication and its severity are largely determined by the extent of the infarction - the size of the myocardium affected by ischemia and necrosis. Therefore, most often, cardiogenic shock develops with MI of the anterior wall of the left ventricle, the apex of the heart and the anterior part of the interventricular septum, that is, with occlusion of the left coronary artery, which supplies most of the myocardial mass with blood, as well as with damage to all three main coronary arteries (which causes involvement more than 40% of the mass of the left ventricular myocardium into the infarction zone). Cardiogenic shock with right ventricular myocardial infarction is much less common.
The biggest problem with cardiogenic shock is the following vicious circle: severe depression of systolic function and a decrease in blood pressure (BP) cause ineffective coronary perfusion, as a result, coronary blood flow is further deteriorated, and myocardial ischemia and necrosis progressively worsen, which further impairs the pumping function of the left ventricle . If the mass of necrotic myocardium is 40-50% or more, then, as a rule, cardiogenic shock becomes areactive (torpid), that is, one in which the introduction of inotropes and vasopressors has no effect. Mortality in this group of patients approaches 100%.
Are there any positive trends in solving the problem of cardiogenic shock in recent years? What opportunities can modern medicine offer for the routine management of patients with this complication? We have selected the main evidence and practical recommendations on this pressing issue and present a summary of them in this article.
Cardiogenic shock yesterday and today
The incidence of cardiogenic shock in patients with ACS is difficult to accurately determine, since different authors use different definitions and criteria for diagnosing shock. According to rough estimates ten years ago (GUSTO-I, 1996; GUSTO-III, 1999; RJ Goldberg et al., 1999), cardiogenic shock developed in 7-10% of patients with ACS. Later, various population studies showed a slightly lower incidence of cardiogenic shock in ACS - from 3.2 to 8.6% (TRACE, 2003; NRMI, 2005; J. Fang et al., 2006; GRACE, 2007). In some of these studies, the positive dynamics in the risk of cardiogenic shock noted over the past few years was clearly associated with the implementation of modern evidence-based recommendations for the management of patients with ACS [2, 3], primarily with the wider use of coronary revascularization methods, especially surgical , as well as hemodynamic support using intra-aortic balloon counterpulsation.
The most valuable thing about the achievements of modern cardiology is that they are expressed not only in a lower risk of cardiogenic shock, but also in a decrease in mortality from this complication. More recently, cardiogenic shock was actually a death sentence. Before the introduction into practice of modern methods of treating MI (urgent revascularization, intra-aortic balloon counterpulsation), the development of cardiogenic shock doomed almost all patients to death - about 85-95% (E. Braunwald, 1988). As a number of studies in recent years have shown (NRMI, 2005; J. Fang et al., 2006; AMIS Plus, 2008), mortality in cardiogenic shock can be reduced to 30-40%, although in practice the real mortality figures even in developed countries of the world are still remain at the level of 50-60%. The latest European Society of Cardiology (ESC) guidelines for the management of patients with heart failure (HF) (2008) [4] indicate that the in-hospital mortality rate for people with cardiogenic shock is 40-60%.
Thus, the results of the American National Registry of Myocardial Infarction (NRMI) showed that in-hospital mortality among patients with cardiogenic shock that developed in connection with ST-elevation MI decreased from 60.3% in 1995 to 47. 9% in 2004 (p<0.001). This increase in patient survival was associated with a doubling in the number of percutaneous coronary interventions (PCI) used for urgent coronary revascularization (27.4% in 1995 and 54.4% in 2004, p < 0.001). Similar data were obtained in the Swiss AMIS Plus registry - a decrease in mortality in cardiogenic shock over the past 10 years was noted from 62.8 to 47.7% (p = 0.01), which is associated with a significant increase in the number of PCI for ACS (from 7. 6 to 65.9%, p=0.01) [5].
In 1999, the SHOCK study [6] showed that urgent revascularization (angioplasty or coronary artery bypass grafting) for ACS complicated by cardiogenic shock, although it does not lead to a significant reduction in early (1-month) mortality compared with the drug treatment group, but statistically significantly improves the prognosis in the long term. The mortality rate of such patients during the first six months after MI decreased from 63.1 to 50.3% (p=0.027), and within 1 year - from 66.4 to 53.3% (p<0.03). The greatest benefits of revascularization were observed in the subgroup of patients younger than 75 years. Based on the results of this study, it was strongly recommended to increase the use of surgical methods of early coronary artery revascularization in patients with cardiogenic shock due to ACS [2].
Thrombolysis is less effective for revascularization in cases of cardiogenic shock because low perfusion pressure prevents adequate delivery of fibrinolytic to the coronary arteries. However, thrombolytic therapy, according to the GUSTO-I study, reduces the incidence of cardiogenic shock to 7.2%, and the mortality rate for this complication to 55%. Other studies have also confirmed a significant improvement in survival of patients with cardiogenic shock with the use of thrombolysis compared with conservative therapy (without revascularization). Therefore, thrombolysis in conditions of unavailability of PCI and urgent coronary artery bypass grafting is the optimal treatment method, especially considering that thrombolysis can be carried out in a much shorter time, including at the prehospital stage, which can be life-saving for patients with cardiogenic shock. For the realities of domestic medicine, this is currently a more acceptable treatment strategy, although not optimal, of course, compared to PCI and coronary artery bypass surgery.
Along with this, it was confirmed that in patients with MI who suffered cardiogenic shock and survived early, long-term clinical outcomes do not differ significantly from those in patients without a history of cardiogenic shock (SHOCK, 2006; M. Singh et al., 2007) . Therefore, measures that improve the early (in-hospital) survival of patients with cardiogenic shock actually equalize the further risks of patients, regardless of the presence of a history of shock.
AMIS Plus Registry: 10 Years of Progress in the Treatment and Prevention of Cardiogenic Shock
To illustrate the above data, I would like to talk in more detail about the results that were obtained within the Swiss registry for MI (Acute Myocardial Infarction in Switzerland, AMIS Plus) [5].
The register included more than 23.6 thousand patients with ACS (with and without ST segment elevation) from 70 clinics in Switzerland. Data were analyzed from January 1, 1997 to December 31, 2006. The authors assessed changes in the incidence of cardiogenic shock and mortality from this complication during the study period and determined the dependence of these changes on the nature of patient treatment.
It turned out that over the past 10 years, the incidence of cardiogenic shock in patients with ACS has decreased from 12.9 to 5.5% (p = 0.001). This happened due to a decrease in the risk of developing cardiogenic shock during hospital treatment (from 10.6 to 2.7%, p<0.001), while the frequency of registration of already developed cardiogenic shock at the time of admission to the hospital did not change significantly - about 2-2.3%
Although cardiogenic shock was 2 times more common in patients with ACS with ST elevation than without ST elevation, the reduction in the risk of its development over the past decade was approximately the same in both cohorts of patients.
In-hospital mortality in patients with cardiogenic shock also decreased significantly - from 62.8 to 47.7% (p = 0.01), and this also applied to patients admitted with cardiogenic shock (from 73.8 to 46.6%, p = 0.009), and those in whom cardiogenic shock developed after hospitalization (from 60.9 to 48.9%, p = 0.094), although, as can be seen, in the latter subgroup of patients the differences did not reach statistical significance.
Analysis of AMIS Plus data [5] indicates that positive trends in the dynamics of the risk of cardiogenic shock and associated mortality in patients with ACS correlate with the improvement of inpatient care for such patients, in particular with the widespread introduction of surgical revascularization methods. It should be noted that in the studied clinics during the study the number of PCIs performed significantly increased (from 7.6 to 65.9%, p = 0.01) and the use of intra-aortic balloon counterpulsation, while the number of thrombolysis decreased and the number of aortocoronary operations shunting has remained virtually unchanged.
The analysis showed that the reduction in both in-hospital mortality in patients with ACS in general and in-hospital mortality in the subgroup of patients with cardiogenic shock was significantly affected by the wider use of PCI.
In addition, the therapeutic treatment of patients with cardiogenic shock has significantly improved: during the studied period of time, the use of acetylsalicylic acid (from 80.4 to 89.2%), clopidogrel (from 11.7 to 65.5%), increased in this cohort of patients. inhibitors of IIb/IIIa glycoprotein platelet receptors (from 11.8% in 1999 to 35.6% in 2006), lipid-lowering drugs (from 14.3% in 1999 to 77.8% in 2006) , β-blockers (from 32.7 to 40%).
The AMIS Plus registry [5] also studied risk factors for the development of cardiogenic shock. It turned out that independent predictors of this complication were older age, ST segment elevation, tachycardia, and low systolic blood pressure. In contrast, the use of lipid-lowering drugs and PCI were significantly associated with a lower risk of cardiogenic shock.
- Practical recommendations for the management of patients with cardiogenic shock
Diagnostics
Quick diagnosis of cardiogenic shock allows you to take the necessary measures in a timely manner and prevent the death of the patient. Therefore, it is very important to know the signs that most likely indicate the development of this complication.
To assess the severity of acute HF in patients with MI, the classifications of T. Killip (1967) and JS Forrester (1977) are used [4]. Both classifications imply the division of patients into 4 groups (stages, classes) depending on the severity of systemic hemodynamic disorders and pulmonary congestion. The differences between them are that the JS Forrester classification takes into account not only clinical signs, as in the T. Killip classification, but also some indicators of central hemodynamics (wedge pressure in the pulmonary artery, cardiac index). According to T. Killip's classification, the state of cardiogenic shock corresponds to a decrease in blood pressure <90 mm Hg. Art. and the presence of signs of peripheral vasoconstriction (oliguria, cyanosis, sweating); according to the JS Forrester classification - signs of reduced perfusion of body tissues in combination with high “wedge” pressure in the pulmonary artery.
In the ESC guidelines for the management of ST-elevation MI, updated at the end of 2008 [1], cardiogenic shock is defined as a decrease in systolic blood pressure <90 mmHg. Art., an increase in the filling pressure of the ventricles of the heart (and, accordingly, the “jamming” pressure of the pulmonary artery) >20 mm Hg. Art., a decrease in cardiac index <1.8 l/min/m2. The diagnosis of cardiogenic shock is established if other possible causes of hypotension (hypovolemia, vasovagal reflex, electrolyte imbalance, side effect of pharmaceuticals, myocardial tamponade) are excluded. But it should be remembered that cardiogenic shock accounts for about 80% of all shock conditions that complicate the course of myocardial infarction, that is, this pathology is the most likely when it comes to significant hypotension and hypoperfusion in heart attack patients.
According to the new ESC guidelines for the management of patients with heart failure (2008) [4], cardiogenic shock does not have clear diagnostic criteria, although in typical cases it can be considered when blood pressure decreases below 90 mmHg. Art. (or mean blood pressure drops by more than 30 mm Hg), and diuresis is absent or sharply reduced (<0.5 ml/kg/h). Within a short time, clinical signs of organ hypoperfusion and pulmonary congestion appear. Heart rhythm disturbances often develop.
Thus, for the initial diagnosis of cardiogenic shock (at the patient’s bedside), it is enough to detect threatening clinical signs, measure blood pressure and exclude other probable causes of hypotension. A decrease in the volume of urine output, which can be assessed after bladder catheterization, quickly confirms whether the diagnostic search is moving in the right direction. In addition, invasive testing of central hemodynamic parameters is extremely important both for diagnosing shock and for monitoring the effectiveness of treatment. For this purpose, catheterization of the right heart and pulmonary artery (balloon “floating” Swan-Ganz catheter) is recommended. In this case, the pressure in the right atrium and ventricle, pulmonary artery, pulmonary artery wedge pressure, and cardiac output are measured. These data allow you to most accurately assess the state of cardiac function and make it possible to notice its minimal deterioration or improvement, as well as exclude many other possible causes of hypotension.
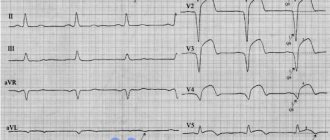
It is important to recall that right ventricular myocardial infarction sometimes manifests as cardiogenic shock, but the approaches to treating both conditions are significantly different. Therefore, if cardiogenic shock is suspected, differential diagnosis with infarction of the right ventricular wall is also necessary. To this end, identification of distension of the jugular veins (especially during inspiration), confirmation of the absence of moist rales in the lungs, ECG signs of right ventricular myocardial infarction (for example, ST segment elevation in lead V4, characteristic changes in the recording of the ECG chest leads on the right), ultrasonographic evidence may help. right-sided infarction.
Prevention and treatment
To date, the only approach that has clearly confirmed the possibility of reducing the risk of cardiogenic shock in patients with ACS is the earliest possible revascularization of the coronary vessels. Clinical trials and large registries show that the currently recommended strategy of urgent reperfusion is the best way to reduce the number of patients with cardiogenic shock.
Treatment of cardiogenic shock is a difficult task, but not hopeless. As shown above, some therapeutic interventions for ACS have already proven their significant benefits not only in preventing cardiogenic shock, but also in improving survival if this complication develops. This applies primarily to the strategy of early revascularization, the use of which is associated with better outcomes for patients with manifest cardiogenic shock. A reduction in in-hospital mortality in patients with cardiogenic shock using PCI was convincingly shown in the AMIS Plus registry (2008) [5], and a positive effect on more long-term outcomes (6 months) - in the SHOCK study (1999) [6]. Based on the data obtained in the SHOCK study, the developers of American guidelines for the treatment of MI (American College of Cardiology, ACC; American Heart Association, AHA) classified emergency revascularization for cardiogenic shock as class I recommendations [2].
In the 2008 ESC guidelines for the management of patients with STEMI (1), early revascularization with PCI is the recommended strategy in the event of cardiogenic shock (grade I recommendation, level of evidence B). If PCI cannot be performed or is available only after some delay, immediate coronary artery bypass grafting may be indicated in patients with cardiogenic shock, especially if there are other indications for cardiac surgery (mitral regurgitation, left ventricular wall rupture, etc.). If both PCI and coronary artery bypass grafting are not possible in the near future, early revascularization with thrombolysis is necessary.
Early and effective elimination of myocardial ischemia and prevention of the formation of necrosis or significant limitation of its size ensures rapid restoration of systolic function of the heart and thereby breaks the vicious circle of “depression of cardiac output → decreased perfusion → additional deterioration of the myocardium.”
In addition, to interrupt this same vicious circle, hemodynamic support measures are very important to maintain blood pressure at a level that ensures adequate perfusion of vital organs, primarily the myocardium itself (90-100 mm Hg). For this purpose, inotropic drugs, vasopressors, and intra-aortic balloon counterpulsation are used.
The ESC guidelines for the management of patients with STEMI (2008) [1] and for the management of patients with HF (2008) [4] for the treatment of cardiogenic shock, in addition to early revascularization, recommend the use of oxygen therapy, mechanical ventilation depending on the level of blood gases, assessment hemodynamic conditions using cardiac catheterization, administration of inotropes (dopamine, dobutamine), the use of intra-aortic balloon counterpulsation and mechanical devices to ensure left ventricular function.
To date, there is no evidence that inotropes improve survival in patients with cardiogenic shock. But inotropes can either improve the patient's clinical condition and bring him out of shock, or at least stabilize his hemodynamics until more effective methods (intra-aortic balloon counterpulsation, surgery, special mechanical devices) can be used. The most popular inotrope is dopamine, especially since in low doses it effectively improves renal perfusion without significantly affecting systemic hemodynamics. Dobutamine has its benefits - although it is a slightly weaker inotrope than dopamine, it reduces pulmonary artery pressure. But it should be remembered that the administration of inotropes, especially dopamine in high doses, increases the risk of developing tachycardia and arrhythmia, so they should be used with caution in patients with an accelerated heart rate and require ECG monitoring. A combination of low doses of dopamine with higher doses of dobutamine is often used - this allows, with minimal risk of side effects, to improve systemic hemodynamics and especially renal perfusion.
The main criteria for the effectiveness of inotropic therapy are an increase in systolic blood pressure above 90 mmHg. Art., increase in cardiac index >2 l/min/m2, decrease in pulmonary artery “wedge” pressure to 20 mm Hg. Art., increased diuresis. It is important that the heart rate does not exceed 100 beats/min. If tachycardia or cardiac arrhythmias develop, the dose of inotropes should be reduced if possible.
If inotropes in standard doses are not effective enough, the following options for changing tactics are possible: increasing the dose of the inotrope used, a combination of two inotropes, adding vasopressors, using intra-aortic balloon counterpulsation, special mechanical devices to support left ventricular function.
Vasopressors (norepinephrine) are not recommended as first-line therapy for acute heart failure, but may be indicated in the case of cardiogenic shock if inotropes in combination with adequate fluid therapy are not sufficiently effective in stabilizing hemodynamics. In this case, the use of vasopressors must be done with great caution, since cardiogenic shock is usually accompanied by peripheral vasoconstriction and high peripheral vascular resistance. In addition, norepinephrine promotes lactic acidosis, increases pressure in the pulmonary artery (can provoke pulmonary edema) and is characterized by the fact that tolerance to it quickly develops. Therefore, even if you had to resort to norepinephrine, it is important to stop it as soon as possible. The use of epinephrine for the treatment of cardiogenic shock is not recommended; indications for its administration should be limited to cases of cardiac arrest only.
A method of temporary hemodynamic support that significantly improves the survival of patients with cardiogenic shock is intra-aortic balloon counterpulsation. However, it should be noted that the evidence demonstrating the clinical benefits of the method is somewhat contradictory. However, the SHOCK study showed a decrease in mortality in patients with cardiogenic shock when using counterpulsation, including in combination with thrombolytic therapy [6].
If the patient does not respond to standard therapy, the use of mechanical devices to maintain left ventricular function is indicated, although the evidence base for this strategy requires further research. Like intra-aortic balloon counterpulsation, the use of these devices, which partially or completely replace the pumping function of the left ventricle, allows you to gain time and maintain the patient’s hemodynamics until it stabilizes or until it becomes possible to solve the problem in a more radical way.
If cardiogenic shock is caused by a rupture of the myocardial wall, avulsion of the papillary muscle, mitral regurgitation or other serious changes in the structure of the heart, then the only chance to save the patient is immediate surgical intervention.
Among medications, the administration of inhibitors of platelet glycoprotein receptors IIb/IIIa may also be useful. These drugs have demonstrated certain advantages in the prevention of the “no-reflow” phenomenon after revascularization and are therefore most indicated for patients for whom adequate reperfusion is life-saving, that is, primarily for patients with cardiogenic shock. For example, in the CAPTURE study (1996), the use of absiximab in combination with urgent coronary angioplasty, the combined end point (death + recurrent MI + repeated urgent angioplasty) was 8.5% compared with 20.4% in the comparison group (p = 0.05 ). The EPIC, EPILOG, and EPISTENT studies confirmed similar benefits of combining abciximab with PCI.
Some innovative approaches are also being studied, for example, the use of nicorandil, NO synthase inhibitors, Na+/K+ pump inhibitors, glucose-insulin-potassium mixture, etc. But the feasibility of their use in patients with cardiogenic shock still needs to be confirmed in fairly large randomized studies.
Before removing the patient from a state of cardiogenic shock, beta-blockers, calcium antagonists, angiotensin-converting enzyme inhibitors, cardiac glycosides, and glucocorticosteroids should not be used without specific indications. Infusion therapy for cardiogenic shock should be very careful and carried out in small volumes (in contrast to the strategy for treating right ventricular myocardial infarction, when the patient is indicated for rapid volume-replenishment therapy).
Literature
1. Van de Werf F., Bax J., Betriu A. et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J 2008; 29: 2909-2945.
2. Antman EM, Anbe DT, Armstrong PW et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004; 110: 588-636.
3. Van de Werf F., Ardissino D., Betriu A. Et al. Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2003; 24: 28-66.
4. Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology, Dickstein K., Cohen-Solal A., Filippatos G. et al.; ESC Committee for Practice Guidelines, Vahanian A., Camm J., De Caterina R. et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008; 29 (19): 2388-442; Eur J Heart Fail 2008; 10 (10): 933-89.
5. Jeger RV, Radovanovic D, Hunziker PR et al.; for the AMIS Plus Registry Investigators. Ten-Year Trends in the Incidence and Treatment of Cardiogenic Shock. Ann Intern Med 2008; 149 (9): 618-26.
6. Hochman JS, Sleeper LA, Webb JG et al.; for The SHOCK Investigators. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med 1999; 341 (9): 625-34.
7. Davies CH Revascularization for cardiogenic shock. QJ Med 2001; 94: 57-67.
8. Topalian S., Ginsberg F., Parrillo JE Cardiogenic shock. Crit Care Med 2008; 36 (1 Suppl): S66-74.
9. Sanborn TA, Feldman T. Management strategies for cardiogenic shock. Curr Opin Cardiol 2004; 19 (6): 608-12.
10. Lee KW, Norell MS Cardiogenic shock complicating myocardial infarction and outcome following percutaneous coronary intervention. Acute Card Care 2008; 10 (3): 131-43.
11. Mann HJ, Nolan PE Jr. Update on the management of cardiogenic shock. Curr Opin Crit Care 2006; 12 (5): 431-6.
12. Duvernoy CS, Bates ER Management of cardiogenic shock attributable to acute myocardial infarction in the reperfusion era. J Intensive Care Med 2005; 20 (4): 188-98.
Based on materials from Madicine Review
Diagnostic methods
Diagnosis of cardiogenic shock requires immediate action. Doctors will check for signs and symptoms of shock and then order additional tests to determine the cause of your condition. Methods for diagnosing cardiogenic shock include:
- Blood pressure measurement. People with shock often have low blood pressure. If a person in shock is taken to hospital by ambulance, blood pressure is measured before admission to hospital.
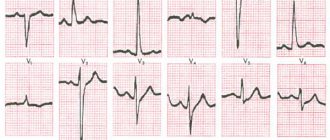
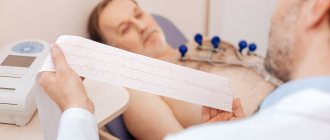
- Electrocardiogram (ECG). This is the first test to diagnose a heart attack. It is often done at the same time as a survey about symptoms. The test involves recording the electrical activity of the heart using electrodes attached to the skin. The pulses appear as "waves" displayed on a monitor or printed on paper. Because the heart muscle cannot conduct electrical impulses normally when damaged, an ECG can help determine whether you are having a heart attack or are in the process of having one.
- X-ray of the chest organs. A chest x-ray will help your doctor evaluate the size and shape of your heart and its blood vessels.
- Blood tests. Blood tests can determine if you have kidney or liver damage, detect signs of heart infection, and also determine if you are having a heart attack. Another type of blood test (arterial blood gas test) is also ordered to determine the amount of oxygen in the blood.
- Echocardiogram. This test uses sound waves to produce images of the heart. During echocardiography, sound waves are sent to the heart from a wand-shaped device (transducer) placed on the chest. Sound waves bounce off the heart and back through the chest and are processed electronically to produce a video image of the heart. An echocardiogram can help identify areas of damage to your heart and problems with the heart's pumping function.
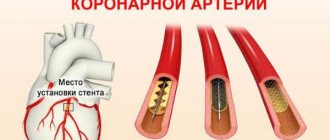
- Catheterization of coronary arteries (angiography, coronary angiography). This test will look for narrowing and blockage of the coronary arteries. Liquid contrast dye is injected into the arteries of the heart through a long, thin tube (catheter) that is passed through an artery in the leg or arm to the arteries of the heart. As the contrast agent fills the arteries, they become visible on X-rays, revealing areas of blockage.
Additionally, during catheterization, your doctor may remove the blockage in the artery through coronary angioplasty and stenting. Angioplasty uses tiny balloons that are inserted through a blood vessel into the coronary artery to widen the blocked area. After angioplasty, a mesh tube (stent) is placed inside the artery to maintain sufficient lumen and prevent re-narrowing in the future.
How to treat cardiogenic shock during myocardial infarction?
N
Despite significant advances in the treatment of patients with acute myocardial infarction (MI), which has significantly reduced mortality in this disease,
cardiogenic shock
(CS)
still remains the main cause of death in patients with MI even in the so-called “thrombolytic era”
. CABG occurs on average in 5-10% of patients with MI. According to Golbert (1991), the mortality rate in patients with MI complicated by CABG in the period from 1975 to 1988 was 78%. And the results of the National Registry of Myocardial Infarction (NRMI 2), which tracked the outcomes of myocardial infarction in 23 thousand patients with CABG in 1400 US hospitals from 1994 to 2001, showed that mortality in recent years has decreased slightly and amounted to about 70%.
CABG is a complication of MI associated with a decrease in cardiac output with adequate intravascular volume, leading to hypoxia of organs and tissues. As a rule, shock develops in patients as a result of serious dysfunction of the left ventricle due to significant damage to the myocardium. At autopsy in patients who died from CABG, the size of the MI ranges from 40 to 70% of the mass of the left ventricular myocardium. This article will discuss the principles of treatment of patients with true CABG. Other clinical variants of CABG, for example, those associated with the development of arshock or hypovolemia, as well as with internal or external myocardial ruptures and acute mitral regurgitation, require other pathogenetic approaches to treatment.
Criteria for diagnosing cardiogenic shock:
- systolic blood pressure is less than 90 mmHg. for 1 hour or more;
- signs of hypoperfusion - cyanosis, cold, moist skin, severe oliguria (urination less than 20 ml per hour), congestive heart failure, mental disorders;
- heart rate above 60 beats. per minute;
- hemodynamic signs - wedge pressure in the pulmonary artery more than 18 mm Hg, cardiac index less than 2.2 l/min/sq.m.
Standard treatment for cardiogenic shock
Patients with MI complicated by CABG must be in an intensive care unit and require careful constant monitoring of a number of parameters: general clinical condition; blood pressure level - preferably by a direct method, using, for example, catheterization of the radial artery; water balance - with mandatory measurement of hourly diuresis; ECG monitoring. If possible, it is advisable to monitor central hemodynamic parameters using a Swan-Ganz catheter or at least central venous pressure.
Conventional therapy for CABG includes oxygen therapy
.
Patients should receive oxygen through intranasal catheters or a mask, and in cases of severe respiratory dysfunction, transfer to artificial ventilation. As a rule, patients require therapy with inotropic drugs: intravenous infusion of dopamine
at a rate necessary to control blood pressure levels (on average, it is 10-20 mcg/kg per minute);
in case of insufficient effectiveness and high peripheral resistance, a dobutamine infusion
of 5-20 mcg/kg per minute is started.
Subsequently, it is possible to replace one of these drugs with norepinephrine in increasing doses from 0.5 to 30 mcg/kg per minute or adrenaline. In some cases, these drugs help maintain blood pressure at a level of at least 100 mm Hg. Art. diuretics
are usually prescribed . The use of nitro drugs and other peripheral dilators should be avoided due to their hypotensive effect. CABG is an absolute contraindication for the use of b-blockers.
It must be said that standard drug therapy, as a rule, turns out to be either ineffective or gives a short-term effect, so we will not dwell in detail on the detailed characteristics of well-known drugs - we will discuss those treatment methods that, according to modern ideas, can change the prognosis in patients with CABG.
Thrombolytic therapy
Most often, CABG develops with thrombotic occlusion of a large subepicardial coronary artery, leading to myocardial damage and ischemic necrosis. Thrombolytic therapy (TLT) is one of the modern methods of treatment
, allowing to restore perfusion in the ischemic focus and save viable (hibernated) myocardium.
The first encouraging results were obtained in the large-scale study GUSTO-I
(1997), where more than 40 thousand patients with MI were examined.
It turned out that in patients receiving tissue plasminogen activator (t-PA) therapy, shock developed in the hospital in 5.5% of cases, and in the group treated with streptokinase - in 6.9% of cases (p<0.01). The 30-day mortality rates were 57% and 58%, respectively. That is, t-PA therapy can prevent the development of CABG in patients with MI in the hospital. The next generations of tissue plasminogen activator - alteplase and retiplase, which have a number of advantages over t-PA (rapid thrombus destruction and ease of administration), were studied in the GUSTO-III
(1999). When alteplase and retiplase were administered, shock developed in a hospital setting in 5.3 and 5.5% of cases, and 30-day mortality was 65% and 63%, respectively. Thus, the next generation of thrombolytics were not as effective as expected in preventing the development of CS in hospital patients. Among patients included in GUSTO-I and GUSTO-III, upon admission to hospital, signs of CABG were recorded in 0.8% and 11%, respectively. Their mortality rate was: in the t-PA group - 59%, streptokinase - 54%, retiplase - 58%. Thrombolytic therapy slightly reduces the mortality rate of patients with MI complicated by CABG, and t-PA appears to reduce the incidence of its development. Research in this direction continues. It is possible that not only the use of new thrombolytics (mutant molecules, etc.), but also other methods of optimizing the treatment of patients can improve the outcome of the disease. It is known that combined therapy with thrombolytics and low molecular weight heparins, for example, enoxaparin (ASSENT-3, AMI-SK, HART II), significantly improved the prognosis of MI, reducing mortality, the number of recurrent MIs and the need for revascularization. It is possible that treatment of CABG using thrombolytic drugs and low molecular weight heparins will be more effective, although at present this is only a hypothetical consideration.
The use of thrombolytics for cardiogenic shock can improve survival in patients with MI, and in some cases prevent the development of this complication. However, the use of this treatment method alone is unlikely to significantly change the existing situation. This is due to the fact that low systemic pressure leads to low perfusion pressure in the coronary arteries and a significant decrease in the effectiveness of thrombolysis.
Intra-aortic balloon counterpulsation
Intra-aortic balloon pumping (IABP) is used to stabilize the condition of patients with CABG and increase the effectiveness of thrombolytic therapy. This is due to the fact that IABP improves myocardial perfusion in diastole, reduces systolic afterload, and does not change the myocardial oxygen demand. The GUSTO-I study showed a decrease in mortality by the 30th day of MI and after 1 year of the disease in cases where IABP was used in patients with CABG. Analyzing the results of NRMI-2
, compared the effectiveness of treatment of patients with CABG with and without IABP. It should be noted that these data were obtained as a result of the treatment of CABG in more than 20 thousand patients, not in a specially designed study, but in US healthcare practice over the past 6 years. Data on the percentage of patients who received thrombolytics, primary angioplasty (TBCA) or who did not receive “reperfusion” therapy are presented in Figure 1. It turned out that in the group receiving thrombolytic therapy, the use of IABP significantly reduced hospital mortality from 70% to 49%. The use of IABP during primary angioplasty did not significantly change in-hospital mortality. Mortality with CABG (Fig. 2) in patients after primary coronary angioplasty was 42% and was lower than with any other treatment methods.
Rice. 1. Percentage of patients treated with TLT or primary TBKA, or without these interventions, depending on the implementation of IABP NRMI 2
Rice. 2. In-hospital mortality in the group of patients with TLT or primary TBKA depending on the IABP NRMI 2
Thus, the use of IABP during CABG allows not only to stabilize the condition of patients during its implementation, but also to significantly improve the effectiveness of thrombolytic therapy and patient survival.
Non-drug restoration of coronary blood flow
The possibilities of non-drug restoration of blood flow in MI complicated by CABG, primarily using primary coronary angioplasty and emergency CABG, have been actively studied in the last decade. It is known that with successful primary angioplasty it is possible to achieve a more complete restoration of coronary blood flow, a smaller diameter of residual stenosis and improved survival of patients with CABG (S-MASH, GUSTO-1).
The most convincing results were obtained in the multicenter SHOCK
, conducted in 30 centers in the USA and Canada from 1993 to 1998, 302 patients with true CABG were randomized into groups of intensive drug treatment (n=150) and early revascularization (n=152). Patients of groups 1 and 2 received therapy with inotropic drugs in 99%, IABP in 86%, and thrombolytics in 63% and 49% of cases, respectively. In the second group, 97% underwent emergency coronary angiography and early revascularization was achieved in 87% of cases (intracoronary intervention - 64%, surgical - 36%).
The mortality rate in patients in the drug treatment group by the 30th day of MI was 56%, and in the early revascularization group it was 46.7%. By the 6th month of the disease, mortality in group 2 was significantly lower (63% and 50%, respectively), and these differences persisted until the 12th month of the disease (Fig. 3). Analysis of the effectiveness of aggressive management of patients with MI with CABG showed that the survival results of patients were better in cases of revascularization
in almost all subgroups (Fig. 4). The exceptions were elderly patients (over 75 years of age) and women in whom drug treatment was preferable. The SHOCK study was brilliantly designed. For example, the average time from decision to angioplasty was 0.9 hours, and to coronary surgery was 2.7 hours.
Rice. 3. Mortality of patients in the drug treatment (group 1) and early revascularization (group 2) groups SHOCK Study
Rice. 4. Comparative risk of 30-day mortality in subgroups of the SHOCK Study
Thus, early revascularization in patients with MI complicated by CABG apparently leads to restoration of the function of the hibernated myocardium and allows for a significant reduction in mortality in these patients. It should be noted that 55% of patients with CABG in the SHOCK study were transferred to specialized centers, where they underwent appropriate invasive interventions. This tactic for managing patients with CABG may be the most promising in our country.
Metabolic therapy
When a coronary artery is occluded, serious disturbances in the structure and function of the myocardium occur. Disorders of myocardial metabolism that develop during prolonged ischemia and systemic hypotension, even with restoration of coronary blood flow, can interfere with the normalization of recovery of cardiac function. In this regard, numerous attempts have been made to restore myocardial metabolism in patients with CABG using a number of drugs - glucose-insulin-potassium mixture, adenosine, Na+H+ channel blockers, L-carnitine. It was assumed that they would help increase the viability of ischemic myocardium. Despite the theoretical premises, today, from the standpoint of evidence-based medicine, no data has been obtained that would allow recommending the use of metabolic drugs in clinical practice for the treatment of patients with CABG.
New approaches to treatment
Encouraging results have been obtained using the latest generation of antiplatelet drugs - blockers of IIb-IIIa glycoprotein platelet receptors. Use of eptifibatide
in the subgroup of patients with CABG in the
PURSUIT
(2001) in patients with acute coronary syndromes without ST elevation led to a significant increase in survival compared to the control group. Perhaps this effect is partly due to the ability of this group of drugs to improve blood circulation in the microvasculature by eliminating platelet aggregates.
Treatment after discharge from hospital
Significant impairments in myocardial contractility persist in the majority of surviving patients with MI complicated by CABG. Therefore, they need careful drug monitoring and active therapy after discharge from the hospital. The minimum possible therapy, the purpose of which is to reduce the processes of myocardial remodeling and manifestations of heart failure (ACE inhibitors, diuretics, b-blockers and cardiac glycosides), reduce the risk of thrombosis and thromboembolism (acetylsalicylic acid, coumarin derivatives - warfarin, etc.), should be used by the treating physician doctor in relation to each patient, taking into account the developing clinical situation.
Medications
Medicines to treat cardiogenic shock are prescribed to improve blood flow through the heart and improve the pumping function of your heart.
- Aspirin. Aspirin reduces blood clotting and maintains blood flow through the narrowed artery. Take aspirin on your own while waiting for emergency services to arrive, only if your doctor tells you to do so if you are experiencing symptoms of a heart attack.
- Thrombolytics. These drugs help dissolve a blood clot that is blocking blood flow to the heart. The sooner you are given a thrombolytic drug during a heart attack, the better your chance of survival and less damage to your heart. Thrombolytics are prescribed if emergency catheterization of the heart arteries and stenting cannot be performed.
- Antiplatelet agents. Doctors in the emergency room may give you other drugs that are similar to aspirin to prevent new blood clots from forming. These include drugs such as clopidogrel (Plavix) and other platelet glycoprotein IIb/IIIa receptor blockers.
- Other blood thinning drugs. You will likely be prescribed other medications. These include heparin, to reduce the likelihood of dangerous blood clots. Heparin is given intravenously or subcutaneously for the first few days after a heart attack.
- Inotropic drugs. Dopamine or epinephrine is prescribed to improve and maintain heart function.
Treatment
Treatment of cardiogenic shock is aimed at restoring damaged heart muscle and other organs caused by a lack of oxygen.
Emergency medical care
Emergency medical care is necessary to treat most patients in cardiogenic shock. Treatment of cardiogenic shock is carried out in intensive care. You will be given oxygen to breathe using a mask, this is done to reduce damage to organs and tissues. Medicines and fluids will be given through intravenous catheters.
Drug treatment
Drug treatment of cardiogenic shock is aimed at improving blood flow through the arteries supplying the heart and increasing the contractility of the heart.
Aspirin.
Aspirin reduces blood clotting and helps blood flow through the narrowed area in the artery. Patients who were already taking aspirin before the development of cardiogenic shock, its administration in the acute stage of the disease is accompanied by a more favorable course.
Thrombolytics.
These drugs are so named because they help dissolve a blood clot that is blocking blood flow to the heart. The earlier these drugs are administered after the onset of acute myocardial infarction, the higher the survival rate and the less damage to the heart. Thrombolytics are usually prescribed if emergency cardiac catheterization is not possible.
"Superaspirin."
Emergency doctors may give you another drug that works similarly to aspirin and also prevents the formation of new clots in the blood vessels. These drugs include: clopidogrel (Plavisk) and other drugs that block platelet glycoprotein IIb/IIIa receptors.
Other blood thinning drugs. You may be given another drug, such as heparin, which will prevent clots from forming in your blood vessels. Heparin is administered intravenously or by injection into the subcutaneous fat and is prescribed for several days after myocardial infarction.
Inotropic drugs.
You may be prescribed drugs such as dopmin, adrenaline, to improve and maintain the contractility of the heart until other treatments are completed.
Therapeutic manipulations
Therapeutic manipulations for cardiogenic shock are aimed at restoring blood flow through the coronary arteries supplying the infarction zone. They include:
Angioplasty
Angioplasty and stenting.
These treatment methods are aimed at influencing areas of stenosis of the coronary arteries, restoring blood flow through these arteries and, as a result, improving the course of cardiogenic shock. Emergency angioplasty corrects a narrowed or occluded coronary artery, allowing blood to flow more freely to the heart. The doctor installs a special catheter through a puncture (puncture) of the femoral artery into the narrowed segment of the coronary artery. This catheter is equipped with a special balloon. When the catheter is inserted into the desired section of the coronary artery, it expands and opens the closed artery. At this time, a stent may be implanted into the artery to reduce the incidence of re-narrowing of the coronary artery. Your doctor may implant a drug-eluting stent; these stents keep the artery open for a long time.
Intra-aortic balloon counterpulsation.
Depending on your condition, your doctor may choose this treatment method. A special balloon is inserted into the aorta, which inflates and deflates, simulating the contractile function of the heart, helping blood flow to other organs and tissues.
Surgery
If medications and medical procedures are not effective in treating cardiogenic shock, then surgery may be recommended.
Coronary artery bypass surgery.
The essence of this surgical treatment is to create bypass vessels, shunts, which use veins and arteries between the aorta and the narrowed part of the coronary artery. As a result, blood flow to the heart is restored. Your doctor may recommend coronary artery bypass surgery some time after an acute myocardial infarction to help your heart recover.
Surgery
, aimed at repairing heart damage. Sometimes damage to the heart, such as a rupture of one of the heart chambers or damage to the heart valves, can cause cardiogenic shock. If cardiogenic shock is caused by a heart injury, then your doctor will recommend surgical treatment aimed at eliminating the heart defect.
Artificial heart.
This is a mechanical device that is implanted in the abdominal cavity and helps in the functioning of a weakened heart. An artificial heart helps prolong and improve the lives of patients with end-stage heart failure and patients awaiting a heart transplant.
Heart transplant.
If the heart is severely damaged and no treatment helps, the last resort to treat cardiogenic shock is a heart transplant.
Surgical interventions
Surgery to treat cardiogenic shock aims to restore blood flow through the heart. They are performed in specialized cardiac centers. Such interventions include:
- Angioplasty and stenting. Typically, when blood flow through the blocked artery is restored, the signs and symptoms of cardiogenic shock improve. Emergency angioplasty works to clear blocked arteries, allowing blood to flow freely to your heart. Doctors insert a long, thin tube (catheter) that is threaded through an artery in your leg or arm to the blocked artery in your heart. This catheter is equipped with a special balloon. When installing a stent in the area of narrowing, the balloon is inflated to restore the lumen of the artery. In addition, a metal mesh stent may be inserted into the artery to keep it open for a long time and restore blood flow to the heart. Doctors install stents that slowly release drugs into the blood to keep the artery wide enough.
- Balloon pump. Depending on your condition, doctors may place a balloon pump in your heart's main artery (aorta). The balloon pump inflates and deflates to mimic your heart and keep your blood flowing.
Shock
Which doctors should I contact?
At the first signs of shock, you should immediately call an ambulance.
Treatment of shock
A patient with shock of any type should be taken to the intensive care unit according to the expected profile of the disease. In case of injury, in the presence of signs of internal bleeding, as well as in situations where the cause of shock could not be determined at the prehospital stage, hospitalization in an anti-shock operating room (or another operating room intended for the treatment of victims with shock) is indicated.
Main goals of therapy:
- interrupt pain impulses;
- normalize the volume of circulating blood and the rheological properties of blood;
- correct metabolism;
- eliminate the causes of organ disorders.
1. Ensuring airway patency.
Oxygen therapy is carried out through a mask. In cases of severe shock or inadequate ventilation, intubation of the airway with mechanical ventilation is necessary. 2. Installation of intravascular catheters for rapid infusion of solutions and blood components, and, if necessary, administration of large quantities of medications (including catecholamines).
3. Etiological treatment.
4. Support the circulatory system and oxygen transport:
- discontinuation of antihypertensive drugs, if any were used;
- in most types of shock, the main importance is to replenish the intravascular volume by infusion of plasma substitutes and plasma expanders, solutions of crystalloids and colloids;
- infusion of vasopressor drugs to eliminate hypotension;
- patients with low cardiac output in the absence of cardiac arrhythmias are prescribed a continuous intravenous infusion of dobutamine; with concomitant hypotension, vasoconstrictor drugs are simultaneously used;
- oxygen therapy to reduce hemoglobin oxygen saturation.
5. The main method of correcting lactic acidosis is etiotropic treatment that supports the functions of the circulatory system.
6. Monitoring vital signs.
7. Prevention of bleeding from the gastrointestinal tract and thromboembolic complications.
8. Correction of hyperglycemia.
After resuscitation, specific treatment for the underlying disease is prescribed.
Additional supportive care depends on the type of shock. Complications
The danger of shock is a decrease in blood supply to vital tissues. The prognosis depends on the cause of shock and the timeliness of treatment.
If left untreated, shock is fatal.
The development of hypovolemic shock is accompanied by a mortality rate of up to 70%, depending on its severity. With timely assistance provided in full, the mortality rate does not exceed 25%. Distributive shock is characterized by varying mortality rates, depending on the etiological factor, and can reach 70% in the case of septic shock. Obstructive shock associated with fairly effectively removable causes is accompanied by a mortality rate not exceeding 15%. With pulmonary embolism, the mortality rate exceeds 30%. Even with treatment, mortality from cardiogenic shock after myocardial infarction remains very high and reaches 70%.
Prevention of shock
Shock is a condition, a process that cannot be considered separately from known nosological forms. Therefore, shock cannot be the cause of death; the cause of death becomes the factor that caused the state of shock. Prevention of shock involves timely treatment of diseases that can lead to its development.
Sources:
- Great Medical Encyclopedia (BME), edited by Petrovsky B.V., 3rd edition, volume 27.
- Clinical guidelines (protocol) for providing emergency medical care for shock in children. Russian Society of Emergency Medical Care Union of Pediatricians of Russia, 2015, p. 21.
- Moroz V.V., Bobrinskaya I.G., Vasiliev V.Yu. et al. Shock. Educational and methodological manual for students, residents, graduate students and doctors. - Moscow, 2011.
IMPORTANT!
The information in this section cannot be used for self-diagnosis and self-treatment. In case of pain or other exacerbation of the disease, diagnostic tests should be prescribed only by the attending physician. To make a diagnosis and properly prescribe treatment, you should contact your doctor.
Operations
If drug treatment and the listed surgical interventions are ineffective, the doctor will recommend surgery:
- Coronary artery bypass grafting. Bypass surgery involves sewing veins or arteries to bypass the area of blockage or narrowing of the coronary artery. This restores blood flow to the heart. The doctor will suggest this surgery after the heart has recovered from a heart attack.
- Surgeries to repair heart damage. Sometimes the cause of cardiogenic shock is a rupture of one of the chambers of the heart or damage to the heart valve. To correct these problems, the doctor will suggest surgical treatment.
| Intensive therapy | Site's home page | ||
Amniotic embolism
Amniotic embolism is an obstetric disaster that, fortunately, is quite rare. The frequency of this complication, according to various authors, ranges from 1 case in 8000 to 1 in 80,000 births. Amniotic embolism is the most unpredictable and, in many cases, almost unpreventable cause of maternal mortality, with rates reaching 86%. In the structure of maternal mortality, this pathology occupies from 1.2 to 16.5%. Such a wide range of data on incidence and mortality is due to the fact that, unfortunately (or rather, fortunately), not a single doctor can “boast” of extensive personal experience in managing patients with amniotic embolism. Despite the fact that interest in this complication of childbirth has been growing in recent years, doctors’ ideas about the clinical physiology of amniotic embolism and the intensive care of this complication are still very far from the desired perfection. Even though amniotic embolism is rare, it does not matter for the woman in labor and her doctors who are faced with this formidable complication that among other complications of childbirth, the percentage of amniotic embolism is small: the patient who died is 100% for herself and her family. Therefore, it seems to us very important that obstetricians and anesthesiologists are well aware of the current state of the problem of amniotic embolism.
Brief history of the problem
Apparently, the first purposeful description of amniotic fluid embolism was the dissertation of our compatriot N.E. Kasyanov “On the issue of pulmonary embolism by placental giants,” defended in St. Petersburg in 1896. True, in 1893. Ch. G. Schmorl discovered trophoblast cells in the lungs of a woman who died in childbirth and suggested that these cells enter the uterine bloodstream into the heart, and then into the lungs, where they are deposited, creating an obstacle to blood flow in the pulmonary circulation. However, this fairly well-reasoned hypothesis remained unnoticed. The next case of amniotic embolism was reported only 33 years later by the Brazilian doctor LRMeyer. Another year later, experimental work by RMWarden showed significant changes in the cardiovascular system in animals that occur after intravenous administration of amniotic fluid. These perfectly executed experiments had no clinical significance: they were obtained as a “by-product” in the study of the pathogenesis of eclampsia, and not amniotic embolism. This was followed by another break of a decade and a half, until in 1941 two researchers from the University Hospital of Chicago, PESteiner and CCLushbaugh, began a systematic study of amniotic embolism. The authors described this syndrome in 8 women who died suddenly in childbirth, and only after this amniotic embolism was recognized by medicine as a nosological form of the disease. It may seem that in their subsequent works these authors are guilty of overdiagnosis of amniotic embolism, citing a very high incidence of embolism - 1 in 3000 births. However, if we recall the cases of unexpected coagulopathic bleeding and pulmonary edema during childbirth, some of which, as we now know, are caused by undiagnosed amniotic embolism, then the statistics given by the authors will not seem so implausible. However, the “discovery” of amniotic embolism in 1941 flooded the medical literature with this diagnosis, since it was attributed to most cases in which a pregnant or postpartum woman died suddenly. In 1958, fetal epithelial cells were found in the uterine veins without clinical symptoms of amniotic embolism. Therefore, the detection of desquamated epithelial cells in blood samples obtained from the central vein or pulmonary artery, in the absence of clinical manifestations, should not be considered a pathognomonic sign of amniotic embolism, because a small number of them can be detected in the absence of this complication. According to modern ideas about the clinical physiology of amniotic embolism, the cause of the disaster does not lie in fetal scales, which means their detection in the bloodstream cannot always confirm or reject this diagnosis. For a very long time, published materials about amniotic embolism resembled extremely pessimistic scientific, and sometimes unscientific, fiction. And only in the last two decades, experimental and clinical work on amniotic embolism began to be devoted to the mechanisms of development of this formidable complication and its intensive therapy and prevention. One thing is indisputable - with amniotic embolism, amniotic fluid enters the maternal bloodstream. When and how does this happen, that is, what are the physiological mechanisms of amniotic embolism?
Clinical physiology
Conditions for embolism
Using radioisotope methods, it has been shown that during contractions during normal childbirth, amniotic fluid does not enter the maternal bloodstream. For this to happen, two conditions are necessary:
1) a significant excess of amniotic pressure over venous pressure;
2) gaping of the venous vessels of the uterus.
As for the first condition - the excess of amniotic pressure over venous
, - then in the absence of labor, amniotic pressure is about 8
mm water column
, and venous pressure is about 10
mm water column
.
At the height of contractions, these indicators are equal to 20 and 40 mm of water column, respectively,
that is, the first condition for the occurrence of amniotic embolism is not present either at rest or at the height of contractions. However, there are a number of situations that cause an imbalance of these parameters, which makes it possible to identify a group at risk of amniotic embolism. A decrease in venous pressure can sometimes be a consequence of hypovolemia associated with obstetricians' enthusiasm for diuretics in the treatment of edema in pregnant women without proper clinical and physiological understanding of the causes of their occurrence. Hypovolemia with low venous pressure can often accompany pregnancy in patients with diabetes mellitus, heart defects and severe forms of preeclampsia. Amniotic embolism occurs more often in multiparous women. Previously, it was believed that changes in the cardiovascular system that occur during pregnancy undergo complete reversal after childbirth. However, in 1991, ELCapeless and IFClapp showed that the decrease in peripheral vascular resistance that occurs after pregnancy is quite persistent. Therefore, during subsequent pregnancies and childbirths, multiparous women may experience a growing discrepancy between the increasing capacity of the peripheral vascular bed and the volume of circulating blood. As a result, relative hypovolemia and a decrease in venous pressure may sometimes occur, and then the likelihood of amniotic embolism increases. The unreasonable prescription of vasodilators and antihypertensive drugs to pregnant women without appropriate timely correction of volemic disorders leads to a similar result. Amniotic pressure can exceed venous pressure during rapid labor, breech presentation, large fetus or multiple pregnancy, with a rigid cervix and untimely opening of the amniotic sac. Among the factors predisposing to amniotic ambolism are stimulation of labor with oxytocin. It should be borne in mind that during a cesarean section, an infusion of oxytocin at a rate of 10 drops/min can itself lead to the development of arterial hypotension. There was even a report linking castor oil, used to stimulate labor, with the occurrence of amniotic embolism. Ingestion of castor oil increases the concentration of prostaglandin E and thus can induce contractions and potentiate the action of various contractile agents. Therefore, both castor oil and other factors that cause excessively strong contractions and increased intrauterine pressure can contribute to the occurrence of amniotic embolism.
Gaping of the vessels of the uterus - the second indispensable condition of amniotic embolism - is observed with premature placental abruption and with its presentation, with any surgical intervention on the uterus - caesarean section, manual examination of the uterus and separation of the placenta, as well as with postpartum atony of the uterus. Some authors associate amniotic embolism with traumatic non-penetrating uterine ruptures, the entry of amniotic fluid into the bloodstream through damaged endocervical veins or decidual sinuses. The conclusion from these clinical and physiological considerations is specific: various pathologies of pregnancy and childbirth, as well as concomitant hypovolemia of any etiology, including iatrogenic ones, are fraught with the danger of amniotic embolism.
We must pay attention to an amazing paradox: amniotic fluid is a clever invention of nature, designed to preserve the life and growth of the fetus, that is, for the normal development of pregnancy. But the imperfection of this important device is striking, fraught with a mortal threat to the mother and fetus if the amniotic fluid enters the maternal bloodstream, which, as we see, may not be so rare.
Patho- and thanatogenesis
Let's consider the composition of amniotic fluid. Amniotic fluid is a colloidal solution containing mucoproteins with a high carbohydrate content, a large amount of lipids and protein in a concentration of 210-390 mg%
.
Various biologically active substances are present in fairly high concentrations - adrenaline (76 µmol/l
), norepinephrine (59
µmol/l
), thyroxine and estradiol [Barnes].
Amniotic fluid is rich in histamine, levels of which increase in preeclampsia. It also contains profibrinolysine and thrombokinase-like substances. Adding one drop of amniotic fluid to a test tube of blood speeds up the clotting time by half. Amniotic fluid contains many products of protein and fat metabolism, biologically active substances, including cytokines and eicosanoids, as well as various mechanical impurities - epidermal scales, lanugo, cheese-like lubricant. With intrauterine infection of the fetus, the amniotic fluid can become infected, and the entry of infected amniotic fluid into the maternal bloodstream causes even more severe coagulopathy. The clinical physiology of amniotic embolism falls into two main forms - cardiopulmonary shock
and
coagulopathy
, but often these forms differ only in the time and degree of their manifestation.
Cardiopulmonary shock
For a long time there was an opinion that pulmonary arterial hypertension is caused by obstruction of the pulmonary capillaries by mechanical impurities (scales, vellus hair, mucin, etc.), but since in 1 ml
amniotic fluid contains on average about 500-600 cells, then in order for it to create a barrier to pulmonary blood flow, it would be necessary for the lungs to filter about seven liters of amniotic fluid, which requires the amniotic fluid of at least six pregnant women. The hypothesis of an increase in pulmonary vascular resistance with the occurrence of cardiopulmonary shock under the influence mainly of prostaglandin F2a seems more plausible. In animal experiments, the development of pulmonary hypertension and cardiopulmonary shock has been repeatedly noted with intravenous administration of amniotic fluid taken during childbirth, but not in mid-pregnancy. The main difference in the composition of amniotic fluid at these moments is the high concentration of prostaglandins at the time of birth. True, amniotic fluid embolism has been described in the early stages of pregnancy with high rupture of the membranes, but in the early stages amniotic embolism is more manifested by coagulopathy and, to a lesser extent, by cardiopulmonary shock. In 1985, special studies of SLClark ea revealed acute left ventricular failure and a sharp decrease in cardiac output with relatively normal pulmonary vascular resistance in amniotic embolism. A hypothesis arose about the direct toxic effect of amniotic fluid on myocardial contractility, and to test it, isolated animal hearts were perfused with filtered amniotic fluid. This caused pronounced spasm of the coronary vessels and a decrease in cardiac output, that is, heart failure due to direct myocardial ischemia. It is appropriate to remember that prostaglandins F2a and E, as well as thromboxane, cause spasm of the coronary vessels, while prostacyclin dilates them and improves the pumping function of the heart. Rare symptoms of amniotic embolism include the appearance of an unpleasant taste in the mother’s mouth immediately after separation of the placenta, short-term chills and hyperthermia, although most likely these “trifles” are simply rarely paid attention to by specialists intoxicated by the power of their science. Anesthesiologists working in maternity wards are familiar with difficult-to-control postpartum tremors, which can hardly be considered only from the standpoint of hypoergosis, especially when it is followed by hypotonic or coagulopathic bleeding. During a caesarean section, these symptoms are masked by anesthesia. The chain of changes described ends with either ventricular fibrillation or cardiopulmonary shock with the development of components of non-cardiogenic pulmonary edema. The gloomy description of thanatogenesis of cardiopulmonary shock during amniotic embolism has one small silver lining: we mentioned that there are no insurmountable mechanical obstacles to pulmonary and coronary blood flow, and death occurs from reactions triggered by biologically active substances. Coping with them is the main task of the anesthesiologist-resuscitator in the early stages of amniotic embolism.
Coagulopathy
If the phase of cardiopulmonary shock can be successfully overcome, then most patients develop one or another degree of coagulopathy caused by the thromboplastic properties of the amniotic fluid. Disseminated intravascular coagulation syndrome may be the only clinical manifestation of amniotic fluid embolism, and may also precede or complete cardiopulmonary shock. You just need to remember that amniotic embolism is the clinical and physiological basis of many “unexplained” coagulopathic bleeding in obstetric practice. Bleeding can begin without RVS syndrome, due to the fact that amniotic fluid also causes uterine atony. Clinical manifestations and functional diagnosis of the coagulopathic form of amniotic embolism are not specific.
Intensive therapy
Unfortunately, intensive care for cardiopulmonary shock during amniotic embolism most often turns into intensive care. That is why it is necessary that near a woman in labor who is suspicious of the possibility of this complication, there is always a person who knows resuscitation techniques. And it doesn’t matter what he is called in the hospital’s staff list - obstetrician, anesthesiologist, resuscitator or therapist: we will be considered glory after the patient’s safe discharge. But only one person must command, because, as H.V. Hufeland rightly noted, “... one doctor is better than two, two are better than three, etc., with an increase in the number of doctors, the probability of recovery always decreases.”
. When the heart stops due to compression syndrome of the inferior vena cava, venous return practically stops, no matter how hard the heart massage is performed. Therefore, performing pulmonary-cardiac resuscitation in the supine position of a pregnant woman reduces its effectiveness due to a sharp decrease in blood supply to the myocardium due to a decrease in venous return. During resuscitation in late pregnancy, the table should be tilted slightly to the left to reduce aortocaval compression. We believe that amniotic embolism is too formidable a complication of the daily practice of obstetricians and anesthesiologists working next to them to remember about it only when it occurs. Based on knowledge of clinical physiology, identification of risk factors for amniotic embolism, timely elimination of physiological mechanisms contributing to the development of this complication, readiness and equipment for resuscitation and intensive care for both cardiopulmonary shock and coagulopathy - this is the simple basis for success in preserving the lives of patients with amniotic embolism. After successful resuscitation or in cases of amniotic embolism that did not require it, intensive care can be roughly divided into three groups of actions:
I. Suppression of reactions that caused cardiopulmonary shock.
II. Treatment of coagulopathy.
III. Prevention and treatment of multiple organ failure.
We adhere to the following procedure for intensive care of amniotic embolism. Catheterization of the subclavian vein with mandatory monitoring of central venous pressure after eliminating aortocaval compression by tilting the operating table to the left;
Following actions:
* at CVP < 8 cm water column.
correction of hypovolemia is carried out by infusion of colloids and crystalloids in a 2:1 ratio at a rate of 5 to 20
ml/min
, depending on changes in central venous pressure; in case of bleeding, the infusion therapy includes transfusion of fresh frozen plasma or fresh blood;
* at central venous pressure > 8 cm water column
. use drugs with a positive inotropic effect (Dobutrex, etc.), starting with minimal doses; At the same time, glucocorticoids can be administered, which stabilize adrenergic receptors;
* with increasing signs of respiratory failure and PaO2 < 70 mm Hg
. perform tracheal intubation and transfer the patient to mechanical ventilation;
* as early as possible, after monitoring the blood clotting properties, administer heparin at the rate of 50-100 units/kg
simultaneously intravenously in combination with large doses of proteolytic enzyme inhibitors: this can significantly reduce the likelihood of coagulopathy.
Further intensive therapy for the coagulopathic form of amniotic embolism is not specific and requires only a scrupulous clinical and physiological approach. What in critical care medicine does not require this? It is necessary to take blood from the central vein, sputum or tracheal washings, and then examine them for the presence of elements of amniotic fluid, although, as already noted, their presence is not an absolute argument for confirming the diagnosis. An amniotic embolism can masquerade as another extremely serious pathology, and no matter how our intensive care ends, who knows how much effort will still have to be made at the morning conference, proving that it was an amniotic embolism and not something completely different.
| Intensive therapy | Site's home page | ||
Prevention
The best way to prevent cardiogenic shock is to prevent heart attacks. Lifestyle changes used to treat heart disease can help prevent a heart attack. These lifestyle changes include:
- Control of high blood pressure (treatment of hypertension). One of the most important steps to reduce your risk of heart attack and cardiogenic shock is to control your blood pressure. Regular exercise, managing stress, maintaining a healthy body weight, and limiting salt and alcohol intake will help control high blood pressure. In addition to recommendations for lifestyle changes, your doctor may prescribe medications to treat hypertension, such as diuretics, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers.
- To give up smoking. Quitting smoking reduces the risk of having a heart attack. Several years after quitting smoking, a smoker's risk of developing a stroke is the same as a non-smoker's.
- Maintaining normal body weight. Excess weight contributes to the risk factors for heart attack and cardiogenic shock: high blood pressure, cardiovascular disease and diabetes. Losing just 10 pounds of weight will lower your blood pressure and cholesterol levels.
- Reduce cholesterol and saturated fat in your diet. Eating less cholesterol and fat, especially saturated fat, will reduce your risk of heart disease.
- Regular exercise. Exercise reduces your risk of having a heart attack. Exercise lowers blood pressure, increases high-density lipoprotein (HDL) levels, and improves the overall health of your blood vessels and heart. They will help you lose weight, control diabetes and reduce stress. Get regular exercise such as walking, jogging, swimming or cycling for 30 minutes every day.
Intensive therapy of arrhythmogenic shock
Arrhythmogenic shock is a type of circulatory disorder in which adequate blood supply to organs and tissues is impaired due to an imbalance in the heart rate. Arrhythmia can be primary or secondary. Primary disorders include rhythm and conduction disorders caused by abnormalities in the development of the conduction system. Secondary arrhythmia is associated with cardiomyopathy, fibroslastosis, organic lesions and myocardial metabolic disorders, and electrolyte disturbances. Based on the location of the pathological pacemaker, supraventricular (atrial and nodal) and ventricular arrhythmias are distinguished. There are also tachy- and bradyarrhythmias . The main pathogenetic mechanism of tachyarrhythmic shock is a shortening of the diastolic filling time of the heart and a decrease in stroke output against the background of a reduction in the diastolic period of coronary blood flow.
In bradyarrhythmic shock, a decrease in cardiac output cannot be compensated by an increase in stroke output, since the volume of diastolic filling of the ventricles is limited by the ability of the myocardial wall to mechanical stretch.
A preliminary diagnosis of arrhythmia is established based on palpation of the pulse in the femoral or carotid arteries, auscultation of the heart and the presence of hypertension. Symptoms such as a sudden change in the child’s condition, restlessness or lethargy (blockade of the atrioventricular junction), loss of consciousness (Edams-Stokes syndrome), acrocyanosis, pallor of the skin, and “marbled” skin pattern can be used to suspect arrhythmia. An accurate diagnosis is established based on ECG data.
Arrhythmogenic shock can develop against the background of excessive sinus, atrial and ventricular tachycardia, ventricular fibrillation, bradyarrhythmia (idioventricular rhythm, atrioventricular block II - III degree), ventricular extrasystoles.
Treatment of arrhythmogenic shock involves emergency restoration of heart rhythm at a rate that ensures adequate cardiac output. A prerequisite for the treatment of tachy- and bradyarrhythmic shock is the elimination of arrhythmogenic factors: the negative influence of the vagus nerve, hypoxia, acidosis, alkalosis, and electrolyte disturbances. The use of antiarrhythmic drugs should be preceded by correction of hypo- and hypervolemia, anemia, hypoglycemia and mandatory atropinization at the rate of 0.01-0.03 mg/kg body weight. The priority means of emergency treatment of tachyarrhythmic shock is electrodepolarization (2 W/s per 1 kg of body weight), which allows you to gain time and select the optimal antiarrhythmic pharmacological agent. For supraventricular tachyarrhythmia, it is preferable to administer isoptin - 0.1 mg/kg over 1 minute. The same dose can be prescribed again at 15-minute intervals. Lidocaine is prescribed at a dose of 1 mg/kg and administered over 10 minutes. Mexitil is effective for ventricular tachyarrhythmia and extrasystole. The drug is administered at a dose of 5 mg/kg over 15 minutes, a maintenance dose of 5–20 mcg/kg per minute.
In case of bradyarrhythmic shock and there is no effect from the administration of atropine sulfate, the drug of choice is Isuprel (isoproterenol, isadrin, novodrin). If there is no effect from drug therapy, cardiac pacing is indicated.
All children in a state of arrhythmogenic shock are hospitalized in the intensive care unit.