GLYCOSIDES,
a group of carbohydrate-containing substances formed during the condensation reaction of cyclic mono- and oligosaccharides with alcohols, phenols, thiols and amines, widely present in living organisms, especially in plants.
Many glycosides that have no natural analogues have also been synthesized. Glycosides are characterized by the ability to hydrolyze (i.e., split in a reaction with water) with the formation of one or more sugar residues and a non-carbohydrate substance, the so-called aglycone. Hydrolysis is carried out in warm water in the presence of specific enzymes or by boiling with dilute acids. Some types of glycosides are also hydrolyzed when heated with dilute alkali solutions. Also on topic:
ORGANIC CHEMISTRY
The term "glycoside" comes from the Greek. "glikos" meaning "sweet". This class is sometimes mistakenly called glucosides, but glucosides are only those glycosides whose hydrolysis releases only glucose (dextroglucose, or dextrose) as the only sugar component. The names of natural glycosides have the suffix -in, and the root is derived from the scientific or folk name of the plant or plant product in which the glycoside was first discovered: for example, gitagin from Agrostemma githago
(cockle), hederin from
Hedera helix
(ivy).
Saponin glycosides
(saponins) are a class of substances that, like soap, form foam when their aqueous solutions are shaken. Hence their name: “sapo” means “soap” in Latin. As a rule, saponins are amorphous, soluble in water and alcohol, neutral substances with an irritating, pungent taste. When hydrolyzed, they produce aglycones (sapogenins) with a fairly large molecular weight and relatively high amounts of sugars. Saponins are widely distributed in the plant world, especially among plants of the Rosaceae and Carnation families (soapwort of the genus Saponaria
).
Saponins act on the body in a characteristic way: 1) when they get on the nasal mucosa, they cause sneezing; 2) cause the formation of hematomas (hemolysis); 3) are a deadly poison for fish and lower animals; 4) significantly reduce the surface tension in liquids that serve as solvents. Saponins and saponin-containing materials are widely used in pharmacy, medicine and technology. They are used as detergents, especially for silk and other valuable fabrics, as poisons for fish and insects, and in fire extinguishers (to stabilize foam). Examples of saponins are digitonin from foxglove, sarsaponin from sarsaparilla (smilax officinalis or smilax chinensis), and trillin from trillium (crow's eye, a plant in the lily family).
Here are collected articles, abstracts, textbooks on various areas of chemistry. All works are checked by the administrator before publication. If you have interesting information that you are ready to share with Internet users, send it to us and we will definitely publish it.| List of medicines presented in Russia. Contains information on 15,767 medicines, including synonyms of names. | ||
| Auto-equalizer - selection of chemical reaction coefficients | www.webqc.org |
| Online equalizer of chemical reactions. Selects the coefficients of a given reaction. Works with classical molecular reactions, redox half-reactions, and reactions in ionic form. | ||
| Adsorption of polymers on solid surfaces | unknown |
| Adsorption interaction at the interface and properties of boundary layers, boundary layers of polymers on solid surfaces, the influence of adsorption interaction on the molecular mobility of polymer chains in boundary layers, changes in the properties of boundary layers as a consequence of a decrease in molecular mobility, the influence of a solid surface on the supramolecular structures of polymers, adsorption connection polymers with adhesion, the influence of the interface on synthesis reactions and the structure of three-dimensional polymers. | ||
| Alchemy | unknown |
| Brief article on alchemy | ||
| Aldehydes and ketones | L.A. Tsvetkov |
| To make the place of aldehydes in the series of hydrocarbon oxidation products clear to students, when drawing up chemical equations one should not avoid using the names and formulas of the acids into which aldehydes are converted. The formulas of acids can be given dogmatically in advance... | ||
| Anthropogenic soil pollution | E.E.Matveev |
| Classification of soil contaminants, ways in which contaminants enter the soil, the importance of protecting soil cover. | ||
| Astatine | unknown |
| The history of the discovery of astatine, as well as its physical and chemical properties, is briefly outlined. | ||
| Atomic-molecular science in chemistry | G. P. Khomchenko |
| Atomic-molecular science was developed and first applied in chemistry by the great Russian scientist M.V. Lomonosov. The main provisions of his teaching are set out in the work “Elements of Mathematical Chemistry” (1741) and a number of others. The essence of Lomonosov’s teaching boils down to the following... | ||
| Acetylene. Acetylene production | L.A. Tsvetkov |
| Experiments in producing acetylene and studying its properties are demonstrated simultaneously. You should not prepare acetylene for the lesson in advance and store it in the gasometer due to the danger of explosion. The most accessible way to produce acetylene is the interaction of calcium carbide with water... | ||
| Benzene as a solvent | L.A. Tsvetkov |
| 1 ml of benzene is poured into one test tube, and the same amount of water into the other. A small piece of fat or a few drops of vegetable oil are placed in test tubes. The test tubes are shaken... | ||
| [ ] |
Prevalence.
Glycosides are found in the bark, fruits, roots, tubers, flowers and other parts of plants. Sometimes one plant contains several different glycosides. They are formed where biosynthesis is actively taking place, for example in leaves and green stems, and are transported in dissolved form to places of accumulation - roots and seeds. Most plant pigments are glycosides. Many tannins are also glycosides. It was initially assumed that glycosides are formed only in plants, but it is now known that they can also arise in the body of animals during the digestion process, when some substances harmful to the body combine with glucuronic acid (which is related to glucose and plays the same role as glucose in plant glycosides) are excreted in the urine.
Mechanism of action
Cardiac glycosides are complex nitrogen-free compounds of plant origin with a selective cardiotonic effect (able to increase myocardial contractility, stroke and minute blood volume without increasing oxygen consumption).
The glycoside molecule consists of two parts:
- Glycone is a sugar that determines the pharmacokinetics of the drug (ability to dissolve in water, fats, acids, passage through the cell membrane, absorption rate in the digestive tract, strength of bond with plasma proteins, affinity with receptors).
- Aglycone is a structure that is responsible for the mechanism of action and pharmacodynamics (direct clinical effects of taking a substance).
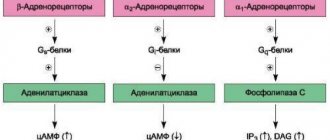
Entering the body, SGs interact with receptors called endodigins, which are located in the myocardium, skeletal muscles, smooth muscle fibers, liver, kidneys, and blood cells.
The cardiotonic mechanism of action of cardiac glycosides is associated with the ability to:
- inhibit the activity of Na-K-ATPase (the enzyme responsible for the operation of the sodium-potassium pump), reducing cell repolarization;
- forming complexes with Ca2+ ions to transport them inside the cardiomyocyte;
- stimulate the release of ions from the sarcoplasmic reticulum into the free cytoplasm.
As a result of these processes, the concentration of functionally active Ca2+ inside the cardiomyocyte increases. This ion ensures the neutralization of the troponin-tropomyosin complex and the release of the actin protein, which interacts with myosin to form the basis of cardiac contraction. In addition, Ca2+ ions activate myosin ATPase, which supplies the contractile process with the necessary energy.
The influence of Ca2+ ions on cardiac activity:
- Increased strength of heart contractions;
- Increased heart rate;
- Increased myocardial excitability and pacemaker automaticity.
Consequently, under the influence of cardiac glycosides, the work of the heart becomes more economical, as they optimize the use of ATP and the need for oxygen.
Pharmacological effects of taking glycosides:
- Positive inotropic effect. The force of myocardial contractions increases, systole shortens, as a result of which the stroke volume of blood and cardiac output return to normal. Partially due to the release of catecholamines and increased tone of the sympathetic nervous system.
- Negative chronotropic effect. Prolongation of diastole (the time of myocardial rest and filling of the coronary vessels with blood) and a decrease in heart rate.
- Negative dromotropic effect. Slowing down the passage of the impulse through the conduction system of the heart (from the sinus to the atrioventricular node).
- Diuretic action. In patients with decompensated heart failure and fluid retention in the body, due to improved renal circulation and inhibition of reabsorption of Na+ ions in the distal tubules, daily diuresis increases.
- Sedative effect.
- Increased intestinal motility and gallbladder tone.
Hemodynamic effects from taking cardiac glycosides:
- Strengthening and shortening the duration of systole;
- Increased minute volume of blood, left ventricular ejection fraction;
- Prolongation of diastole;
- Decrease in heart rate (effect on the vagus nerve);
- Approaching normal heart sizes;
- Decrease in venous pressure;
- Optimization of blood supply to the heart muscle (improvement of subendocardial blood flow, rheological properties of blood);
- Decreased circulating blood volume and myocardial oxygen consumption;
- Normalization of pressure in the vessels of the pulmonary circle, reducing the risk of pulmonary edema, improving gas exchange, oxygen saturation of the blood;
- Elimination of edema.
Functions.
Of the several theories proposed to explain the role of glycosides in plant physiology, the following three are the most plausible. 1) In unripe fruits, glycosides, due to their bitter taste, serve to protect against being eaten by animals. As fruits ripen, the colorless bitter glycosides are broken down, releasing pigments that give the fruit its attractive color, aromatics that give it flavor, and sugars that make it sweet. All this attracts various animals, birds and insects, which leads to effective seed dispersal. 2) According to another theory, glycosides are a means of removing toxic substances by binding them and converting them into inert forms (detoxification). 3) The third theory states that glycosides are a form of storage of sugars as a nutritional reserve. Their breakdown is a quick way to provide the plant with sugars.
Cardenolides
Cardenolides are found in living LRs in the form of “primary” (genuin) glycosides as the final products of biosynthesis and contain the maximum amount of sugar residues. After harvesting and drying, “secondary” glycosides are formed as a result of the action of enzymes and shortening of the glycoside chain.
MPs containing cardenolides: foxglove leaf, strophanthus seed, spring adonis herb, lily of the valley herb, spreading icterus herb.
Foxglove - Digitalis
The genus Foxglove belongs to the Norichnikov family - Scrophulariaceae. Many of them (200 genera and 300 species) contain cardiotonic glycosides, but few are of economic importance.
All types of foxgloves are usually perennial (less often biennial) herbaceous plants. Foxgloves used in medicine belong to two sections: Grandiflorae - plants with flowers located in a one-sided raceme and characterized by a bell-shaped or thimble-shaped corolla (digitalis purple, large-flowered and ciliated), and Globuliflorae (foxgloves woolly and rusty), which are characterized by multilateral brush, spherical tube of the corolla and a strongly protruding lobe of the lower lip.
Foxglove Leaves - Folia Digitalis
Plants. Foxglove purple - D.purpurea L.; large-flowered foxglove - D.grandfflora Mill.
Raw materials - Folia Digitalis - are obtained from the described two types of foxglove - purple and large-flowered, moisture no more than 12%, total ash - 1.8%, darkened leaves 1%, organic and mineral impurities - 0.5% each, other parts 1% .
Medicinal raw materials. Foxglove leaves are fully developed rosette leaves of the cultivated plant Foxglove purpurea, collected in the 1st year of life, immediately kept at a temperature of 55-60 °C, and then quickly dried. Currently, raw materials from foxglove grandiflora are practically not collected. Shelf life is 1 year according to list B. Biological standardization of raw materials is monitored annually.
Benign raw materials of foxglove purpurea and grandiflora have 50-60 ICE in 1 g. Packaging - fabric bales or bales (50 kg of whole raw materials), in bags (25 kg of chopped medicinal plant).
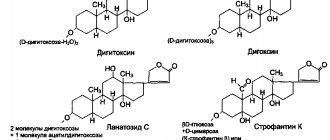
Chemical composition. All organs of the LR contain up to 3% glycosides (26 substances). The active ingredients of the leaves are cardenolides. Native (primary) glycosides are purpureaglycosides A, B and glucogitaloxin. In all native glycosides, the carbohydrate part is represented by three molecules of D-digitoxose and one of D-glucose.
Aglycones: digitoxigenin, gitoxygenin and gitaloxygenin, respectively.
Steroidal saponins are present. They have no effect on the heart, but increase the absorption of cardiac glycosides, so decoctions and herbal preparations work better than pure glycosides.
During enzymatic hydrolysis, which occurs in two stages, secondary glycosides are formed - digitoxin, gitoxin and gitaloxin.
In addition to the listed compounds, the leaves contain flavonoid glycosides, which, like the glycosides themselves, have a diuretic effect (relieving edema).
Application. Cardiotonic drugs are obtained from the raw materials: leaf powder, dry extract – cordigit, infusion, digitoxin. Digitalis preparations increase diuresis. They have a pronounced cumulative property, so they are taken for 5 days with a break of 1-2 days. Prescribed for stage II-III coronary insufficiency, heart defects, tachycardia, edema.
Leaves of foxglove woolly - Folia digitalis lanatae .
Plant. Foxglove woolly - D.lanata Ehrh.
Chemical composition. Contains more than 18 glycosides. The active ingredients of the leaves are cardenolides. The primary glycosides are more complex than those of foxglove purpurea and are called lanatosides (or digilanides): A, B, C, D and E. More complex glycosides in chemical composition
Application. Cardiotonic drugs are obtained from raw materials: digoxin (acetyl digoxin), celanide (lantoside C), lantoside (sum of saponins). They accumulate less, are absorbed faster and have a stronger diuretic effect than digitalis purpurea preparations. All drugs are poisonous and are taken only as prescribed by a doctor.
In addition to the characterized types of foxglove, foxglove ciliata, from which the grass was harvested, and foxglove rusty (including Shishkin's foxglove) are allowed for use. Leaves from the latter species were harvested.
Strophanthus seeds - Semina Strophanthi
Various types of strophanthus. Most often, the following species are included in the pharmacopoeias of different countries: Strophanthus kombe Oliv.; bristly strophanthus - Strophanthus hispidus; attractive strophanthus - Strophanthns gratus. Kutrov family - Apocynaceae.
Chemical composition. In the seeds of the main industrial species, Strophanthus Combe, the main primary glycoside is K-strophanthoside1, which contains up to 3%. A total of 12 substances were found.
Medicinal raw materials. Seeds
1 g of strophanthus seeds must contain at least 2000 ICE. List A.
Application. From the seeds of Strophanthus Combe (imported raw materials from Africa), the domestic industry produces the following preparations.
1. Strophanthin K (Strophanthinum K) consists of the primary glycoside K-strophanthoside and the secondary glycoside K-strophanthin-β. It has high biological activity (43,000-58,000 ICE per 1 g). When administered intravenously (0.05% - 0.2-0.5), the effect appears within 5-10 minutes. Does not have a cumulative effect.
Glycosides are not stable and the drug is ineffective when taken orally. Used for acute cardiovascular failure, including due to acute myocardial infarction; in severe forms of chronic circulatory failure. List A.
2. Strophanthidin acetate is a pure aglycone esterified with acetic acid. The drug has an activity of 18000-20000 LED. Gives a cardiotonic effect, characteristic of drugs of the digitalis group, but without signs of cumulation. Available in the form of a 0.05% solution in ampoules of 1 ml. List A.
Crystalline G-strophanthin is used as a standard in the biological standardization of cardiac glycosides.
Spring adonis herb - Herba Adonidis vernalis
Adonis (adonis) spring - Adonis vernalis L.; kaz. Gulzhapyrak; Ranunculaceae family. There are about 30 species of annual and perennial plants, in the CIS – 11 species, in Kazakhstan – 8.
Identification and quantification.
Glycosides are recognized by identifying their breakdown products - sugars and aglycones. For this, conventional methods of separation and identification of organic compounds are used: various types of chromatography, mass spectrometry, nuclear magnetic resonance spectroscopy, etc. To quantify the content of glycosides in raw materials, free sugars are determined before and after hydrolysis: the increase in the amount of free sugars corresponds to the number of glycosidic bonds destroyed by hydrolysis. Knowing the composition of glycosides, it is possible to estimate their content in the sample. see also
STEROIDS; TANNINS.
Indications and contraindications
Indications for use of SG:
Acute and chronic heart failure caused by impaired contractile activity. (Most often, glycosides are prescribed to patients with congestive CHF of functional class II, III, or IV).- Atrial fibrillation (tachysystolic form);
- Paroxysmal, supraventricular, atrial tachycardia;
- Vegetative-vascular cardioneurosis.
List of contraindications to the use of cardiac glycosides:
- Severe bradycardia;
- Atrioventricular block II-III degree;
- Morgagni-Adams-Stokes syndrome, WPW;
- Polytopic extrasystole;
- Heart failure with diastolic dysfunction;
- Acute coronary syndrome;
- Sick sinus syndrome (without an established pacemaker);
- Infectious myocarditis;
- Constrictive pericarditis;
- Ventricular tachycardia;
- Electrolyte imbalance: hypokalemia, hypercalcemia;
- Mitral valve stenosis;
- Hypertrophic cardiomyopathy (obstructive form);
- Aneurysm of the thoracic aorta;
- Hypertrophic subaortic stenosis;
- Cardiac tamponade;
- Carotid sinus syndrome;
- Restrictive cardiomyopathy;
- Arrhythmias caused by a history of glycoside intoxication;
- Chronic renal failure with program hemodialysis;
- Hypersensitivity to any cardiac glycosides.
These drugs require individual dose selection (for long-term therapy it is titrated over 7-10 days) and regular monitoring of the patient’s clinical condition (ECG, blood electrolytes - K, Ca, Mg).
If intravenous administration is necessary, the dose of the drug is divided into 2-3 doses.
Dose adjustment is required for patients with:
Diseases of the thyroid gland. In case of hypothyroidism, the dose of cardiac glycosides should be reduced. In the case of thyrotoxicosis, relative resistance to SG is observed.- Malabsorption syndrome, short bowel. Due to impaired absorption of the drug, an increase in dose is required.
- Severe respiratory pathology (increased sensitivity to glycosides).
- Electrolyte imbalance. High risk of developing digitalis intoxication and arrhythmia.
In elderly patients and debilitated patients, the elimination period of the drug is prolonged, which increases the risk of side effects and overdose.
Interaction of glycosides with other medicinal substances, requiring dose adjustment:
- Adrenomimetics – Epinephrine, selective β-agonists (incompatible, risk of arrhythmias);
- Aminazine reduces the effectiveness of SG;
- Anticholinesterase drugs - Prozerin, Physiostigmine (increase bradycardia);
- Glucocorticosteroids - Hydrocortisone, Prednisolone (increase the frequency of side effects);
- Diuretics – Furosemide, Trifas (strengthen the effect of SG);
- Paracetamol (reduced excretion of glycosides by the kidneys);
- β-blockers and Ca2+ channel antagonists (the occurrence of bradycardia and complete heart block).
Side effects and symptoms of overdose
During treatment with cardiac glycosides, like any other medications, there is a risk of developing adverse reactions:
- Rhythm and conduction disturbances (sinus bradycardia, blockades, ventricular fibrillation);
Blood system disorders – eosinophilia, thrombocytopenia, agranulocytosis;- Allergic manifestations - skin itching, hyperemia, rashes (erythematous, papular), urticaria, Quincke's edema.
- Gynecomastia (enlargement of the mammary glands) in men due to the estrogenic activity of SG;
- Mental disorders - depression, auditory and visual hallucinations, memory disorders, confusion;
- Neurological symptoms - migraines, asthenia, vertigo, sleep disturbances, nightmares, apathy or nervous agitation, myalgia;
- Blurred vision (result of retrobulbar neuritis), photophobia, glow effect around objects, impaired color perception (seeing everything in the yellow-green or gray-blue range);
- Lack of appetite, abdominal pain, nausea, insufficiency of visceral blood flow, intestinal ischemia.
If the recommended doses are exceeded, SGs exhibit toxicity and are capable of disrupting metabolic processes and causing Ca2+ retention in the cytoplasm due to problems with its removal from the cell. At the same time, the amount of ATP, glycogen, proteins, potassium, magnesium decreases and metabolism shifts to the anaerobic side. Myocardial relaxation in diastole is disrupted, stroke volume drops, and pre- and afterload increases.
Failure to comply with the rules of treatment with glycosides (independently increasing the dose or reducing the intervals between doses) is fraught with the development of overdose symptoms:
- Arrhythmias, bradycardia, AV block, extrasystoles, ventricular fibrillation;
- Nausea, diarrhea, lack of appetite, feeling of a full stomach;
- Headaches, vertigo, psychomotor agitation;
- Decreased visual acuity, impaired color perception, scotomas (blind spots), distortion of the size of an object;
- Impaired consciousness, syncope.
Treatment of signs of glycoside poisoning
If symptoms of overdose develop, the following measures must be taken:
- immediately stop taking glycosides;
- Induce vomiting, rinse the stomach;
- Take activated carbon (at the rate of 1 tablet per 10 kg of weight) or another sorbent;
- Seek medical attention immediately.
In a hospital, a patient with an overdose of cardiac glycosides is administered:
- Subcutaneous administration of Unithiol (antidote) 0.05 g per 10 kg of weight 4 times a day, cholestyramine;
Infusion of potassium solution (without insulin) or combined drugs - Asparkam, Panangin;- Use of salt-carrying preparations;
- For bradycardia - administration of Atropine;
- In case of arrhythmia - intravenous Lidocaine, Difenin, Phentoin, Amiodarone;
- In case of severe blockade, an artificial pacemaker is installed;
- Vitamin preparations – Nicotinamide, Thiamine, Tocopherol, Pyridoxine;
- Drugs that increase metabolism - Riboxin, Cytochrome C, methyluracil;
- Oxygen therapy.
The mechanism of action of Unithiol (a universal antidote) is associated with the property of reducing the toxic effect of glycosides on the functional activity of ATPase and normalizing energy metabolism in the myocardium.
In case of life-threatening overdose of glycosides (mortality during intoxication reaches 40%), the administration of antidigoxin serum, Digibind, and immunoglobulins that bind FH is indicated. Dialysis and exchange blood transfusion are ineffective.
Considering the frequency of side effects, overdose and their connection with a violation of the regimen of cardiac glycosides, the doctor should focus the patient’s attention on strict adherence to the prescriptions, and the patient should definitely study the instructions for the drug before using it. It is strictly forbidden to independently replace the glycoside with an analogue without prior approval from the attending physician.
conclusions
Glycosides are drugs for the treatment of chronic heart failure with impaired contractile function. A particularly good effect is observed in patients with a combination of CHF and atrial fibrillation. In this case, a diagram of slow digitalization is shown.
Prescribing cardiac glycosides (most often Digoxin) to patients improves the course of the disease, quality of life, increases resistance to physical stress, without changing the myocardial oxygen demand. While taking SG, patients experience decreased weakness, shortness of breath, improved sleep, edema and cyanosis disappeared, tachycardia returned to a normal rhythm, diuresis increased, and ECG readings stabilized.