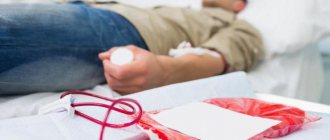
Sometimes cancer patients require blood transfusions. If the doctor said that he plans to prescribe a blood transfusion, the patient usually has a lot of questions. Why is the procedure needed? Did something terrible happen? How safe is blood transfusion? Can you get HIV and other dangerous infections from a donor? How will the body react to someone else's blood? Will there be any complications? Is it possible to refuse the procedure or replace it with something else?
Below you will find answers to many questions.
- What you need to know about blood?
- In what cases do cancer patients need blood transfusions?
- What are the types of blood transfusion?
- Red blood cell transfusion
- Plasma transfusion
- Platelet transfusion
- Cryoprecipitate transfusion
- Leukocyte transfusion
- How is the blood transfusion procedure performed?
- From whom can a blood transfusion be given?
- How is donor blood tested?
- Are there any alternatives?
- How safe is it?
- Is it possible to refuse a blood transfusion?
- Price
What you need to know about blood?
Doctors and scientists often refer to blood as the body's internal environment. It washes all organs. Blood performs many important functions: it carries oxygen, nutrients and hormones, removes waste products of metabolism, provides immune protection, and helps regulate body temperature.
Blood consists of two main parts:
- The liquid part is plasma. It is a solution of salts, ions, proteins and other substances.
- Blood cells. Erythrocytes (red blood cells) contain hemoglobin and transport oxygen. Leukocytes (white blood cells) provide nonspecific and immune protection. Platelets (blood platelets) form a clot when bleeding needs to be stopped.
Book a consultation 24 hours a day
+7+7+78
Indications for autohemotherapy:
Due to the fact that autohemotherapy causes persistent activation of rehabilitation and protective mechanisms, intensive healing of wounds and injuries, accelerated recovery from inflammatory processes, it is very popular and is included in treatment regimens for various dermatological and cosmetic problems among all ages and categories. Autohemotherapy gives good results in the treatment of indolent and recurrent diseases, has a positive effect on the body as a whole and on the skin in particular:
- getting rid of purulent and inflammatory processes;
- acceleration of reparative processes;
- improvement of metabolism;
- strengthening the immune system;
- improvement of blood and lymph flow;
- normalization of the endocrine system;
- removal of waste and toxins from the body;
- restoration of performance and vitality.
In what cases do cancer patients need blood transfusions?
The cause may be the malignant tumor itself or side effects of antitumor treatment.
Some types of cancer, especially tumors of the gastrointestinal tract and tumors of the female genital area (vagina, cervix, uterus), can cause internal bleeding.
With a long course of cancer, various disorders occur in the body, which cause the so-called anemia of a chronic disease.
Some malignant tumors affect the red bone marrow (the main organ of hematopoiesis), or organs that are necessary to maintain a normal number of blood cells (spleen, kidneys). These types of cancer may also require blood transfusions.
Donor blood is needed by patients after complex operations that are accompanied by large blood loss.
Chemotherapy and radiation therapy affect not only tumor cells, but also other rapidly dividing cells in the body. Sometimes they cause quite a lot of damage to the red bone marrow. It disrupts the production of blood cells, which threatens anemia, bleeding, and severe infections against the background of decreased immunity. Blood transfusion helps normalize the patient's condition and prevent complications.
What does transfusion help with?
Solid tumors lead to significant changes in the hematopoietic system. Under their influence, anemia and abnormalities in the blood clotting system can develop.
The disintegration of tumors leads to depletion of the bloodstream and blood reserve of the body. Surgical treatment also leads to massive bleeding. All of the above factors lead to the fact that the body’s own reserve is depleted and it requires a blood transfusion from a donor. Due to insufficient blood volume, treatment may be delayed because In case of anemia and thrombocytopenia, chemotherapy cannot be administered.
Chemotherapy drugs can have side effects on blood germs and worsen thrombocytopenia. That is why constant monitoring of red and white blood indicators and coagulation properties is necessary. If any deviations from the norm are noted, blood transfusion is prescribed according to all rules.
Red blood cell transfusion
The main function of erythrocytes (red blood cells) is the delivery of oxygen to tissues and the return transport of carbon dioxide to the lungs. A condition in which the number of red blood cells in the blood decreases is called anemia. Actually, this is an indication for red blood cell transfusion. The doctor makes a decision depending on how quickly the anemia increases:
- With chronic anemia, which increases gradually, there is no need to rush. The doctor monitors the patient’s condition, controls the level of red blood cells and hemoglobin. If these indicators decrease significantly, or the patient’s condition worsens, red blood cells are transfused. For heart and lung diseases that impede oxygen delivery to tissues, transfusions may be required even if there is a relatively small decrease in hemoglobin levels.
- Acute blood loss requires immediate action. This usually happens during surgery. If the doctor is planning a complex operation during which the patient will lose a lot of blood, blood transfusion may be performed in advance.
Evaluation of the effectiveness of the procedure
With the help of properly prescribed blood transfusion, it is possible to normalize the condition of a cancer patient and prevent severe complications of the disease. The result of a properly organized blood transfusion for oncology is visible almost immediately after its completion:
- hemoglobin levels increase;
- platelet count increases;
- the blood is saturated with oxygen;
- the feeling of weakness and chronic fatigue disappears;
- it becomes possible to carry out radiation therapy, chemotherapy, or perform surgery;
- Feeling better after blood transfusion for blood cancer.
The procedure increases the chances of a positive treatment outcome. The duration of the effect of blood transfusion depends on the nature of the oncological process and the individual characteristics of the patient. A positive result is more stable in the absence of constant internal bleeding.
Prescribing and performing blood transfusions in cancer patients requires high professionalism. The further condition of the patient depends on the procedure, especially during blood transfusion for stage 4 cancer, so it is necessary to undergo it in a safe place. Oncology provides a range of services related to oncology. Contact our center, here you can get advice, undergo an examination or additional examination, and receive the necessary treatment.
Plasma transfusion
Plasma, the liquid part of blood, appears as a clear, yellowish liquid. It contains blood clotting factors - substances that are necessary for the formation of a blood clot and stop bleeding. Plasma also contains substances that protect the body from infection.
Plasma can be stored frozen for up to 12 months. When necessary, it is thawed and the resulting fresh frozen plasma is transfused into the patient.
The main indication for plasma transfusion in cancer patients is increased bleeding. The procedure is also necessary for DIC syndrome (disseminated intravascular coagulation syndrome) - a serious condition in which blood clots form in small vessels, ultimately depleting the entire supply of platelets and coagulation factors, and there is a risk of severe bleeding.
Yours is better than someone else's
Instead of donor blood, the patient can be transfused with his own blood - doctors call this technique autotransfusion. Blood is taken from the patient in advance, two to three days before surgery, and stored under appropriate conditions. In this case, there is no risk of contracting blood infections, an immune reaction to transfusion is eliminated, and there is no risk of complications.
In addition, a little bloodletting before surgery has a good effect on the condition of the body. And a psychologically sick person also has a better attitude towards transfusion of his own blood than someone else’s.
Autotransfusion is especially indicated for those who have a rare blood type, who do not tolerate transfusion well, as well as for those with impaired liver or kidney function. This procedure itself has such a beneficial effect on the body that it is even used as stimulation for athletes before competitions.
In some cases, a whole blood transfusion is not necessary because the body is missing one of its components, such as red blood cells, platelets, or plasma. In these cases, those blood components that are necessary are prepared.
Article on the topic
Promotion of blood donation: plans include new benefits and free parking
Platelet transfusion
Platelets, or blood platelets, take part in the formation of a blood clot and stop bleeding. Their levels may drop due to chemotherapy, radiation therapy, or if the tumor has replaced normal red bone marrow tissue. Platelet transfusions are usually required for cancer patients in one of three cases:
- if the level of platelets in the blood has fallen below a critical value;
- if there is increased bleeding, risk of bleeding;
- if you are undergoing surgery during which significant blood loss is expected.
How is the blood transfusion procedure performed?
Although blood transfusion is formally equated to surgical interventions, this procedure is not at all scary and is practically painless. Blood transfusion is carried out through a needle that is inserted into a vein. It is no more painful than a regular intravenous injection. If the patient already has a central venous catheter, donor blood can be administered through it.
The procedure can take different times, depending on which blood components are transfused: from 30-60 minutes (platelet transfusion) to 2-4 hours (red blood cell transfusion).
Scheme of autohemotherapy
With the classic treatment option, blood is taken from a vein (volume from 5 to 25 ml) and immediately injected into the gluteal muscle. If you miss the moment, clots will appear that can no longer be used. 1-2 days – break between procedures. As a rule, the result is achieved after 8-12 injections. It is unacceptable to administer more blood than the specified volumes; this can cause inflammatory reactions, chills, and muscle pain. In addition to the classic version, there are others - stepwise, with ozone, the use of blood subjected to various chemical influences, laser treatment.
With ozone
This method is more modern, superior in efficiency to the classical one. On average, treatment requires no more than 5-7 procedures. Course – 1-2 times a week. Before use, the blood is mixed with ozone in a certain concentration. Specialists use:
- Minor autohemotherapy . About 10 ml of blood is drawn from a vein into a syringe containing an ozone-oxygen mixture and injected into the patient.
- Major autohemotherapy . In a sterile container, stir 100 to 300 ml of the mixture and approximately 100-150 ml of blood. After mixing, use as directed.
- The benefits of an exercise bike: what muscles work and how to exercise correctly
- How to lose weight in a week at home. Diets and exercises for quick weight loss
- How to treat obsessive-compulsive disorder and fears
Stepped
Stepped autohemotherapy involves the introduction of a small amount of blood - about 0.1-0.2 ml. It is pre-mixed with several homeopathic medicines. As a rule, the procedure takes 4 stages. For injections, you can use one syringe, the main thing is that after each injection a small amount of blood remains in it. From stages 2 to 4, the contents are intensively shaken and administered to the patient.
Drugs for stepwise autohemotherapy are selected individually for each person. Sometimes it is enough to use complex remedies containing nosodes; a little less often, ampoule homeopathic monopreparations and symptomatic medications are prescribed. Stepped autohemotherapy has proven itself as a proven way to get rid of viral infections, arthrosis, chronic eczema, migraines, and toxic liver damage.
How is donor blood tested?
A person who donates blood for the first time must fill out a questionnaire, undergo an examination by a therapist, a dermatovenerologist, and take tests for blood type, Rh factor, and infections: HIV, viral hepatitis B and C, syphilis, cytomegalovirus. Sometimes the examination program can be expanded.
If signs of a particular infection are found in the donor’s blood, it is discarded and not used in the future.
The compatibility of the blood of the donor and the recipient is checked using a special test - a cross-blood compatibility test.
Who discovered the human blood type
{banner_banstat0}
The Austrian immunologist Karl Landsteiner succeeded in identifying the class of human biological material in 1900. At this time, only 3 types of antigen were identified in the membranes of erythrocytes - A, B and C. In 1902, it was possible to identify the 4th class of erythrocytes.
Karl Landsteiner was able to make another important achievement in medicine. In 1930, the scientist, in tandem with Alexander Wiener, discovered the Rh factor of blood (negative and positive).
Are there any alternatives?
Sometimes blood disorders can be corrected with medications. For example, colony-stimulating factors are used to increase the number of white blood cells.
However, in cases where blood transfusion is necessary, there are no alternatives. There are no blood substitutes that can provide similar effects. That is why donation is constantly promoted in all countries of the world, including Russia, and donor days are held periodically. It is important. This helps save the lives of many people.
How safe is it?
Can you get an infection from donated blood? Donated blood goes through rigorous testing, but there are still risks, even though they are negligible. Thus, the likelihood of becoming infected with HIV through donated blood is lower than the likelihood of being struck by lightning during a person’s lifetime. The risk of contracting hepatitis C is 1 in 2,000,000. Doctors and scientists are constantly working to reduce the risks to zero.
Can incompatible blood be transfused? Before blood transfusion, the recipient's blood type and Rh factor must be determined; the doctor must make sure that the blood of the donor and recipient are compatible.
But the blood of different people can differ not only in group A0 and Rh factor. It is very difficult to take into account all the nuances. Therefore there is a slight risk of an allergic reaction. Most often it manifests itself in the form of fever, chills, and rash. Such complications are rarely life-threatening. In order to provide assistance to the patient, if necessary, a medical professional is constantly monitoring his condition during the procedure.
An allergic reaction can occur not only immediately during the transfusion, but also within 48 hours after it. You should immediately tell your doctor if your body temperature rises above 38°C, chills, rash, itching, redness of the skin, shortness of breath, difficulty breathing, nausea, lower back pain, blood in the urine, weakness. The most dangerous symptom is chest pain, which requires immediate attention. If you are at home, you should immediately call an ambulance.
Is it possible to refuse a blood transfusion?
The patient always has the right to refuse prescribed treatment, be it chemotherapy, surgery or blood transfusion. But you need to remember some points:
- A doctor will not prescribe a procedure, especially one as serious as a blood transfusion, just like that. If the doctor decides to perform a blood transfusion, then there are good reasons for this, and first of all it is in the interests of the patient.
- Large blood loss during surgery and significant blood disorders can lead to death or serious complications and impair the effectiveness of antitumor treatment.
- There are some risks during blood transfusion, but they are negligible, and the procedure often helps save the patient's life.
Euroonko clinics officially cooperate with the largest blood banks in the country. We operate on the basis of a license for “transfusiology in outpatient and inpatient settings” issued by the health departments of Moscow and St. Petersburg.
Classification and characteristics of blood groups and Rh factor
Group antigens are classified according to a single AB0 system (a, b, zero). The established concept divides the composition of blood cells into 4 main types. Their differences are in alpha and beta agglutinins in plasma, as well as the presence of specific antigens on the membrane of red blood cells, which are designated by the letters A and B.
Table "Characteristics of blood classes"
| Variety | Description |
| 1 group or zero | There are no antigens in blood cells. Plasma contains alpha and beta agglutinins |
| Group 2 (A) – the most common | Group A antibodies are present in the membrane of blood cells, and only beta-agglutinin is present in the plasma itself |
| Group 3 (B) | Red blood cells contain B antigen, but plasma contains only alpha antibody |
| Group 4 (AB) | Blood cells in their membrane contain antigens of both groups (A and B), but there are no agglutinins in the plasma |
People's nationality or race does not affect group membership.
Rh factor
{banner_banstat1}
In addition to the AB0 system, biological material is classified according to the blood phenotype - the presence or absence of a specific antigen D, which is called the Rh factor (Rh). In addition to protein D, the Rh system covers 5 more main antigens - C, c, d, E, e. They are contained in the outer membrane of red blood cells.
The Rh factor and the class of blood cells are formed in the child in the womb and are passed on to him from his parents for life.
Price
- Consultation with a transfusiologist - 4,500 rubles.
- Complete blood count (CITO) - RUB 1,800.
- Compatibility test before blood transfusion - 2,300 rubles.
- Erythrocyte suspension, depleted of leukocytes (filtered) - RUB 21,760.
- Transfusion of blood components - 3,900 rub.
- The cost of fresh frozen plasma (1 dose 250 ml) is 20,100 rubles.
- Platelet concentrate (1 dose) - 68,000 rub.
In accordance with the legislation of the Russian Federation (Law of the Russian Federation of June 9, 1993 N 5142-I “On the donation of blood and its components”), the procurement of blood, the receipt of blood components and their storage are carried out exclusively by state budgetary institutions. Blood transfusions to our patients are carried out on the basis of licenses for “transfusiology in outpatient and inpatient settings”.
Book a consultation 24 hours a day
+7+7+78
Kathleen Sazama MD, JD
Allegheny University of Health Sciences, Philadelphia, PA.
Complications and side effects occur in less than 0.2% of all blood transfusions (this rate is higher in patients who regularly receive blood transfusions). Most often they are minor and transient. However, certain side effects can lead to significant impairment of life functions and even death. Because blood transfusions cannot be completely safe, informed consent should be obtained from patients based on their understanding of the risks, benefits, and possible alternatives before transfusion is performed. Systems for rapid identification and management of life-threatening side effects of transfusion of each blood component used, including autologous blood collection and reinfusion, should be available in any hospital. New federal guidelines require reporting such incidents annually and periodically, every 45 days, as errors and accidents. The transmission of infectious diseases, which can be fatal in some cases, will not be discussed further beyond the following:
- The transfusion risk that patients fear most, human immunodeficiency virus (HIV) infection, is one of the least likely side effects of transfusion (1 in 800,000 transfusions). The risk of transmission of hepatitis viruses, hepatitis B (1 case in 50-250 thousand) and hepatitis C (1 case in 10-100 thousand transfusions), is much higher.
- Bacterial contamination of platelets, detected by competitive methods in 1 in 900 to 2,000 cases but causing any symptoms much less frequently, is the subject of an ongoing national study by the Centers for Disease Control and Prevention. Methods for detecting bacteria in platelets before they are released as a transfusion drug are under active study.
- Transmission of agents such as syphilis, malaria, babeshiosis (piroplasmosis), Chagas disease (South American trypanosomiasis), and parvovirus B-19 has been periodically reported, but is not common.
Most transfusion reactions can be classified according to the time of onset of symptoms and the mechanisms of their occurrence, immunological or non-immunological. These reactions occur with the use of both allogeneic and autologous blood components, the latter usually associated with errors in patient identification, but even the correct use of autologous blood can lead to adverse effects, including fatal ones.
Table 1. Adverse effects of blood transfusion
Acute
| Deferred | |
| Immunological | |
| Fever without hemolysis (febrile non-hemolytic transfusion reaction) | Alloimmunization |
| Urticarial rash | Delayed hemolysis |
| Acute lung injury | Platelet refractoriness |
| Acute hemolysis | Immunomodulation/suppression |
| Anaphylaxis | Graft-versus-host disease |
| Fatal acute hemolysis | Post-transfusion purpura |
| Non-immunological | |
| Bacterial infection of platelets | Hepatitis C |
| Hypervolemia | Hepatitis B |
| Chemical effects, hypothermia, coagulopathy | Human T-lymphotropic virus type 1 |
| Non-immune hemolysis | Human immunodeficiency virus type 1 |
| Sepsis (red blood cells) | Hemosiderosis (red blood cells only) |
Table 2. Immediate management of acute adverse effects of blood transfusion.
| 1st stage |
| STOP TRANSFUSION |
| Stage 2: Patient management |
| Maintain intravenous access with saline or its equivalent. Consult with your healthcare provider regarding treatment/intervention. Prescribe treatment. |
| 3rd stage |
| Notify the blood transfusion service and the doctor. Receive and start filling out the form notifications about reactions to blood transfusions. Carry out and document checking and comparing the patient's data with the information attached to the vessel with the blood component. Disconnect the blood component container and transfusion system and dispose of them as advised by the blood transfusion service or as required by your facility. Obtain patient blood samples; some institutions also require a urine test. Send tests, test results and notification of the reaction to the blood transfusion (and, if required, the transfusion system and the transfused blood component) to the blood transfusion service. |
*If the only manifestation of the reaction is a urticarial rash, blood transfusion may be resumed after the rash resolves. In some institutions, transfusion is restarted after antipyretics are administered to patients with a febrile reaction, but this practice is not recommended.
ACUTE ADVERSE EFFECTS
Immunological
Febrile non-hemolytic transfusion reaction (FNHR)
FNGRT is a diagnosis of exclusion. Fever, the most commonly observed side effect of all blood transfusions (0.1 to 1% of cases), may be a minor symptom, easily relieved by antipyretics, or a signal of the onset of serious consequences, including death from sepsis or hemolysis. Therefore, the appearance of fever during the transfusion of any blood components should be considered as a threatening sign. The transfusion should be stopped while a possible hemolytic or septic reaction is identified. Although some institutions allow transfusion to be resumed after hemolysis and sepsis have been ruled out, most still recommend withholding the febrile blood component. It is now believed that the mechanism of fever during blood transfusion is usually related to the injected cytokines (interleukin-6 and tumor necrotizing factor) that accumulate due to the metabolism of leukocytes in blood components during storage, but interaction of recipient antibodies (HLA or granulocytes) may also play a role. with injected leukocytes. The widespread practice of premedicating all patients to prevent FNGRT should probably be abandoned now that a more effective intervention can be selectively applied. Because only about 15% of recipients experience recurrent FNGRT, the use of any preventive measures is not recommended until a recurrent episode of fever occurs. In documented cases of recurrent febrile reactions, leukoreduction is recommended using either pre-storage or bedside filtration, depending on the local cost of such methods. The definition of fever varies among institutions and may contribute to differences in reported cases. Using the definition of a temperature rise above normal (its level before transfusion) by more than 1.5-2° C (more than 2.7-3.8° F) will allow appropriate treatment of the patient and more efficient use of blood. When fever is the only symptom, antipyretics can be used. If there are chills, especially shaking chills, meperidine (Demerol) may be needed for symptomatic treatment.
Urticarial rash without other signs or symptoms
The appearance of an itchy rash during a transfusion of blood components (platelet or plasma transfusion) is common. The mechanism is related to the release of histamine after degranulation of basophils or mast cells due to the interaction of immunoglobulin E (Ig E) with transfused plasma proteins. Because a localized or generalized itchy rash resolves quickly with antihistamines, 50 mg of diphenhydramine (Benadryl) is usually used to treat this side effect. Once the rash and/or itching has resolved, the transfusion can be completed safely. This is the only adverse effect of transfusion where resumption of transfusion is considered safe after symptoms resolve. Since these reactions are a manifestation of idiosyncrasy, premedication is not recommended. Only rarely can patients experience a recurrence of this reaction, usually when using blood components from the same donor.
Transfusion-related acute lung injury
The abrupt onset, usually within 2 to 4 hours after transfusion, of acute respiratory distress syndrome, hypotension, and fever with documented hypoxemia and bilateral pulmonary edema may indicate the occurrence of a potentially life-threatening complication called transfusion-related acute lung injury (TRAL). These symptoms define adult respiratory distress syndrome (ARDS), which can occur in numerous clinical situations. In cases associated with transfusion, the prognosis of ARDS is much more favorable compared to other causes, with mortality limited to 10% with proper diagnosis and treatment. Rapid connection of respiratory support with mechanical ventilation and oxygen therapy is necessary. The mechanism of this reaction is not completely clear. It is believed that HLA antibodies and/or antibodies from donor granulocytes react with the recipient's pulmonary vascular cells, causing complement activation and granulocyte aggregation with subsequent damage to the lung parenchyma.
Acute hemolysis
Acute hemolysis of transfused red blood cells is usually associated with misidentification of the patient's blood, most often during phlebotomy before laboratory testing or at the time of blood order. In the vast majority of cases, the error is an ABO mismatch, although sometimes other antigens are to blame (Kell, Kidd, and Rh antibodies have been reported). A common clinical situation is the transfusion of group A(II) blood into a patient with group O(I), but other options are known. These errors occur with a frequency of 1 in 20 thousand transfusions, with a probability of death with such an error of 1 in 12-30 cases. All such errors in the United States, regardless of outcome, must be reported to the Food and Drug Commission. Hemolysis may be without clinical manifestations, with only unexplained hyperbilirubinemia or anemia. However, it can also be severe and life-threatening due to disseminated intravascular coagulation, shock, hypotension, or acute renal failure. Cases have been published of the classic picture of the sudden onset of acute pain in the side, the appearance of bright red urine, fever, and often the patient’s words “something is wrong.” Fever (body temperature more than 1°C above normal) and chills are common. In patients under anesthesia or in a coma, signs of hemolysis may include the onset of unexplained bleeding from injection sites and the appearance of hemoglobinuria. In some patients, for reasons that are not entirely clear, a very small volume (less than 25 mL) of incompatible red blood cells can cause severe hemolysis and death, while others tolerate multiple transfusions without visible clinical symptoms. If signs of hemolysis occur, you need to act according to the above steps. Each facility should have a formal transfusion response protocol that guides the transfusion staff, the attending physician, and the hospital administration (or equivalent group for out-of-hospital transfusions). Rapid and vigorous hydration and the use of vasoactive drugs such as dopamine may help. Since the vast majority of these complications are due to incorrect patient identification, improvements to patient identification systems are strongly recommended.
Anaphylaxis
is a life-threatening adverse effect, usually occurring within minutes of the start of transfusion, characterized by sudden respiratory distress, shock, hypotension and angioedema, and sometimes gastrointestinal symptoms. Rapid administration of adrenaline (0.3 ml diluted 1:1000 intramuscularly) and respiratory support are necessary. The most common mechanism for this reaction is the administration of plasma containing Ig-A to a patient who is Ig-A deficient and has anti-Ig-A antibodies. Ig-A deficiency is relatively common, affecting 1 in 200 to 500 people in the US, but few have anti-Ig-A antibodies. Because this is a rare situation, most cases are diagnosed only when a reaction develops. Subsequent treatment alternatives include the use of plasma-depleted blood components or Ig-A-deficient plasma.
Non-immunological complications
With the exception of the problem of bacterial contamination of platelets, other adverse effects are rare. They should be considered in appropriate clinical situations as described below.
Bacterial infection of platelets
A national study is being conducted into this side effect, which should provide additional data on identifying and avoiding this problem.
Hypervolemia
This problem typically occurs in two clinical situations: during massive blood replacement to control bleeding and in very young or elderly patients. Clinical signs include dyspnea, tachycardia, and hypertension from pulmonary edema due to congestive heart failure, with onset of symptoms typically occurring within 6 hours of transfusion. Treatment should be with diuretics and oxygen therapy; Sometimes therapeutic phlebotomy may be required.
Chemical effects
Citrate intoxication
Citrate toxicity is a rare adverse effect. Citrate is an anticoagulant used in the collection of whole blood and blood components because it binds calcium and prevents clotting. With massive transfusions, when blood components are transfused in 1 package in 5-10 minutes, the recipient may experience hypocalcemia, which will manifest itself as arrhythmias, muscle spasms and tetany. These patients require close cardiac monitoring and sometimes a slow infusion of calcium bicarbonate.
Hyperkalemia and hypokalemia
Hyperkalemia can only occur in very young children who are transfused with red blood cellulose that is past its expiration date. With massive blood transfusions, you can also encounter hypokalemia; it is treated with potassium administration. The mechanism of development of hypokalemia is associated with the metabolism of citrate into bicarbonate, which transfers potassium into the cells.
Hypothermia and coagulopathy
In patients with severe bleeding receiving massive blood replacement, usually defined as replacement of the entire blood volume in 24 hours, dilution coagulopathy can be anticipated. This is uncommon in practice, probably because extravascular reservoirs of essential clotting factors exist that can maintain levels of at least 20–25% of normal, such as platelet and plasma clotting factors such as von Willebrand factor, factors V, VIII, and fibrinogen. If available, laboratory tests should be done promptly to assess the need for clotting factor replacement. However, in the presence of shock and severe blood loss with hypothermia, some institutions continue to use a standard blood replacement protocol to avoid microvascular coagulopathy.
Non-immune hemolysis
Patients may have hematuria with or without fever because the transfused red blood cells are destroyed by external factors during or before entering the patient's bloodstream. Red blood cells are lysed under the influence of unfavorable chemical (for example, osmotic) or thermal factors. Therefore, red blood cells should be transfused using only 0.9% saline or other FDA-approved isosmotic fluid. For example, the use of a 5% glucose solution is contraindicated because, when mixed with red blood cells, their rapid uptake of glucose will cause intracellular hyperosmolality with rapid accumulation of water, leading to lysis. Also, when erythrocytes are frozen without using appropriate doses of cryoprotectants or when they are heated above recommended temperatures, they lyse. Storage of red blood cells has strict temperature requirements that can only be achieved using carefully monitored refrigerators and approved shipping containers.
Sepsis
Although morbidity and mortality from bacterially contaminated red blood cells continue to be reported, the incidence is less than 1 in 1,000,000 cases. However, this risk is known to be higher for autologous blood components and should be taken into account when they are retransfused.
DELAYED ADVERSE EFFECTS
Immunological
Alloimmunization
The most common adverse effect occurring days or months after transfusion is the development of alloantibodies to antigens present on the cells of the transfused blood that are foreign to the recipient. Because we typically test only red blood cells (not platelets) and only the most common and highly reactive antigens, namely A, B and D, other antigens (over 50) presented on these cells can potentially trigger an immune response. In fact, in many busy trauma centers, it is common practice to administer D-positive RBCs to D-negative (or D-unknown) male patients or D-negative women past childbearing age. This practice is caused by a chronic shortage of D-negative red blood cells (which are stored for D-negative women under 45-50 years of age to prevent hemolytic disease of the newborn). Alloimmunization can also develop in women in connection with pregnancy. The mere presence of alooantibodies usually does not harm the patient (as is the case with the presence of immune-stimulated antibodies to various childhood infections), but such antibodies can cause problems in two clinical situations. In the first case, if antibodies are present but not detected prior to transfusion, and red blood cells containing the corresponding antigen are transfused, such red blood cells will be destroyed and a delayed hemolytic reaction to transfusion may occur (discussed below). The second clinical situation involves the detection of alloantibodies prior to transfusion, which leads to a delay in transfusion while searching for a suitable antigen-negative component. This most often occurs in patients with hemoglobinopathies (eg, sickle cell disease or thalassemia) who receive frequent blood transfusions. Typically, the blood transfusion service will inform the treating physician and/or patient of the presence of alloantibodies so that appropriate treatment can be planned. In most cases of alloimmunization, transfusion is performed without delay, but sometimes only blood components from very rare donors can provide a therapeutic benefit to alloimmunized patients. This may require careful planning and collaboration.
Delayed hemolysis
When a patient experiences unexplained hyperbilirubinemia, anemia, or failure to increase hemoglobin after 5 to 10 days of adequate red blood cell transfusion, delayed hemolysis should be suspected. If alloantibodies are not detected prior to transfusion of antigen-containing red blood cells, they will react with antigens on the surface of the red blood cells. This antigen-antibody complex on red blood cells, which usually does not add complement, is removed from the vascular bed. (Occasionally, short-term intravascular hemolysis occurs). A positive direct antiglobulin test (DAT) or the presence of alloantibodies that are now sufficiently immunostimulated to be detected are often detected by the blood transfusion service when testing new blood samples. These antibodies are often directed against antigens of the Kidd (Jka), Duffy (Fya) or Rh (E, c, C) systems. Typically, a delayed hemolysis reaction does not require treatment, but all subsequent transfusions should be performed using red blood cells free of the antigens of interest.
Platelet refractoriness
For certain medical conditions, usually related to hematologic or oncologic conditions, patients may receive regular platelet transfusions. Typically, compatibility testing or selection of antigen-negative donors is not performed until the patient becomes platelet refractory. Patients whose platelet levels do not rise after an appropriate dose of transfusion may be refractory to platelet transfusion for immune or nonimmune reasons. If sources of platelet loss, destruction, or consumption (eg, bleeding, sepsis, fever) are excluded, immune destruction due to antiplatelet (most often anti-HLA-la) or anti-HLA antibodies may occur. For patients with nonimmune platelet destruction, supportive care should be given to the underlying disease state and continued random or single donor platelet transfusions. If long-term platelet transfusion is indicated in a patient with known immune compromise, consultation is indicated to find special devices or tests to obtain suitable platelets.
Immunomodulation/suppression
A growing body of literature, debated by some investigators, suggests that transfusions may alter the immune status of some surgical patients, as evidenced by earlier recurrence of malignancies after resection and/or increased rates of infectious complications in the postoperative period. Currently, the only approach is standard treatment of the malignancy or infection. The issue of using “leukoreduced” erythrocytes and platelets is being discussed.
Graft-versus-host disease
Some patients may develop rash, fever, nausea or vomiting, and unexplained peripheral blood cytopenia 3 to 15 days after transfusion; These are manifestations of graft-versus-host disease (GVHD). GVHD, almost always fatal in blood transfusions, can occur when the recipient is immunologically compromised (eg, congenital immunodeficiency conditions) or when the recipient receives blood components containing an HLA antigen identical to one of the recipient's own HLA haplotypes. If the blood component is homozygous for the HLA haplotype (for example, contains two copies of exactly the same HLA antigen), and the recipient is heterozygous for the HLA haplotype (has two different HLA antigens), GVHD may occur. The only effective measure against GVHD is its prevention by gamma irradiation of cellular components of the blood at a sufficiently high dose (2500 cGy) to suppress mitosis of donor lymphocytes. Because a particular HLA pattern is more likely to occur among biological relatives than among strangers, all blood donations that are purposefully donated are irradiated to prevent this catastrophic disease. There is no effective treatment for GVHD.
Post-transfusion purpura
Women who have been pregnant or who have received blood transfusions have the highest risk of developing post-transfusion purpura (PTP) (male to female ratio: 1:26). Patients with PTP develop severe thrombocytopenia (numbers usually less than 10 thousand per ml) within 5-10 days after a platelet or red blood cell transfusion. This thrombocytopenia can last from days to weeks and is treated with plasma exchanges, intravenous immunoglobulin, and/or high-dose steroids. It is generally believed that anti-HPA-la (antiplatelet) antibodies cause immune destruction of both transfused and autologous HPA-la-negative platelets (the latter by an unknown mechanism). If transfusion is required, the use of HPL-la-negative platelets is recommended, although they are more rapidly destroyed.
Non-immunological
Hemosiderosis
Patients who receive red blood cell transfusions for a long time develop iron accumulation (hemosiderosis). Hemosiderosis sometimes manifests itself as cardiomyopathies, cirrhosis and “bronze diabetes”. 250 mg of iron after each transfused dose of red blood cells accumulates in the reticuloendothelial system until the tissues are saturated; iron then begins to be deposited in other places such as the heart, skin, pancreas and other endocrine organs. Some benefit may be obtained from the use of the iron chelating agent, deferoxamine.
Conn's Current Therapy 1999, 51st ed.