Pharmacological properties of the drug Lorista h/hd
Pharmacodynamics. Losartan is an antihypertensive agent, a selective antagonist of angiotensin II receptors (AT1 type). It binds to AT1 type receptors located in various tissues, including the myocardium, vascular smooth muscle, adrenal cortex and kidneys, blocks the development of the effects of angiotensin II, in particular, reduces arterial vasoconstriction and aldosterone release, wedge pressure in the pulmonary vessels, reduces peripheral vascular resistance, which leads to to a decrease in systemic blood pressure. Losartan does not inhibit the activity of kinase II, an enzyme that catalyzes the breakdown of bradykinin. The maximum effect of losartan is observed 6 hours after taking the drug. This effect lasts for 24 hours, so it is enough to take the drug once a day. The hypotensive effect stabilizes during the first week of treatment, and the maximum hypotensive effect is achieved 3–6 weeks from the start of therapy. Hydrochlorothiazide is a diuretic. Thiazide diuretics inhibit the reabsorption of Na+ and Cl– ions in the distal tubules of the nephrons, increasing the excretion of sodium, potassium, chlorine and water. At the beginning of treatment with hydrochlorothiazide, a decrease in the volume of circulating blood plasma occurs, followed by a decrease in cardiac output and a significant decrease in blood pressure. In response to a decrease in blood pressure and cardiac output, fluid is redistributed from the interstitial space into the intravascular bed, and after 3–4 months there is a gradual normalization of blood plasma volume. With prolonged use of the drug, cardiac output returns to the initial value, and peripheral vascular resistance decreases to a lower level compared to the initial value. Diuresis is usually observed within 2 hours after taking hydrochlorothiazide, reaches a maximum after 3-4 hours and lasts for 6-12 hours. The hypotensive effect appears after 3-4 days of treatment and reaches a maximum after 3-4 weeks. The duration of the hypotensive effect is 12–18 hours. When losartan is combined with hydrochlorothiazide, an additive hypotensive effect is observed, which lasts 24 hours and persists over a long period of treatment. Pharmacokinetics of Losartan. After oral administration, losartan is rapidly absorbed into the gastrointestinal tract. It undergoes significant primary metabolism to form active carboxylic acid metabolites and a number of inactive metabolites. Systemic bioavailability is approximately 33%. The maximum concentration of losartan in the blood serum is achieved within 1 hour, and its active metabolite - within 3-4 hours after administration. 99% of losartan and its active metabolite are bound to plasma proteins, mainly albumin. The clearance of losartan and its active metabolite from blood plasma is about 600 and 50 ml/min, respectively. The renal clearance of losartan and its active metabolite from the kidneys is approximately 75 and 26 ml/min, respectively. When taken orally, about 5% of the dose of losartan taken is excreted in the urine unchanged and 6% in the form of an active metabolite. The half-life is 1.5–2 hours and 6–9 hours, respectively. Approximately 35% is excreted in urine, and approximately 65% in feces. Hydrochlorothiazide. Absorption of hydrochlorothiazide when taken orally is rapid. Its bioavailability is 70%. The maximum concentration in blood plasma is achieved 1.5–5 hours after administration. Approximately 40% of the drug is bound to plasma proteins. About 95% of hydrochlorothiazide is excreted unchanged by the kidneys. Elimination occurs as a result of tubular excretion. The half-life from the body is 5.6–14.8 hours.
Instructions for use of LORISTA®
Angiotensin II type AT1 receptor antagonist for oral administration. Angiotensin II, a powerful vasoconstrictor, is the primary active hormone of the RAAS and an important determinant of the pathophysiology of hypertension. Angiotensin II binds to AT1 receptors found in many organs and tissues of the body (for example, vascular smooth muscle, adrenal glands, kidneys and heart) and causes several important biological effects, including vasoconstriction and the release of aldosterone. Angiotensin II also stimulates the proliferation of smooth muscle cells.
Losartan selectively blocks AT1 receptors. In vitro and in vivo, losartan and its pharmacologically active carboxylic acid metabolite E-3174 block all physiologically significant actions of angiotensin II, regardless of the source or route of synthesis.
Losartan does not have an agonistic effect and does not block other hormone receptors or ion channels important for cardiovascular regulation. In addition, losartan does not suppress ACE (kininase II), an enzyme that destroys bradykinin. As a consequence, there is no potentiation of undesirable effects mediated by bradykinin.
When taking losartan, removal of the negative feedback of angiotensin II on renin secretion leads to an increase in plasma renin activity (PRA). An increase in ARP leads to an increase in the concentration of angiotensin II in plasma. Despite this, antihypertensive activity and suppression of plasma aldosterone concentrations are maintained, indicating effective blockade of angiotensin II receptors. After discontinuation of losartan, the values of ARP and angiotensin II fell within 3 days to baseline values.
Both losartan and its main active metabolite have a much greater affinity for the AT1 receptor than for the AT2 receptor. The active metabolite is 10-40 times more active than losartan.
Hypertension Research
In controlled clinical studies, taking losartan once a day in patients with mild or moderate essential hypertension provided a statistically significant reduction in systolic and diastolic blood pressure. The results of blood pressure measurements 24 hours after dosing relative to the results of measurements 5-6 hours after dosing showed a decrease in blood pressure within 24 hours; the natural circadian rhythm was maintained. The decrease in blood pressure at the end of the dosing interval accounted for 73-80% of the effect observed 5-6 hours after taking the drug dose.
Discontinuation of losartan in patients with hypertension did not lead to a sharp increase in blood pressure (rebound phenomenon). Despite the pronounced decrease in blood pressure, losartan did not have clinically significant effects on heart rate.
Losartan is equally effective in men and women, as well as in older and younger (under 65 years of age) patients with arterial hypertension.
LIFE Study
The LIFE (Losartan Intervention For Endpoint Reduction in Hypertension) study was a randomized, triple-blind, active-controlled trial involving 9193 patients aged 55 to 80 years with hypertension and evidence of left ventricular hypertrophy confirmed by ECG. Patients were randomized to receive losartan 50 mg once daily or atenolol 50 mg once daily. If target blood pressure (<140/90 mmHg) was not achieved, hydrochlorothiazide (12.5 mg) was first added to therapy, and then, if required, the dose of losartan or atenolol was increased to 100 mg once a day. Other antihypertensive drugs, excluding ACE inhibitors, angiotensin II antagonists, or beta-blockers, were added as needed until target BP was achieved. The mean follow-up duration was 4.8 years.
The primary endpoint was a composite endpoint of cardiovascular disease and death due to cardiovascular disease, measuring reductions in the cumulative incidence of cardiovascular death, stroke, and myocardial infarction. BP was significantly reduced to similar levels in the two groups. Losartan therapy resulted in a 13% risk reduction (p=0.021, 95% confidence interval 0.77–0.98) compared with atenolol therapy for patients achieving the composite primary endpoint. This was mainly due to a reduction in the incidence of stroke. Therapy with losartan resulted in a 25% reduction in the risk of stroke compared with therapy with atenolol (p=0.001, 95% confidence interval 0.63-0.89). There were no significant differences in the incidence of cardiovascular death and myocardial infarction between treatment groups.
In the LIFE study, black patients receiving losartan were at greater risk of achieving the composite primary endpoint, i.e. cardiovascular event (eg, myocardial infarction, cardiovascular death) and especially stroke than black patients receiving atenolol. Therefore, the results obtained with losartan versus atenolol in the LIEE study on cardiovascular morbidity and mortality do not apply to black patients with hypertension and left ventricular hypertrophy.
RENAAL Study
The RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Receptor Antagonist Losartan) study was a controlled clinical trial conducted worldwide in 1513 patients with type 2 diabetes mellitus with proteinuria, with or without hypertension. 751 patients received losartan.
The aim of the study was to demonstrate the nephroprotective effect of losartan potassium in addition to the hypotensive effect. Patients with proteinuria and serum creatinine 1.3-3 mg/dL were randomized to receive losartan 50 mg once a day (with dose titration, if necessary, to achieve target blood pressure) or placebo, against the background of traditional antihypertensive therapy, for with the exception of ACE inhibitors and angiotensin II receptor antagonists.
The study drug was titrated to a dose of 100 mg/day, if necessary; 72% of patients took the drug at a dose of 100 mg/day most of the time. As adjuvant therapy, if necessary, the use of other antihypertensive drugs (diuretics, calcium antagonists, α- and β-adrenergic receptor blockers, as well as centrally acting antihypertensive drugs) was allowed in both groups. Patients were followed up to 4.6 years (average 3.4 years).
The primary endpoint of the study was a composite endpoint that included a 2-fold increase in serum creatinine concentration, end-stage renal failure (requirement for dialysis or transplant), or death. Results showed that losartan therapy (327 events) compared with placebo (359 events) resulted in a 16.1% (p=0.022) risk reduction in the number of patients achieving the primary composite endpoint. For the following individual and composite components of the primary endpoint, results showed a significant risk reduction in the losartan group; risk reduction by 25.3% for a 2-fold increase in serum creatinine concentration (p=0.006); 28.6% risk reduction for end-stage renal disease (p=0.002); 19.9% risk reduction for end-stage renal disease or death (p=0.009); a 21% risk reduction for a 2-fold increase in serum creatinine concentration or for end-stage renal disease (p=0.01). There were no significant differences in the incidence of deaths from any cause between the two treatment groups.
In this study, losartan was generally well tolerated:
- the rate of treatment discontinuation due to adverse events was comparable to the rate in the placebo group.
HEAAL Study
The HEAAL (Heart Failure Endpoint Evaluation of Angiotensin II Antagonist Losartan) study was a controlled, worldwide clinical trial of 3834 patients aged 18 to 98 years with heart failure (NYHA class II-IV) who were intolerant to therapy. ACE inhibitors. Patients were randomized to groups receiving losartan at a dose of 50 mg/day or 150 mg/day, against the background of traditional therapy (except for ACE inhibitors).
Patients were followed up for 4 years (median 4.7 years). The primary endpoint of the study was a composite endpoint of death from any cause or hospitalization for heart failure.
The results showed that therapy with losartan 150 mg (828 events), compared with therapy with losartan 50 mg (889 events), resulted in a 10.1% reduction in risk (p=0.027 95% confidence interval 0.82-0.99) in the number of patients achieving the primary composite endpoint. This was mainly due to a reduction in the frequency of hospitalizations for heart failure. Therapy with losartan 150 mg resulted in a 13.5% reduction in the risk of hospitalization for heart failure compared with therapy with losartan 50 mg (p=0.025 95% confidence interval 0.76-0.98). There were no significant differences in the incidence of death from any cause between treatment groups. Renal impairment, hypotension, and hyperkalemia were more common in the 150 mg losartan group than in the 50 mg losartan group, but these adverse events did not lead to significantly more discontinuations in the losaratan group. at a dose of 150 mg.
ELITE I and ELITE II Study
In the 48-week ELITE study in 722 patients with heart failure (NYHA class II-IV), there was no difference between patients treated with losartan and patients treated with captopril in the primary endpoint of long-term change in kidney function. The conclusion of the ELITE study that losartan reduced the risk of death compared with captopril was not confirmed in the subsequent ELITE II study, which is described below.
In the ELITE II study, losartan 50 mg once daily (initial dose 12.5 mg, increased to 25 mg, then 50 mg once daily) was compared with captopril 50 mg 3 times daily (initial dose 12.5 mg, increased to 50 mg once daily). increasing to 25 mg, then 50 mg 3 times a day). The primary endpoint of this prospective study was death from any cause.
In this study, 3152 patients with heart failure (NYHA class II-IV) were followed for almost 2 years (median:
- 1.5 years) to determine whether losartan is superior to captopril in reducing all-cause mortality. The primary endpoint showed no statistically significant difference between losartan and captopril in the reduction of mortality from any cause.
In both comparator-controlled (rather than placebo-controlled) clinical studies, losartan was shown to be tolerable to captopril based on significantly lower rates of discontinuation due to adverse events and significantly lower rates of cough.
Increased mortality was observed in the ELITE II study in a small subgroup (22% of all patients with heart failure) taking beta blockers at baseline.
Two large randomized controlled trials (ONTARGET (Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) and VA NEPHRON-D (The Veterans Affairs Nephropathy in Diabetes)) examined the use of ACE inhibitors in combination with angiotensin II receptor antagonists.
The ONTARGET study was conducted in patients with cardiovascular disease, cerebrovascular disease or type 2 diabetes with evidence of end-organ damage. The VA NEPHRON-D study was conducted in patients suffering from type 2 diabetes mellitus and diabetic nephropathy.
These studies did not show a significant beneficial effect (compared with monotherapy) on renal function and/or cardiovascular outcome and mortality, while an increased risk of hyperkalemia, acute renal failure and/or hypotension was observed. Due to similar pharmacodynamic properties, these results apply to other ACE inhibitors and angiotensin II receptor antagonists. Therefore, ACE inhibitors and angiotensin II receptor antagonists should not be used simultaneously in patients with diabetic nephropathy.
The ALTITUDE (Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints) study was designed to determine the benefit of adding aliskiren to standard ACE inhibitor or angiotensin II receptor antagonist therapy in patients with type 2 diabetes mellitus, chronic renal failure, and cardiovascular disease. The study was stopped early due to an increased risk of adverse outcomes. Stroke and cardiovascular mortality were observed more often in the aliskiren group than in the placebo group. Also, in the group taking aliskiren, undesirable side effects (hyperkalemia, hypotension, renal dysfunction) were more often observed.
Use of the drug Lorista h/hd
The initial and maintenance dose is 1 tablet of Lorista H or Lorista HD 1 time per day. The dose should be adjusted taking into account the degree of blood pressure reduction achieved during 3 weeks of treatment. The maximum recommended dose is 2 Lorista H tablets or 1 Lorista HD tablet per day. For patients with moderately severe renal impairment (creatinine clearance 0.5 ml/s or 30 ml/min), the drug can be prescribed at the usual dose. The drug is not recommended for patients with significant renal impairment, including patients undergoing hemodialysis. The recommended starting dose of losartan for patients with hypovolemia is 25 mg 1 time per day, so treatment with the drug is not recommended until the end of diuretics and elimination of hypovolemia. The drug can be taken both after meals and on an empty stomach. It is recommended to take the drug at the same time of day. If a drug dose is missed, the patient should not double its dose; in this case, he should take the next dose of the drug at the usual time. The duration of treatment is not limited.
Side effects of the drug Lorista h/hd
dizziness, sometimes tachycardia, dry cough, upper respiratory tract infections, diarrhea, edema, abdominal pain, back pain, angioedema, rash, hepatitis, pancreatitis, neutropenia, thrombocytopenia, hypercalcemia and hyperkalemia. Rarely - an increase in the activity of liver enzymes and the level of bilirubin in the blood serum, a decrease in hematocrit and hemoglobin levels is possible; in patients with bilateral renal artery stenosis or with stenosis of the artery of a single kidney, there may be a slight increase in the level of urea and creatinine in the blood serum, but these symptoms do not require cancellation drug.
Indications and contraindications for therapy with Lorista
Both forms of medication are prescribed to patients:
- to reduce the likelihood of developing cardiovascular pathologies;
- to prevent death in patients with high blood pressure and hypertrophic changes in the left ventricle;
- as a component of combination treatment of hypertension.
The instructions indicate the following contraindications to Lorista:
- in the absence of urine entering the bladder;
- insufficient amount of lactase, increased or decreased amounts of potassium;
- hepatic, renal dysfunction, disorders of the conversion of galactose into glucose;
- dehydration, low blood pressure;
- intolerance to the component composition;
- pregnancy, breastfeeding, under age.
Particular care during therapy with Lorista is required for patients:
- with diabetes mellitus, bilateral narrowing of arterial vessels in the kidneys;
- water-electrolyte imbalance - with insufficient amounts of magnesium, potassium, sodium;
- systemic blood pathologies, allergic reactions;
- a large amount of calcium, uric acid in the blood;
- bronchial asthma, gout.
Lorista is not used in conjunction with NSAIDs; in some cases it causes the development of adverse reactions. During drug therapy, the following may occur:
- attack of dizziness, sleep disturbance with insomnia, cephalgia;
- accelerated heart rate, decreased blood pressure (dose-dependent), vasculitis;
- swelling of the nasal passages, pharyngitis, infection of the upper respiratory tract;
- cough, pain in the abdominal area, nausea with vomiting, dyspeptic disorders;
- hepatitis, increased activity of liver enzymes, liver dysfunction;
- muscle and joint pain, anemic conditions, capillary toxicosis;
- swelling in the peripheral areas, chest pain, asthenic syndrome, general weakness;
- nettle fever, anaphylaxis, obsessive itching, Quincke's edema.
Treatment may change the levels of urea, creatinine, potassium, hemoglobin, and hematocrit.
Special instructions for the use of the drug Lorista h/hd
The drug is not recommended for use in patients with severe impairment of liver and kidney function (creatinine clearance 0.5 ml/s or 30 ml/min). In some patients, treatment with hydrochlorothiazide may cause an increase in serum uric acid concentrations and/or the development of gout attacks. Losartan reduces the concentration of uric acid in the blood serum. Thus, the use of losartan in combination with hydrochlorothiazide reduces the severity of hyperuricemia caused by hydrochlorothiazide. The drug should be used with caution in patients with bilateral renal artery stenosis or with stenosis of the artery of a single kidney. It is necessary to regularly monitor the concentration of creatinine in the blood serum. Losartan should be used with caution in the treatment of patients with a history of angioedema of any etiology. If angioedema occurs, it is necessary to stop using the drug and prescribe adequate treatment. During treatment with hydrochlorothiazide, patients with a history of allergic reactions or asthma, as well as patients without allergies or asthma, may develop hypersensitivity reactions, as well as manifestations typical of systemic lupus erythematosus. As with other antihypertensive agents, symptomatic hypotension may occasionally occur during treatment with hydrochlorothiazide. In patients with uncomplicated hypertension (arterial hypertension), it occurs rarely and is more often observed in patients with hypovolemia or electrolyte imbalance. During treatment, it is recommended to monitor serum potassium and calcium levels, especially in patients with impaired renal function and in the elderly. An increase in serum calcium levels during treatment with hydrochlorothiazide may be a sign of latent hyperparathyroidism. Before performing parathyroid function tests, it is necessary to discontinue the use of hydrochlorothiazide. Hyperkalemia, which sometimes occurs during treatment with losartan, is not clinically significant due to the combination of losartan with hydrochlorothiazide and does not require discontinuation of treatment. It is not recommended to use the drug during pregnancy and breastfeeding. During treatment with the drug, almost all patients can perform any work that requires high concentration (for example, driving vehicles and working with potentially dangerous mechanisms). However, losartan, especially at the beginning of treatment, can cause hypotension and dizziness in some patients, which lead to a transient decrease in psychophysical abilities. Therefore, patients should ensure that they respond normally to treatment with the drug.
Lorista®
Hypersensitivity reactions
Patients with a history of angioedema (swelling of the face, lips, pharynx/larynx and/or tongue) should be under strict medical supervision when using Lorista® (see section “Side Effects”).
Embryotoxicity
The use of drugs that affect the RAAS during the second and third trimester of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and mortality. The development of oligohydramnios may be associated with fetal pulmonary hypoplasia and skeletal deformities. Possible AEs in newborns include calvarial hypoplasia, anuria, hypotension, renal failure and death. If pregnancy is diagnosed, Lorista® should be discontinued immediately (see section “Use during pregnancy and breastfeeding”).
Arterial hypotension and water-electrolyte imbalance or decreased blood volume
In patients with reduced blood volume (for example, those receiving treatment with large doses of diuretics), symptomatic arterial hypotension may develop. Correction of such conditions must be carried out before using the drug Lorista® or treatment must begin with a lower dose of the drug Lorista® (see section “Method of administration and dosage”).
Fluid and electrolyte imbalance is common in patients with impaired renal function with or without diabetes mellitus, so careful monitoring of these patients is necessary. In clinical trials in patients with type 2 diabetes mellitus with proteinuria, the incidence of hyperkalemia was greater in the losartan group than in the placebo group. Several patients discontinued therapy due to hyperkalemia (see section “Side effects”, subsection “Laboratory and instrumental data”).
During treatment with Lorista®, it is not recommended to take potassium-sparing diuretics, potassium supplements or potassium-containing salt substitutes.
Aortic or mitral stenosis, HOCM
Like all drugs that have a vasodilating effect, ARA II should be used with caution in patients with aortic or mitral stenosis or HOCM.
IHD and cerebrovascular diseases
Like all drugs that have a vasodilating effect, ARA II should be used with caution in patients with coronary artery disease or cerebrovascular diseases, since a pronounced decrease in blood pressure in this group of patients can lead to the development of myocardial infarction or stroke.
CHF
As with the use of other drugs that act on the RAAS, patients with CHF and with or without impaired renal function are at risk of developing severe hypotension or acute renal impairment.
Since there is insufficient experience with the use of Lorista® in patients with heart failure and concomitant severe renal impairment, in patients with severe heart failure (NYHA functional class IV), as well as in patients with heart failure and symptomatic life-threatening arrhythmias, the drug Lorista® should be used with caution in patients in these groups.
Primary hyperaldosteronism
Since patients with primary hyperaldosteronism, as a rule, do not have a positive response to therapy with antihypertensive drugs that act by inhibiting the RAAS, the use of Lorista is not recommended in this group of patients.
Liver dysfunction
Data from pharmacokinetic studies indicate that the concentration of losartan in the blood plasma in patients with cirrhosis of the liver is significantly increased, therefore patients with a history of impaired liver function should use the drug Lorista® at a lower dose. There is no experience with the use of losartan in patients with severe liver dysfunction, so Lorista® should not be used in this group of patients (see sections “Pharmacological properties” [subsection “Pharmacokinetics”], “Contraindications”, “Dosage and Administration”).
Renal dysfunction
Due to inhibition of the RAAS, changes in renal function, including the development of renal failure, have been observed in some susceptible patients. These changes in renal function may return to normal after treatment is stopped.
Some drugs that affect the RAAS may increase serum urea and creatinine concentrations in patients with bilateral renal artery stenosis or renal artery stenosis of a solitary kidney. Similar effects have been reported with losartan. Such renal dysfunction may be reversible after discontinuation of therapy. Losartan should be used with caution in patients with bilateral renal artery stenosis or renal artery stenosis of a solitary kidney.
Special patient groups
Ethnic characteristics
Analysis of data from the entire population of patients included in a clinical trial to study the effect of losartan on reducing the incidence of the main composite criterion for evaluating the study in patients with hypertension and left ventricular hypertrophy showed that the ability of losartan, compared with atenolol, to reduce the risk of stroke and myocardial infarction, and also reducing the rate of cardiovascular mortality in patients with hypertension and left ventricular hypertrophy (by 13.0%) does not apply to patients of the Negroid race, although both treatment regimens effectively reduced blood pressure in these patients.
In this study, losartan, compared with atenolol, reduced cardiovascular morbidity and mortality in patients with hypertension and left ventricular hypertrophy of all races except blacks. However, in this study, black patients receiving atenolol had a lower risk of the study's primary composite endpoint (i.e., lower combined incidence of cardiovascular death, stroke, and myocardial infarction) compared with race-matched patients receiving losartan.
Children and teenagers
The effectiveness and safety of losartan in children and adolescents under 18 years of age have not been established.
If oliguria or arterial hypotension develops in newborns whose mothers took losartan during pregnancy, symptomatic therapy aimed at maintaining blood pressure and renal perfusion is necessary. Blood transfusions or dialysis may be required to prevent hypotension and/or maintain renal function.
Elderly patients
Clinical studies have not revealed any particularities regarding the safety and effectiveness of losartan in elderly patients (over 65 years of age).
Drug interactions Lorista h/hd
The drug can be used in combination with other antihypertensive drugs. There were no clinically significant interactions between losartan and digoxin, warfarin, cimetidine, phenobarbital, ketoconazole and erythromycin. With simultaneous use of losartan and rifampicin, the metabolism of losartan and the breakdown of its active metabolites may be accelerated, which leads to a decrease in the effectiveness of losartan. The combined use of losartan and spironolactone, amiloride, triamterene and/or potassium supplements may lead to the development of hyperkalemia. As is the case with other antihypertensive drugs, the severity of the hypotensive effect of losartan may be reduced with simultaneous use of NSAIDs (for example, indomethacin), as well as sympathomimetics. Hydrochlorothiazide reduces lithium excretion. The combined use of losartan with lithium preparations may lead to increased side effects of lithium due to increased reabsorption of lithium in the proximal tubules of nephrons. Therefore, it is recommended to monitor the concentration of lithium in the blood serum and, if possible, avoid the simultaneous use of hydrochlorothiazide and lithium preparations. The combined use of barbiturates, narcotic analgesics (morphine) or consumption of alcoholic beverages may enhance the hypotensive effect of hydrochlorothiazide. In addition, the hypotensive effect of hydrochlorothiazide may also be enhanced by concomitant use of other antihypertensive agents. Hydrochlorothiazide, by weakening the effect of antidiabetic agents, can cause the development of hyperglycemia, therefore, when treating patients with diabetes mellitus, blood glucose levels should be regularly monitored and, if necessary, the dose of the antidiabetic agent should be adjusted. The combined use of hydrochlorothiazide and colestipol or cholestyramine leads to a decrease in the absorption of hydrochlorothiazide by 43 and 85%, respectively. The simultaneous use of hydrochlorothiazide and corticosteroids (including ACTH) may cause the development of hypokalemia. With the combined use of hydrochlorothiazide and pressor amines, the corresponding response to the administration of the latter may be reduced, but this effect is usually insignificant. When performing general anesthesia or administering non-depolarizing muscle relaxants (for example, tubocurarine), the risk of hypotension increases. When taking amiodarone concomitantly with hydrochlorothiazide, the risk of developing arrhythmia due to hypokalemia increases. NSAIDs may reduce the diuretic and hypotensive effects of hydrochlorothiazide. Diflunisal increases the concentration of hydrochlorothiazide in the blood plasma and reduces the severity of its hyperuricemic effect. When the drug is used in combination with digitalis glycosides, the likelihood of toxic effects of cardiac glycosides increases.
Lorista, 30 pcs., 12.5 mg, film-coated tablets
More than 20,000 patients took part in studies on the effectiveness and safety of Lorista.
The results of the studies demonstrated the following data:
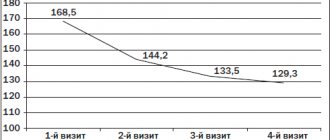
— in the “Take Off” study, Lorista® (losartan from KRKA) significantly reduced uric acid levels by 32.6% in patients with arterial hypertension (AH) and concomitant hyperuricemia and/or gout. 100% of patients participating in the study achieved the target blood pressure level. Therapy with Lorista has a pronounced positive effect on the elasticity of the vascular wall in patients with hypertension1;
— in the open-label multicenter clinical trial LAURA2 (Lorista® and uric acid), the relationship between treatment with Lorista and its fixed combination with hydrochlorothiazide (Lorista® H and Lorista® HD) and hyperuricemia was studied. Based on the results of the study in patients with hypertension and hyperuricemia, Lorista®, Lorista® H and Lorista® ND, due to their apparent ability to lower uric acid levels, can be used as the preferred therapy;
— the EFFECT3 study proved the effectiveness and safety of losartan (Lorista®) in patients with mild and moderate hypertension. In addition, it is important to emphasize the safety of using Lorista (adverse effects in less than 1% of patients), which makes the drug an indispensable assistant in the fight against hypertension;
— as a result of the international study Gemera4, the effectiveness and safety of the use of Lorista® and a fixed combination with hydrochlorothiazide (Lorista® N) in patients with stage 1–2 hypertension was confirmed. 100% of patients achieved CAP.
The results of clinical studies conducted with the drug KRKA Lorista (losartan) and its fixed combinations with hydrochlorothiazide further indicate that the drug contributes not only to the effective and well-tolerated treatment of hypertension, but also to the reduction of cardiovascular risk.
Literature
1. Nedogoda S.V., Ledyaeva A.A., Chumachok E.V., Tsoma V.V., Salasyuk A.S. Possibilities of losartan in angioprotection against hyperuricemia in patients with arterial hypertension. Systemic hypertension. - 2012. - No. 4. - P.50–54.
2. Svishchenko E.P., Bezrodnaya L.V., Gorbas I.M. Clinical and uricosuric efficacy of losartan in patients with arterial hypertension. Results of the open multicenter clinical trial LAURA. Arterial hypertension.- 2012.- 5 (25).- P.25–32.
3. Drapkina O.M., Kozlova E.V. The place of angiotensin receptor antagonists in the treatment of cardiovascular diseases. Study EFFECT: use of Lorista in patients with mild and moderate arterial hypertension in real clinical practice. Problems of women's health.- 2009.- 4(4).- P.17–26.
4. Chazova I.E., Martynyuk T.V. Federal State Budgetary Institution Russian Cardiology Research and Production Complex of the Ministry of Health of the Russian Federation, Moscow. First results of the international clinical trial GEMERA: two therapeutic regimens for the effective treatment of patients with stage 1–2 arterial hypertension.
Overdose of Lorista h/hd, symptoms and treatment
Data on drug overdose are limited. The most likely consequences of an overdose of losartan may be arterial hypotension and tachycardia; It is also possible to develop bradycardia due to parasympathetic (vagal) stimulation. The main symptoms of hydrochlorothiazide overdose are excessive diuresis, significant hypotension with bradycardia, other cardiac arrhythmias, decreased serum electrolyte levels and impaired core function. In case of overdose, the drug should be discontinued immediately. In case of recent overdose, gastric lavage is recommended. It is necessary to monitor the vital functions of the body and, if necessary, carry out symptomatic treatment. Losartan and its active metabolite are not excreted from the body during hemodialysis.