Cardiac markers
The use of cardiac markers in the diagnosis of acute coronary syndrome
To select the correct treatment strategy in patients with suspected acute coronary events, it is necessary to differentiate between myocardial damage and extracardiac syndromes with similar manifestations.
According to statistics, 50% of patients admitted to the hospital with suspected myocardial infarction do not have any abnormalities in the ECG. There are frequent cases of atypical and low-symptomatic variants at the onset of the disease.
10 years ago, along with the ECG, enzyme levels (AST, LDH, Creatine kinase) were measured. A rise in enzyme levels occurs a certain time after the event and indicates the destruction of heart muscle cells. However, these markers are not specific for myocardial damage, and their delayed appearance makes them an imperfect diagnostic tool. As a result, new markers were proposed.
With myocardial necrosis, along with enzymes, various proteins appear that signal damage to cardiomyocytes: myoglobin; cardiac troponins; fatty acid binding protein.
The preferred biomarkers of necrotic myocardial injury are cardiac troponins I (cTnI) and T (cTnT). Once cardiomyocytes are damaged, cardiac troponins are released into the blood within 2–6 hours. Peak concentrations occur after 12–24 hours, varying between individuals. The level of troponins correlates with the area of damage to the heart muscle, and makes it possible to predict the severity of the condition.
The diagnosis of myocardial infarction is made when the level of sensitive and specific biomarkers in the blood increases in the presence of clinical signs of acute ischemia. An increase in troponin levels in patients with acute coronary syndrome is a criterion for differentiating non-ST segment elevation myocardial infarction and unstable angina. The determination of the activity of the cardiac fraction of creatine kinase (KK-MB) has not lost its importance. The use of total CPK for the diagnosis of myocardial infarction is recommended only in the absence of the possibility of studying the values of cardiac troponins and the MB fraction of creatine kinase.
To determine blood troponin levels, it is recommended to use only highly sensitive (hsTn) test kits. Tests for troponins have high specificity and sensitivity regarding myocardial damage; even microscopic areas of necrosis can be diagnosed by their level. Troponin tests must be interpreted over time, in a series of measurements taken over a certain period of time. The disadvantage of these tests is the problem of standardization: different monoclonal antibody matrices are used to produce troponin test kits, so they have different diagnostic ranges and, therefore, diagnostic algorithms. In addition, test results from different manufacturers cannot be compared with each other to assess the situation over time.
One of the new markers for early diagnosis of acute myocardial infarction is FFA, a protein that binds fatty acids. This protein is located in the cytoplasm of cardiomyocytes and is responsible for the transport of fatty acids and other lipophilic molecules. With the development of myocardial necrosis, the membrane of cardiomyocytes is damaged and BFA quickly enters the intercellular space. Having a low molecular weight, the protein reaches the bloodstream in a matter of minutes. The maximum release of BFA into the bloodstream occurs 1–3 hours after clinical signs of damage to the heart muscle. The peak concentration is observed after 6 hours, the value of the blood BSFA level increases 10 times or more. Determination of AFFA helps to improve the diagnosis of acute coronary events, especially in the early stages. However, the question of its use in verifying the diagnosis of acute myocardial infarction remains open.
Myoglobin - interest in this marker remains, despite the fact that it does not have specificity for the heart muscle (90–96% in the absence of trauma and renal failure). Myoglobin increases 1–2 hours after infarction and is the earliest marker of myocardial damage. Myoglobin is also the most sensitive marker for monitoring reperfusion and recurrent events.
In accordance with data from large studies, the simultaneous determination of several markers of myocardial damage increases diagnostic efficiency - the exclusion of myocardial infarction is faster and more reliable.
Assessment of the risk of developing atherosclerosis of coronary and cerebral vessels
Atherosclerosis plays a key role in the development of coronary heart disease and myocardial infarction. The exact cause of atherosclerosis is unknown. However, there are certain traits, conditions, or habits that increase the risk of the disease. These are known as risk factors. The more risk factors, the higher the likelihood of developing atherosclerosis. High blood pressure, insulin resistance and diabetes, unhealthy blood cholesterol levels with high levels of low-density lipoprotein, systemic and local inflammation, smoking, excess weight, etc. are the main risk factors for the disease.
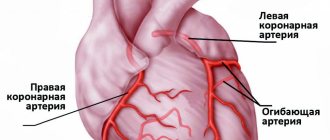
As a result of their influence, the vessel wall is damaged and its function is impaired. In the presence of inflammation, the disease progresses - an atherosclerotic plaque is formed. Plaque structure, rather than size, is a determinant of the outcome of an acute coronary event. In the presence of coronary artery thrombosis, the process is aggravated for the worse. The coronary and carotid arteries are most commonly affected.
Taking steps to control risk factors, annual examinations and follow-up with a doctor can help prevent or delay the onset of atherosclerosis and its complications. It is believed that significant results can be achieved in the latent (latent, preclinical) stage of atherosclerosis.
To assess the risk of developing atherosclerosis of the vessels of the heart and brain, the following diagnostic methods are currently used:
- Angiography of the carotid and coronary arteries. Determining the condition of the inside of the arteries using dyes and special x-rays. Shows whether plaque is blocking arteries and how severe the blockage is. Invasive method.
Non-invasive methods:
- Angiography (CT, MRI, MSCT). Relatively new diagnostic methods. Performed without the use of a contrast agent in case of intolerance. Digital processing occurs using computer and magnetic resonance imaging devices.
- Determination of intima-media thickness. Assessment of the thickness of the intima-media complex of the arterial wall using ultrasound.
- An ECG is a simple, painless diagnostic method that is based on the study of the electrical activity of the heart. After taking an ECG, you can conclude how fast the heart beats and determine its rhythm (stable or irregular). An ECG can reveal signs of heart damage caused by coronary artery disease.
- Determination of the level of biomarkers of atherosclerosis.
Measuring the level of metabolic markers allows us to draw conclusions about the functioning of systems involved in the pathogenesis of atherosclerosis. These indicators include indicators of blood lipid composition, chronic inflammation, hemostasis, markers of glucose utilization and parameters reflecting the metabolism of visceral adipose tissue.
Lipid metabolism indicators
It has been proven that disturbances in lipid metabolism play a central role in the development of atherosclerosis. Dyslipidemia - an increase in the level of low-density lipoprotein cholesterol and a decrease in the level of high-density lipoprotein cholesterol, is one of the precursors of atherosclerosis. However, long before dyslipidemia is diagnosed, the initial stages of atherosclerotic lesions can be suspected. Oxidized low-density lipoprotein cholesterol appears first in the blood during atherogenesis, when foam cells form. Oxidized LDL cholesterol is an important tool in studying the processes of endocytosis of the vascular wall.
ApoB is an important component of many of the most atherogenic lipoprotein particles. Several studies have shown that apoB is a better predictor of cardiovascular disease risk than LDL because it can rise despite normal or low LDL-C concentrations. The apoB/apoA1 ratio is more effective at predicting heart attack risk than apoB or apoA1 measurements alone.
High levels of blood triglycerides also increase the risk of developing atherosclerosis, especially in women. An increase in the indicator indicates that glucose is actively converted into fats and is not used by tissues for energy production. A triglyceride value above 1.7 indicates profound changes in carbohydrate and lipid metabolism. Triglyceride targets are much lower.
Insulin resistance
The degree of insulin resistance is calculated using the HOMA-IR index (Homeostatic Model Assessment of Insulin Resistance) based on analysis of fasting glucose and insulin concentrations. Measuring your waist is an additional way to assess the presence of insulin resistance.
An independent risk factor for cardiovascular disease is homocysteine.
Homocysteine is a sulfur-containing amino acid, an intermediate product of the conversion of methionine. Exceeding target homocysteine levels has a host of adverse effects in the body. As a result of the toxic effect on the endothelium of blood vessels, the elasticity of their walls decreases, which creates the preconditions for the development of atherosclerosis. Homocysteine increases the aggregation ability of blood platelets, thereby increasing the risk of thrombosis.
The normal homocysteine level is 5–15 µmol/l. When the concentration of HC in the blood plasma is 15-30 µmol/l, the degree of hyperhomocysteinemia is considered moderate, 30-100 µmol/l - moderate, more than 100 - severe. Moderate hyperhomocysteinemia before the age of 40 years is usually asymptomatic, while changes in the coronary and cerebral arteries are already occurring. An increase in homocysteine by 5 µmol/l increases the risk of atherosclerotic damage to heart vessels by 80% in women and by 60% in men.
Markers of inflammation
One of the criteria for assessing coronary risk is the level of C-reactive protein in the blood, determined by a highly sensitive method. High hsrpc levels are associated with the risk of atherosclerosis and heart attack. High levels of CRP are a consequence of inflammation in the body. Inflammation is the body's response to injury or infection. Damage to the inner walls of the arteries, combined with inflammation, contributes to the progression of the disease.
People with low hs-CRP levels are less at risk of atherosclerosis than those with elevated hs-CRP levels. In unstable angina, hs-CRP increases in 65–90% of cases. hSRP can be used to judge the risk of recurrent myocardial infarction. CRP level, mg/l.
Interleukin-6 (IL-6) is an inflammatory cytokine that plays a central role in promoting the inflammatory response in atherosclerosis. IL-6 release is stimulated by acute infections, chronic inflammatory conditions, obesity, and physiological stress.
Introduction
Despite the fact that heart failure (HF) with preserved ejection fraction (HFpEF) is a very common disease throughout the world and the number of patients with this pathology is increasing every year, many questions regarding the best diagnostic approach , remain unanswered.
Diagnosis of HFpEF often becomes a clinical challenge, and this is especially true for outpatients at an early stage of the disease without obvious signs of HF [1]. According to the recommendations of the European Society of Cardiology, the necessary components for making a diagnosis of HFpEF are [2]:
increased natriuretic peptide levels (BNP ≥35 pg/ml or NT-proBNP level ≥125 pg/ml);
preserved left ventricular (LV) ejection fraction (EF) more than 50% (LVEF ≥50%);
additional disorders of the structure and/or function of the heart;
left atrial (LA) volume index >34 ml/m2;
LV mass index ≥115 g/m2 for men and ≥95 g/m2 for women;
ratio of E to E′ ≥13, where E is the early diastolic velocity of blood flow on the mitral valve, E′ is the early diastolic velocity of movement of the mitral valve annulus fibrosus according to the results of tissue Doppler sonography;
average E′ in the septum and lateral wall of the LV <9 cm/s.
In case of diagnostic doubt, the patient may undergo stress tests or invasive measurement of elevated LV filling pressure [3].
Growth differentiation factor 15
Another biomarker that may be useful in diagnosing HFpEF is GDF-15, a member of the transforming growth factor superfamily of cytokines and markers of cell damage and inflammation. GDF-15 can be used as a new biomarker to assess myocardial fibrosis. In a study by YM Zhou et al. [42] after linear correlation analysis, GDF-15 expression was found to be positively associated with the degree of cardiac fibrosis in patients with atrial fibrillation. In patients with end-stage nonischemic dilated cardiomyopathy, serum GDF-15 levels correlated with the severity of myocardial fibrosis [43]. GDF-15 expression is highly induced in cardiomyocytes in response to metabolic stress such as cardiac ischemia, tissue injury, or pressure overload conditions [12, 44, 45]. Y. Izumiya et al. [8] found a positive correlation between serum GDF-15 concentrations and HF functional class, as well as plasma BNP levels and serum high-sensitivity troponin T levels in 150 patients with HFpEF.
C. Sinning et al. [9] proposed a formula for calculating an index based on the concentrations of C-reactive protein (CRP), GDF-15, sST2 and NT-proBNP, which allows distinguishing HFpEF from HFrEF:
(CRP + GDF – 15 + sST2) / NT-proBNP.
The prognostic value of GDF-15 has also been confirmed in recent studies. GDF-15 remained a significant independent predictor of HF even after adjustment for important clinical predictors, including high-sensitivity troponin T and NT-proBNP [46, 47]. Overall, GDF-15 may serve as a biomarker to help differentiate between HFpEF and HFrEF, as well as an additional indicator of the prognosis of cardiovascular events.
Adrenomedullin
Noteworthy is a little-studied biomarker associated with sympathetic activity, which has vasodilating, immunomodulatory, antiproliferative and antiapoptotic effects - adrenomedullin (ADM). [39] Currently, there is little data regarding the correlation of ADM and HFpEF. In a large study by F. P. Brouwers et al. [40] reported that the terminal fragment of this molecule, MR-proADM (mid-regional pro-adreomedullin), may help in the diagnosis and identification of new patients with HFpEF, but only after determining the baseline cardiovascular risk profile in the HFpEF population. CM. Yu et al. [41] showed that ADM may specifically respond to diastolic dysfunction.
Diversity of biomarkers of myocardial damage and remodeling
Currently, in the diagnosis of HFpEF, much attention is paid to biochemical studies to assess the activity of myocardial damage and remodeling. It has been shown that plasma biomarkers accurately reflect changes in the hemodynamic load on the heart muscle and myocardial fibrosis, so they may be significant for the diagnosis and management of patients with HFpEF [4]. For example, matrix metalloproteinases, their tissue inhibitors and collagen processing proteins, collagen propeptides and collagen telopeptides reflect changes in collagen homeostasis and the transition from hypertension without end-organ damage to clinically symptomatic HFpEF [4, 5]. Galectin-3 and soluble suppression of tumorigenicity 2 (sST2) reflect the overall degree of fibrosis and the severity of HFpEF, which allows them to be used to determine the early stage of development of heart failure caused by fibrosis. In addition to the above-mentioned markers, growth differentiation factor 15 (GDF-15) and microRNAs are promising new biomarkers for detecting fibrosis [4–7]. Let's take a closer look at the most relevant HFpEF biomarkers.