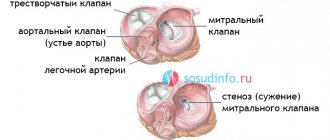
1.What is heart transplantation?
Heart transplant
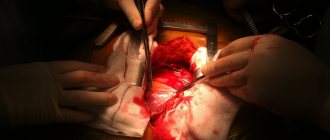
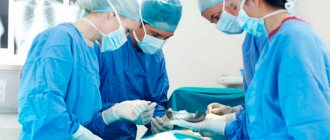
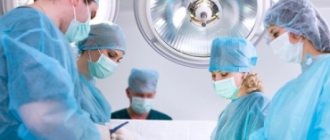
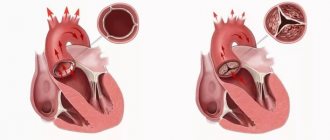
- a procedure in which a surgeon removes a patient's heart and replaces it with a donor one. During surgery, a mechanical pump circulates blood throughout the body while the surgeon transplants a healthy heart from a recently deceased donor.
The surgeon attaches the donor heart to the main blood vessels and attaches wires to it that will temporarily control the heartbeat. The whole procedure takes several hours.
To prevent rejection of the donor heart, the surgeon gives the patient strong medications (immunosuppressants) immediately after the operation. The patient will need to take such drugs for some time.
A must read! Help with treatment and hospitalization!
2.Rehabilitation after surgery
What to expect after heart transplant surgery?
Recovery process after heart transplant surgery
similar to other heart surgeries.
You will most likely spend a couple of weeks in the hospital. In some cases, you will need more time depending on how your health is assessed and whether there are complications after surgery. At the hospital, you will begin a cardiac rehabilitation program.
. Your doctors will check to see if the donor heart is taking root in your body.
Rehabilitation after heart transplant
A rehabilitation program will help you recover and become active again after your heart transplant.
The donor heart can respond to stress in different ways. Your heart rate will not rise as much as before. The heartbeat will also return to normal in a different way. This occurs because some of the nerve connections that control the heart were cut during surgery.
Visit our Cardiology page
3.Indications for heart transplant surgery
Heart transplant surgery is performed when the heart stops working and the patient is on the verge of life and death. Heart transplantation is also performed if the patient has serious heart disease and only surgery provides the best prognosis for recovery. A patient may be recommended for a heart transplant if he:
- End-stage heart failure, coronary artery disease, cardiomyopathy, or congenital heart disease.
- Low chances of living longer than a year without a heart transplant.
- Absence of any other serious medical reasons that reduce life expectancy.
- If the attending physician believes that a heart transplant will increase the length and quality of life.
Some medical centers require heart transplant candidates to provide evidence that they have stopped smoking and/or have been sober for an extended period of time (4-6 months) before being placed on the transplant list.
About our clinic Chistye Prudy metro station Medintercom page!
Postoperative period
This period can be divided into 4 periods.
- The resuscitation period lasts approximately ten days. Here the patient undergoes intensive therapy aimed at preventing rejection of a foreign organ, stopping any other complications such as bleeding, allergic reactions, fluid accumulation in the pericardium, etc. At this time, the patient is very susceptible to any viral, fungal or bacterial infections. The task of medical personnel is to prevent such developments.
- Hospital stay – the patient will have to spend at least 30 days in a medical facility. During this period, an individual medication regimen will be developed and a number of necessary studies will be carried out.
- Post-hospital period - the patient stays at home, takes prescribed medications, is observed by doctors and gradually returns to his usual lifestyle. This recovery time can take about a year.
- The fourth period will last the rest of your life and is characterized by the restoration of partial working capacity and the ability to lead a fairly active lifestyle. A person who has undergone surgery is required to undergo regular examinations that will allow him to monitor the process of the new organ and constantly maintain it. In addition, it is absolutely necessary to lead an absolutely healthy lifestyle and follow the diet recommended by doctors.
4.Risks of heart transplant
The main risks of heart transplant surgery include:
Rejection of a donor heart.
To check the patient's body, surgeons regularly perform biopsies of heart tissue, as well as echocardiography, electrocardiography, or blood tests.
If the patient's body rejects the heart, additional medications (immunosuppressants or steroids) are prescribed to suppress the immune system so that it will accept the donor heart. These medications may have side effects, the most serious of which are various infections and the development of cancer.
Atherosclerosis of the arteries, which could appear in the donor heart
. This is usually a complication and at the same time an important limiting factor that affects life expectancy.
Things to think about
After a heart transplant, you must follow strict life rules, which include taking daily medications and regular medical care. Medical care involves ongoing testing (biopsies) of tissue from the transplanted heart to prevent rejection.
Transplant candidates receive a heart based on the date of registration and the severity of the disease. Also, do not forget that the number of donor organs is limited.
Background
The successes of transplantology are associated with the development of two independent areas of medicine: the improvement of surgical techniques and anesthesia methods, on the one hand, and the achievements of immunology and pharmacology, on the other. The first ensured the successful completion of operations, and the second ensured the successful course of the postoperative period.
First steps
Back in the 16th century, the Italian surgeon Gasparo Tagliacozzi, after a series of unsuccessful experiments with skin transplantation from person to person, presented to the public the successful result of autotransplantation (transplantation of the recipient’s own organ, in this case skin). In 1596, he described his observations of the "strength and power of individuality" in De Curtorum Chirurgia per Insitionem (Replacement Surgery by Transplantation).
However, the idea of organ transplantation aroused widespread interest only 300 years later, when surgeons began practical experiments en masse. Once again faced with the problem of rejection, they enriched medicine with three important conclusions: cross-species organ transplants (xenotransplantations) are always unsuccessful; transplants between members of the same species (allogenetic transplants) in most cases, too, and repeated organ transplants between the same donors and recipients accelerated rejection; if there was a blood relationship between the donor and the recipient, the likelihood of a favorable outcome of the operation increased significantly, and transplantation of one’s own organs (autotransplantation) was almost always successful.
The first successful transplantation described in the literature dates back to the 2nd century AD: the Indian surgeon Shushrata performed a skin graft during rhinoplasty.
And in the 3rd century, according to the chronicles of the Roman Catholic Church, Saints Damian and Cosmas transplanted the limb of a recently deceased Ethiopian to the Roman deacon Justinian, who had lost his leg from gangrene.
dog's heart
In 1905, in a series of experiments on organ transplantation, the turn came to the heart. The first transplantation of the heart of a donor dog onto the vessels of the neck of a recipient dog was carried out by surgeon, biologist and pathophysiologist Alexis Carrel and physiologist Charles Guthrie, Americans of French origin. The donor heart worked for 2 hours.
In 1933, the American physiologist Frank Mann and his colleagues repeated Carrel’s experiment and showed the possibility of a heterotopically (that is, placed in an atypical place) transplanted dog heart functioning for up to 8 days (the dog’s own heart remained in place and continued to work).
Soviet surgeons also kept up with the times. The most significant contribution to the experimental foundations of heart transplantation in the USSR was made by Vladimir Petrovich Demikhov. From 1946 to 1955, in several hundred experiments, he developed 24 (!) variants of the surgical technique of heterotopic heart transplantation into the chest. In some experiments, the donor heart effectively provided blood circulation to the recipient dog for more than 15 hours.
Fighting ischemia
The most obvious problem that stood in the way of surgeons was ischemia: in conditions of insufficient blood flow, both recipient tissue and donor hearts were damaged. Transplantation cannot be performed in a short time, so surgeons and anesthesiologists began to look for ways to extend the operation time. This is how methods of controlled hypothermia were developed and artificial blood circulation (CPB) machines were designed.
Controlled hypothermia can reduce blood loss and also prolong surgery time without increasing the risk of tissue ischemia.
In 1953, American surgeons Wilford Neptune and Brian Cookson and their colleagues performed the first transplantation of the cardiopulmonary complex of a dog under conditions of deep hypothermia (during the operation, the temperature in the room was maintained at about minus 4 ° C).
In 1957, Americans William Webb and Heather Howard repeated this experiment, connecting the recipient dog to an infrared apparatus. They later reported transplantation under the same conditions of an isolated heart. They performed nine anastomoses between the aorta, pulmonary artery, pulmonary and vena cava. True, this transplant functioned for only 7 hours.
By the mid-60s, Norman Shumway and Richard Lower and colleagues from the Stanford University Clinic developed a heart transplantation technique that is still used today: using “local hypothermia” - local cooling to 18-21 ° C. From this point on, there was only one obstacle left for the successful operation - immunological rejection.
Reasons for rejection
In 1952, immunologists Jean Dausset (France) and Baruch Benacerraf (USA), together with geneticist George Snell (USA), discovered that the most important antigens involved in transplant rejection are major histocompatibility complex (HLA) antigens. For this discovery they were awarded the Nobel Prize in 1980.
There are about 150 variants of HLA antigens, and each person has six variants of such antigens on their cell membranes. Consequently, more than a trillion of their combinations are possible - the probability of two people existing with the same HLA antigen complexes is close to zero, of course, unless they are identical twins. Ideally, the donor and recipient have a mismatch of only one antigen out of six. Operations are also performed if the two do not match, but this increases the risk of developing immunological rejection.
In practice, determination of tissue compatibility - histotyping - is carried out using blood lymphocytes.
On the host side, the main effectors of transplantation rejection are cytotoxic CD8 T cells and CD4 T cells. The latter attract inflammatory cells (including macrophages) to the area of transplant rejection. Recognition of transplantation antigens occurs either directly on the transplant cells or in the nearest lymphoid tissue.
Antibodies in the recipient’s body (for example, those that appeared due to a previous whole blood transfusion) can also play a significant role in transplant rejection. By interacting with antigens of the endothelium of blood vessels penetrating the graft, they initiate the complement system and a cascade of reactions leading to blockage of blood vessels.
Immunosuppression
The first substances that were used to suppress the immune response were corticosteroids.
In 1948, the American doctor Philip Hench, using cortisone in the treatment of rheumatoid arthritis, showed that the drug has a pronounced anti-inflammatory effect. In 1952, American surgeon Roger Baker and his colleagues demonstrated that cortisone could also be used for immunosuppression after transplantation.
In 1962, Americans Willard Goodwin and Matt Mims first used azathioprine, an immunosuppressive drug with the greatest activity against T-lymphocytes, to prevent acute rejection of a transplanted kidney. Azathioprine in combination with corticosteroids was also planned for heart transplant patients.
Man's turn
The first human heart was transplanted in 1964. James Hardy and his colleagues transplanted the heart of a large chimpanzee, weighing more than 43 kg, into a 68-year-old patient with severe coronary artery disease in the terminal stage of progressive heart failure. Initially, the graft worked satisfactorily, but an hour after the CP device was turned off, acute heart failure developed due to volume overload of the graft, and the patient died.
The first allogenetic human heart transplant was performed three years later in Cape Town by South African surgeon Christian Barnard, who trained under Norman Shumway. Dr. Barnard's team transplanted the heart of a 25-year-old girl who died in a car accident into a 55-year-old diabetic who had suffered three heart attacks and suffered from congestive heart failure. The transplantation was successful, but on the 18th day after the operation the patient died of bilateral pneumonia. A month later, a second donor heart transplant was performed in the same clinic, after which the recipient lived for a year and a half, dying from chronic rejection.
In the USSR, the first successful heart transplant was performed in 1987 by the famous transplantologist, academician of the USSR Academy of Medical Sciences Valery Ivanovich Shumakov (1931–2008).
The success of the first clinical experiment captivated the medical community, and surgeons around the world turned to solving the problem of heart transplantation. However, most patients died soon after the operation, and the interest of researchers began to fade: if in 1968 there were 100 heart transplants, then in 1970 there were only 18. The main cause of death remained rejection of transplanted organs.
Only four of those operated on between 1967 and 1973 lived with a new heart for more than a year; from 1974 to 1983, the one-year survival rate increased to 60%, but the five-year survival rate was only 21%.
Immunologists and pharmacologists get down to business
In 1983, clinical studies of a new immunosuppressant, cyclosporine, a non-ribosomal polypeptide obtained from soil fungi of the species Beauveria nivea, were successful. It was isolated in 1970 as part of a program to screen new antibiotics. In 1972, the Belgian immunologist Jean-François Borel discovered the ability of cyclosporine to inhibit the culture of lymphocytes in the absence of a general cytostatic effect. In the late 70s, British surgeon Roy Calne conducted the first tests of the effectiveness of cyclosporine after heart and kidney transplantation on experimental animals. Its introduction into widespread practice opened a new era in heart transplantation, as it made it possible to significantly prolong the life of patients after transplantation. In 1994, another new immunosuppressant, tacrolimus (FK-506), a macrolide produced by the actinomycete Streptomyces tsukubaensis, was approved for postoperative therapy. In 1987, Japanese immunologists Shinichi Sawada and Jen Suzuki discovered in in vitro experiments that tacrolimus was 100 times more potent than cyclosporine in suppressing the proliferation of T lymphocytes. In studies in rats, dogs, and primates conducted from 1988 to 1993, tacrolimus provided prevention of transplant rejection at doses 10 to 100 times lower than cyclosporine and with fewer side effects. However, modifications of cyclosporine developed at the same time had similar advantages.
Recent advances in immunosuppression are associated with poly- and monoclonal antibodies (we talked about this in detail in KS No. 5 (118) 2013, article “Pharmaceutical Advances: Monoclonal Antibodies”), which help prevent steroid-resistant rejection, which cannot be stopped even by pulse therapy with methylprednisolone. tissues, which occurs with a frequency of 10–18%.
Currently, a three-component regimen of immunosuppressive therapy is used: cyclosporine A or tacrolimus in combination with methylprednisolone and mycophenolate mofetil (a cytostatic agent that is most effective against lymphocytes). Antilymphocyte antibody drugs (ALG, ATG, OKT-3) are used as induction therapy, as well as for severe signs of rejection or steroid-resistant rejection.