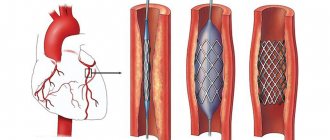
Coronary angiography does not always reveal stenosis of the coronary vessels of the heart
Coronary angiography does not always reveal stenosis of the coronary vessels of the heart
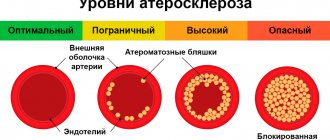
Stenosis of the coronary vessels of the heart is a fairly common disease - it occurs in 30-40% of cases when visiting a cardiologist. The consequences of stenosis are heart failure, the development of coronary heart disease, myocardial infarction and thrombosis. In advanced cases, the disease increases the likelihood of developing a dissecting aneurysm, which can lead to internal bleeding. In the absence of adequate drug therapy, death occurs.
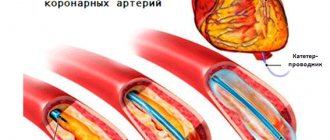
The “gold standard” for detecting coronary artery stenosis is still coronary angiography (CAG), but this method also has its limitations.
As an example, we can cite the medical history of a 58-year-old patient with a history of hypertension, who went to the Medservice clinic. Prior to this, she was hospitalized in the hospital with pain in the chest.
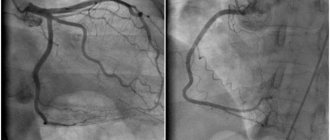
Based on the results of the electrocardiogram, clinical and laboratory data, a diagnosis of myocardial infarction (without q wave) was made. Coronary angiography was performed, where no hemodynamically significant stenoses were detected.
A month after hospitalization, during dynamic observation at the Medservice clinic, the patient underwent echocardiography (EchoCG - ultrasound examination of the heart) to assess the contractility of the myocardium and coronary arteries. In the absence of obvious visual contractility disorders, when assessing myocardial deformation using the left ventricular function analysis (LVAF) method, a zone of segmental disorders in the anterior septal wall of the heart was revealed, as well as a slight acceleration of blood flow in the middle segment of the anterior interventricular branch (LAD). The patient was undergoing treatment.
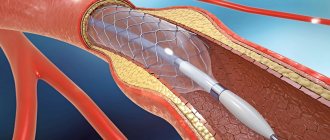
Repeated coronary angiography revealed no hemodynamically significant narrowings. But intravascular ultrasound revealed extensive hemodynamically significant stenosis of the LAD (75%, length 25 mm). The patient underwent stenting of the affected area.
Currently, the patient is still regularly monitored at the Medsrvis clinic. Angina pectoris is not about LVAF; there is an area of impaired contractility, but its area has decreased. Ultrasound examinations of the coronary arteries showed that blood flow in the anterior interventricular branch was normal.
Thus, the use of all available methods in the diagnosis and treatment of patients with coronary artery disease usually gives a complete picture of the patient and helps in correct treatment.
The article presents clinical and diagnostic criteria for a hemodynamically significant ductus arteriosus in premature infants, as well as modern approaches to its correction.
Hemodynamically significant patent ductus arteriosus in preterm infants
The article presents the clinical diagnostic criteria for hemodynamically significant ductus arteriosus in preterm infants, as well as modern approaches to correction.
Patent ductus arteriosus (dutus arteriosus, ductus arteriosus) is a vessel connecting the aorta and pulmonary artery, which has retained its normal structure for the fetus after its closure has expired. PDA is one of the most common defects: according to clinical data, its frequency is 10-18% of all congenital heart defects. A long-term (several weeks) functioning ductus arteriosus in premature infants is usually a sign of morpho-functional immaturity of the cardiovascular system [2, 6]. Cases in which its functioning is accompanied by noticeable (recorded using clinical-instrumental methods) disturbances of central and regional hemodynamics are usually designated by the term hemodynamically significant functioning ductus arteriosus (HSFDA) [1, 4]. The probability of its long-term functioning is greater, the lower the child’s gestational age, his body weight and the more severe the condition of the newborn. According to Doppler echocardiography, in full-term infants the ductus arteriosus completely collapses in 50% of cases on the first day of life, in 90% on the second day, and by 96 hours of life it is not detected in anyone. With a body weight of 1500-2000 g, by this age PDA persists in 7% of children, with a body weight from 1000 to 1500 g - in 21%, and less than 1000 g - in 42% of newborns [3]. In premature infants weighing less than 1200 g who require intensive care, the duct remains open in 85% of cases. According to some data, regardless of gestational age, FAP complicates 35% of long-term mechanical ventilation in newborns [8].
The PDA closes after birth under the influence of oxygen. However, with hypoxia or hyperoxia in the blood, the amount of reactive oxygen radicals increases, stimulating the accumulation of prostaglandin E in the lungs, which relaxes the muscles of the ductus arteriosus, and it remains open. Due to the difference in pressure in the aorta and pulmonary artery, blood discharge develops from the systemic circulation to the pulmonary circulation, i.e. left-right shunt. Due to lower myocardial distensibility in immature children, especially against the background of relatively large volumes of intravenously administered fluid, end-diastolic pressure in the left atrium and ventricle sharply increases. Pulmonary venous pressure increases secondarily, causing pulmonary congestion or congestive pulmonary hypertension. Hence, the younger the child’s gestational age, the faster his heart failure occurs, but the lower the pressure gradient between the pulmonary artery and the aorta and the smaller the value of the left-right shunt. With a wide arterial duct during diastole, retrograde flow occurs through it in the aorta and cerebral vessels, which can, on the one hand, lead to cerebral ischemia and intracerebral hemorrhage, and on the other hand, reduce perfusion pressure and blood flow in tissues [1, 4, 6]. The main antenatal factors contributing to FAP are: prematurity, prenatal use of nonsteroidal anti-inflammatory drugs, use of enzaprostal during labor, and lack of antenatal prophylaxis for RDS. In the postnatal period, the causes of FAP can be asphyxia at birth, RDS, the use of surfactant, pneumothorax, anemia, excessive infusion therapy, phototherapy (a transient vasodilating effect that weakens the primary constriction of the ductus arteriosus) [1].
Depending on the timing of clinical manifestations, complications of gastric FAP can be divided into early (in the first seven days after birth) and late (in the 2nd - 4th weeks of life).
Early complications: aggravation of the severity of RDS against the background of adequate respiratory therapy, the development of intraventricular hemorrhages, pulmonary hemorrhage, the development of enterocolitis, arterial hypotension, decreased diuresis, metabolic or mixed acidosis [4, 6].
Late complications include the appearance of classic signs of congestive heart failure. A number of researchers have shown that HZ FAP is a factor that increases the risk of developing bronchopulmonary dysplasia and retinopathy of prematurity [3, 4]. Its role in the development of periventricular leukomalacia cannot be ruled out [1].
To exclude GC FAP, a clinical examination and instrumental examination (x-ray and echocardiographic) are performed.
- Clinical signs of FAP are increased cardiac impulse, systolic murmur in the 2-3rd intercostal space to the left of the sternum, racing pulse, decreased diastolic pressure, decreased diuresis, metabolic acidosis, bloody discharge from the trachea, and enlarged liver [1, 5].
- X-ray examination is an auxiliary method. Diagnostically significant signs on a chest x-ray: increased vascular pattern, enlarged left atrium and left ventricle, accentuated interlobar pleura.
- Echocardiography and Dopplerography are the most objective methods for diagnosing gastric FAP. ECHO-CG signs of hemodynamically significant left-to-right blood shunting in premature infants appear on days 1-7 (on average 2-3 days) earlier than clinical ones [1, 6].
Indications for conducting ECHO-CG in newborns within the first 48 hours after birth are: gestation period less than 30 weeks, as well as gestation period 31-34 weeks, if the child is undergoing mechanical ventilation, surfactant was administered, and pulmonary hemorrhage has developed [1]. A repeat study is carried out 48 hours after the previous one, if the premature infant experiences: an increase in oxygen demand or “tightening” of ventilation parameters, the development of mixed/metabolic acidosis, infectious toxicosis, the appearance of systolic murmur [5, 6].
The main criteria for the hemodynamic significance of a PDA:
- Diameter of the ductus arteriosus is more than 1.5 mm in newborns weighing < 1500 g or more than 1.4 mm/kg in newborns weighing > 1500 g
- The presence of left-right shunting of blood along the duct
- Presence of retrograde blood flow in the postductal aorta, constituting >50% of antegrade blood flow
Additional criteria for the hemodynamic significance of a PDA:
- Left atrium to aortic root size ratio (LA/Ao) > 1.4
- Diastolic blood flow velocity in the pulmonary artery > 0.2 m/s
- Left ventricular output to superior vena cava flow (LVO/SVC) ratio > 4
- Ratio of left ventricular end-diastolic size to aortic root (LV/Ao) > 2.1
- Vascular resistance index (IR) in the anterior cerebral artery > 0.8
- Presence of diastolic steal or antegrade flow in the renal and/or mesenteric arteries (IR) = 1.0)
A patent ductus arteriosus can be considered hemodynamically significant if all the main criteria and one of the additional ones are met [1, 5].
In more than 50% of premature infants weighing less than 1000 g, closure of the FAP is undeniably required. There are medical and surgical methods of correction, but uncertainty remains regarding the timing and methods of this treatment:
1. Drug treatment is based on suppressing the synthesis of prostaglandins, one of the main factors that keep the duct open. For this purpose, intravenous administration of non-steroidal anti-inflammatory drugs - cycloxygenase inhibitors (indomethacin, ibuprofen) is used [7].
Mandatory conditions for their use are: a course of treatment in the first 3-4 days of life, no later than 7 days of life, the ability to monitor vital functions. The main side effects of the drug are platelet dysfunction, hyperbilirubinemia (displacement of bilirubin from the complex with albumin) and renal dysfunction (blockade of prostaglandin synthesis in the kidneys), therefore, hyperbilirubinemia more than 200 µmol/l, ΟΠΗ and hemorrhagic syndrome are considered contraindications to its use. To prevent oliguria, indomethacin is recommended to be used with furosemide (5 mg/kg) or dopamine (3-4 μg/(kg min) [8].
2. Surgical ligation of the PDA is indicated in the absence of effect from 2-time use of drug therapy, with a wide arterial duct with prolonged pneumonia and in children older than 3 weeks. Direct indications are formulated based on progressively increasing PaCo2 up to 60 mm Hg. Art., a growing need to increase Fio2 to maintain Po2 above 80% and mechanical ventilation for more than 7-9 days. The optimal period for ligation of the ductus arteriosus is the second week of life [4].
Thus, hemodynamically significant FAP remains an acute problem in the care and treatment of premature infants. Timely diagnosis and rational therapy have a huge impact on the prognosis of the life and health of such children.
E.V. Volanyuk
Kazan State Medical Academy
Volyanyuk Elena Valerievna – Candidate of Medical Sciences, Assistant at the Department of Pediatrics and Neonatology
Literature:
1. Degtyarev D.N., Kryuchko D.S., Feoktistova E.V. Tactics for the management of premature infants with a hemodynamically significant functioning ductus arteriosus. Draft methodological recommendations. Moscow, RASPM, 2009. - 22 p.
2. Prakhov A.V. Neonatal cardiology. N. Novgorod: Nizhny Novgorod State Publishing House. honey. Academy, 2008. - 388 p.
3. Prakhov A.V., Gaponenko V.A., Ignashina E.G. Heart disease of the fetus and newborn child. N. Novgorod: Nizhny Novgorod State Publishing House. honey. Academy, 2001. - 188 p.
4. Razumovsky A.Yu., Luzhina M.Yu., Feoktistova E.V. Hemodynamically significant patent ductus arteriosus in low birth weight newborns: a surgeon's view. Moscow, Questions of Practical Pediatrics, 2007. - T. 2, No. 1. - P. 27-32.
5. Vinogradova I.V., Krasnov M.V., Ivanova N.N. Features of the state of the cardiovascular system in newborns with extremely low body weight. Medical almanac. - 2009 No. 4, - pp. 103-106.
6. Degtyarev D.N., Malysheva E.V., Vakueva T.I. Features of postnatal adaptation of premature infants with concomitant perinatal pathology, complicated by the presence of a hemodynamically significant functioning ductus arteriosus. Questions of Practical Pediatrics, 2006. 1 (1): P. - 16-20.
7. Markus Sperandio et al. Effectiveness and Side Effects of an Escalating, Stepwise Approach to Indomethacin Treatment for Symptomatic Patent Ductus Arteriosus in Premature Infants Below 33 Weeks of Gestation, Pediatrics. December 2005; 116: 1361-1366.
8. Bacalari E., Claure A., Consalos A. Patent ductus arteriosus and respiratory outcome in premature infants. Biol Neonate 2005; 88, 192-201.