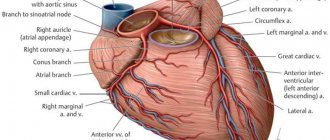
Translation into English of the names and abbreviations of the arteries of the heart
Serzhenko Nadezhda Medical translation agency "Medtran"
Translation of coronary angiography results raises many questions even for an experienced medical translator. The topic is quite difficult for a number of reasons:
- Complex anatomy of the coronary arteries
- A huge variety of anatomical options
- Abundance of terms and abbreviations
- Lack of a unified nomenclature
- Almost every artery has several synonymous names in both Russian and English
A standard situation for a medical translator is that it is necessary to translate an extract from the patient’s medical history, which contains a description of the results of coronary angiography, or, among other medical documents, there is a protocol for angiography of the coronary arteries. If the translator does not have experience in such translations, then two paragraphs of such text may take several hours.
Many translation problems arise due to synonyms (different variants of the names of the same artery). In order to correctly translate the variant name of an artery encountered in a medical history (which does not always have an unambiguous analogue in English), it is often necessary to search and compare the description of the anatomical structure in Russian with the description in English to make sure that the selected English term corresponds to the Russian name for the artery.
To avoid distortion of meaning when translating the names of anatomical formations and angiographic terms into English, we strongly recommend using analogues that are as close as possible to the Russian original. Despite the fact that the same artery may have several names in both Russian and English, the use of synonyms should be kept to a minimum, because this makes verification difficult and is a potential source of error. The translation of a medical text must convey the content of the source text as closely as possible, and the translator does not have the right to interpret the available information at his own discretion. However, for correct translation it is necessary to understand the basics of angiography and know the anatomy of the coronary arteries.
The terms and explanations below are intended to facilitate the work of the translator and help avoid errors when translating angiocoronary angiograms.
Coronary anatomy
Cardiac veins
The vessels that remove deoxygenated blood from the heart muscles are known as cardiac veins. These include the great cardiac vein, the middle cardiac vein, the small cardiac vein, the smallest cardiac veins, and the anterior cardiac veins. The veins of the heart carry blood with low oxygen levels from the myocardium to the right atrium. Most of the blood from the coronary veins returns through the coronary sinus. The anatomy of the veins of the heart is very variable, but it is usually formed by the following veins: the cardiac veins that pass into the coronary sinus: the great cardiac vein, the middle cardiac vein, the small cardiac vein, the posterior vein of the left ventricle, and the veins of Marshall. Cardiac veins that run directly into the right atrium: the anterior cardiac veins, the smallest cardiac veins (Theban veins).[5]
Coronary arteries
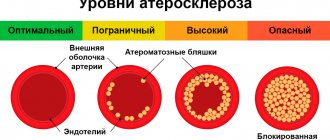
The vessels that deliver oxygen-rich blood to the myocardium are the coronary arteries. When the arteries are healthy, they are able to self-regulate to maintain coronary blood flow at a level that meets the needs of the heart muscle. These relatively narrow vessels are usually affected by atherosclerosis and can become blocked, causing angina or acute cardiovascular disease. Coronary arteries that run deep into the myocardium are called subendocardial. The coronary arteries are classified as the “end circulation” because they represent the only source of blood supply to the myocardium; There is very little excess blood supply, which is why blockage of these vessels can be so critical.
The aortic sinuses
The aortic sinuses, or sinuses of Valsalva, are the pockets between the semilunar valves of the aorta and its wall. The names of the sinuses correspond to the names of the coronary arteries branching from them: the right coronary artery departs from the right coronary sinus, the left coronary artery departs from the left coronary sinus, and the posterior sinus of Valsalva is called non-coronary (non-coronary sinus), since the coronary arteries do not depart from it. The aortic sinuses facing the pulmonary artery are called “facing aortic sinuses”.
The aortic valve has three leaflets, each having a cusp or cup-like configuration. These are known as the left coronary cusp, the right coronary cusp and the posterior non-coronary cusp. Just above the aortic valves there are anatomic dilations of the ascending aorta, also known as the sinus of Valsalva. The left aortic sinus gives rise to the left coronary artery. The right aortic sinus which lies anteriorly, gives rise to the right coronary artery. The non-coronary sinus is positioned on the right side.
The aortic sinuses that are adjacent to the pulmonary valve (facing the pulmonary valve) are described as the 'facing' aortic sinuses.
Right aortic sinus (1st facial sinus, right sinus of Valsalva). Right coronary sinus, right anterior sinus, right sinus of Valsalva, right-facing sinus (anat.: anterior aortic sinus).
The right coronary artery arises from the right aortic sinus.
Left aortic sinus (2nd facial sinus, left sinus of Valsalva). Left coronary sinus, left anterior sinus, left sinus of Valsalva, left-facing sinus (anat.: left posterior aortic sinus).
The left aortic sinus is the origin of the left coronary artery.
Non-coronary aortic sinus (non-facial aortic sinus, posterior sinus of Valsalva). Non-coronary aortic sinus, posterior sinus of Valsalva, non-facing aortic sinus (anat.: right posterior aortic sinus, sinus aortae posterior dexter).
Non-facing aortic sinus is that aortic sinus that does not face the pulmonary artery. The first one from it, when oriented counterclockwise, is called the “1st facial sinus”, and the next one is the “2nd facial sinus” (Terminology developed by a group of researchers from Leiden University (A. Gittenberger: de Groot et al., 1983)).
Non-coronary aortic sinus is an aortic sinus from which the coronary arteries do not arise. Typically (in most people), the posterior (non-facial) aortic sinus is also non-coronary. However, there are many variations in the anatomical structure of the coronary arteries, both normal and pathological, so it is important to understand the difference between the terms “facial” and “coronary” (see comments on the figure).
A diagram explaining the definition of terms.
a: the nonfacial sinus of the aorta (H) is darkened, 1 and 2 - the 1st and 2nd facial sinuses (light), from which the coronary arteries arise;
b: in the case of the origin of the coronary arteries from one facial sinus of the aorta, the second (shaded) may turn out to be non-coronary. Thus, the terms “facial” and “coronal”, “non-facial” and “non-coronal” are not synonymous. Source: Bokeria L. A., Berishvili I. I. Surgical anatomy of the coronary arteries. M.: Publishing house NTsSSKh im. A. N. Bakuleva RAMS, 2003.
Function
Nutrition of the papillary muscles
The papillary muscles attach the mitral valve (the valve between the left atrium and the left ventricle) and the tricuspid valve (the valve between the right atrium and the right ventricle) to the wall of the heart. If the papillary muscles do not function properly, the mitral valve may leak during left ventricular contraction. This causes some of the blood to move "backwards", from the left ventricle to the left atrium, rather than forward to the aorta and the rest of the body. This leakage of blood into the left atrium is known as mitral regurgitation. Similarly, blood may leak from the right ventricle through the tricuspid valve into the right atrium, which is described as tricuspid regurgitation or tricuspid regurgitation.
The anterolateral papillary muscle most often receives two blood supplies: the left anterior descending (LAD) artery and the left circumflex artery (LCX).[4] Therefore, it is more resistant to coronary diseases. ischemia (lack of oxygen-rich blood). On the other hand, the posteromedial papillary muscle is usually supplied only by the PDA.[4] This makes the posteromedial papillary muscle significantly more susceptible to ischemia. The clinical significance of this is that myocardial infarction involving the CPC is more likely to cause mitral regurgitation.
Changes in diastole
During compression, the ventricular myocardium (systole), subendocardial coronary vessels (vessels that enter the myocardium) are compressed due to high ventricular pressure. This compression results in transient retrograde flow (ie, blood flows back toward the aorta), which further impedes myocardial perfusion during systole. However, the epicardial coronary vessels (vessels that run along the outer surface of the heart) remain open, causing blood flow in the subendocardium to stop during ventricular contraction. As a result, myocardial perfusion occurs primarily during cardiac relaxation (diastole), when the subendocardial coronary vessels are open and under lower pressure. Flow never reaches zero in the right coronary artery because the pressure in the right ventricle is below diastolic blood pressure.
Changes in oxygen demand
The heart regulates the amount of vasodilation or vasoconstriction of the coronary arteries due to the heart's need for oxygen. This makes it difficult to fill the coronary arteries, the pressure remains the same. Impaired oxygen delivery, caused by decreased blood flow before the heart's demand for oxygen increases, leads to tissue damage. ischemia, a state of oxygen deficiency. Short-term ischemia is associated with severe chest pain known as angina. Severe ischemia can lead to death of the heart muscle from hypoxia, for example, during a myocardial infarction. Chronic mild ischemia causes weakening of heart contractions, known as myocardial hibernation.
In addition to metabolism, the coronary bloodstream has unique pharmacological characteristics. Among them, its reactivity to adrenergic stimulation stands out.
Coronary arteries (coronary arteries)
The term “coronal” comes from the Greek “wreath, crown,” and “coronary” comes from the Latin “crown.” Both terms refer to the arteries of the heart and are absolute synonyms.
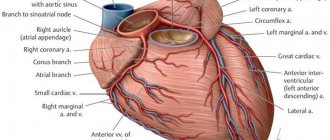
Right coronary artery and its branches
RCA - right coronary artery (RCA - right coronary artery).
Right coronary artery (RCA), right main coronary artery. The right coronary artery arises from the right aortic (1st facial) sinus, most often in the form of a trunk running posteriorly along the right atrioventricular groove, encircling the tricuspid valve, and heading towards the cross of the heart. The RCA typically arises from the right sinus of Valsalva (RSV) of the ascending aorta, passes anteriorly and to the right between the right auricle and the pulmonary artery and then descends vertically in the right atrioventricular sulcus. When the RCA reaches the acute margin of the heart, it turns to continue posteriorly in the sulcus onto the diaphragmatic surface and base of the heart.
Single-plane anatomical diagram of the structure of the coronary arterial tree and the cardiac complex. A - left coronary artery (LCA) system, B: right coronary artery (RCA) system.
1 - first facial sinus of the aorta, 2 - second facial sinus of the aorta.
A - aorta, LA - pulmonary artery, ASA - right atrial appendage, LAA - left atrial appendage, LAD - anterior interventricular branch, OB - circumflex branch, DV - diagonal branch, VTK - obtuse edge branch, ASU - sinus node artery, CA - conal artery, BOK - branch of the acute edge, a.AVU - artery of the atrioventricular node, ZAMV - posterior interventricular branch. Source: Bokeria L. A., Berishvili I. I. Surgical anatomy of the coronary arteries.
M.: Publishing house NTsSSKh im. A. N. Bakuleva RAMS, 2003. CA – conal artery (branch of the conus arteriosus). Conus branch, infundibular branch, conus arteriosus branch.
The conus artery is the first major branch of the right coronary artery, but it can arise as an independent orifice from the 1st facial sinus of the aorta. The conus artery supplies the conus arteriosus and the anterior wall of the right ventricle and may participate in the blood supply to the anterior interventricular septum.
The artery has a variable distribution, but usually supplies a region of the anterior interventricular septum and the cone of the main pulmonary artery (hence its name). Although an acute occlusion of the tiny artery has been shown to result in ST elevation, another more important role it serves in pathophysiology is that of a route of collateral circulation. The conus artery has been shown to collateralise with the more distal acute marginal branch in RCA stenosis/obstruction, and collateralise with the left anterior descending artery (LAD) in LAD stenosis/obstruction, providing a potentially vital collateral pathway.
ASU – artery of the sinus node (branch of the sinus node, artery of the sinoatrial node (a.SNA), branch of the sinoatrial node). Sinoatrial nodal artery (SANa), sinus node artery, sinoatrial nodal branch, SA nodal artery, right SA node branch.
The sinus node artery is the main artery providing blood supply to the sinoatrial node, and its damage leads to irreversible disturbances in heart rhythm. The ASU is also involved in the blood supply to most of the interatrial septum and the anterior wall of the right atrium.
The artery of the sinus node, as a rule, arises from the dominant artery (see types of blood supply to the heart). With the right type of blood supply to the heart (in approximately 60% of cases), the ASU is the second branch of the right coronary artery and departs from the RCA opposite the origin of the conus artery, but can also arise from the 1st facial sinus independently. With the left type of blood supply to the heart, the artery of the sinus node arises from the circumflex branch of the left artery.
The sinoatrial nodal artery (SANa) supplies blood to the sinoatrial node (SAN), Bachmann's bundle, crista terminalis, and the left and right atrial free walls. The SANA most frequently originates from either the right coronary artery (RCA) or the left circumflex branch (LCX) of the left coronary artery (LCA).
Kugel's artery (great auricular artery). Kugel's artery, atrial anastomotic branch, Kugel's anastomotic branch (Lat.: arteria auricularis magna, arteria anastomotica auricularis magna, ramus atrialis anastomoticus).
Kugel's artery is an anastomosing between the systems of the right and left coronary arteries. In 66% of cases, it is a branch of the LCA or the SPU artery arising from it, in 26% - a branch of both coronary arteries or the SPU artery, arising from them simultaneously, and in 8% of cases - a branch of smaller branches arising from the right and left coronary arteries arteries to the atria.
ADVa. – adventitial artery.
The third branch of the PCA. The adventitial artery can be a branch of the conus artery or arise independently from the aorta. It goes up and to the right and lies on the anterior wall of the aorta (above the sinotubular junction), heading to the left and disappearing into the fatty sheath surrounding the great vessels.
AOK - artery of the acute edge (right marginal artery, right marginal branch, branch of the acute edge). Acute marginal artery, right marginal branch, right marginal artery.
The acute margin artery is one of the largest branches of the RCA. It descends from the RCA along the sharp right edge of the heart and forms powerful anastomoses with the LAD. Participates in nutrition of the anterior and posterior surfaces of the acute edge of the heart.
a.PVH - artery of the atrioventricular node (artery of the atrioventricular node). AV node artery, AV nodal artery (branch), AVN artery.
The artery (branch) of the atrioventricular node arises from the RCA in the area of the cross of the heart.
Posterior interventricular branch, posterior interventricular artery, posterior descending artery. Posterior descending artery (PDA), posterior interventricular artery (PIA).
The posterior interventricular branch can be a direct continuation of the RCA, but is more often a branch of it. It passes in the posterior interventricular groove, where it gives off posterior septal branches, which anastomose with the septal branches of the LAD and supply the terminal sections of the conduction system of the heart. With the left type of blood supply to the heart, the LAD receives blood from the left coronary artery, departing from the circumflex branch or LAD.
Posterior septal branches, inferior septal (septal) branches. Posterior septal perforators, posterior septal (perforating) branches.
The posterior (“lower”) septal branches arise from the LAD in the posterior interventricular groove, which anastomose with the “anterior” septal (septal) branches of the LAD and supply the terminal sections of the conduction system of the heart.
Posterolateral branch of the left ventricle (posterolateral left ventricular branch). Right posterolateral artery, posterolateral artery (PLA), posterior left ventricular (PLV) artery.
In approximately 20% of cases, the RCA forms the posterolateral branch of the left ventricle.
Left coronary artery and its branches
LCA – left coronary artery (LCA – left coronary artery, OS LCA – main trunk of the left coronary artery, trunk of the left coronary artery, main trunk of the left coronary artery).
Left coronary artery (LCA), left main coronary artery (LMCA), main stem of the left coronary artery, left main stem. As a rule, the left coronary artery arises with one trunk from the left (2nd facial) sinus of the aorta. The left artery trunk is usually short and rarely exceeds 1.0 cm, bends around the pulmonary trunk from behind, and at the level of the nonfacial sinus of the pulmonary artery is divided into branches, usually two: LAD and OB. In 40-45% of cases, the LCA, even before dividing into the LAD and OB, can give off the artery that supplies the sinus node. This artery can also arise from the OB of the LCA. The LMCA typically originates from the left sinus of Valsalva (LSV), passes between the right ventricle outflow tract and the left auricle and quickly bifurcates into the LAD and the LCX arteries. Its normal length varies from 2 mm to 4 cm.
Trunk of the left coronary artery - division into LAD and OB
Source: Coronary anatomy and anomalies.
Robin Smithuis and Tineke Willems. Radiology department of the Rijnland Hospital Leiderdorp and the University Medical Center Groningen, the Netherlands. LAD – anterior interventricular branch (anterior descending artery, left anterior descending artery, left anterior interventricular artery). Left anterior descending artery (LAD), anterior interventricular artery (AIA), anterior descending coronary artery.
The anterior interventricular branch arises from the left artery trunk and follows down along the anterior interventricular septum. In 80% of cases, it reaches the apex and, going around it, moves to the back surface of the heart.
Right ventricular branch
The right ventricular branch is a non-permanent branch of the LAD and arises from the LAD on the anterior surface of the heart.
Septal branches of the LAD (septal branches of the LAD, “anterior” septal branches). Septal perforators, the septal branches (arteries), the septal perforator branches, perforator branches.
The septal branches of the LAD vary greatly in size, number, and distribution. The large first septal branch of the LAD (also known as the anterior septal branch, anterior septal artery, 1st SV) supplies the anterior part of the interventricular septum and participates in the blood supply to the conduction system of the heart. The remaining septal branches of the LAD (“anterior”) are usually smaller in size. They communicate with similar septal branches of the cervical vein (“lower” septal branches).
Diagonal branch of the LAD (DV - diagonal branches, diagonal arteries). Diagonal arteries (DB - diagonal branches), the diagonals.
Diagonal branches arise from the LAD and follow along the anterolateral surface of the left ventricle. There are several of them, designated by numbers from top to bottom: 1st, 2nd, 3rd diagonal arteries (branches). They supply blood to the anterior part of the left ventricle. The first diagonal branch is usually one of those branches that feeds the apex.
Median artery (intermediate branch) Intermediate artery, intermediate branch, ramus intermedius (RI), median (intermedian) branch.
In approximately 20-40% of cases, the LMCA trunk is divided not into two, but into three branches: the “diagonal branch” departs from the LMCA trunk along with the OB and LAD, and in this case it is called the median artery. The median artery is the equivalent of the diagonal branch and supplies the free wall of the left ventricle.
The ramus intermedius (RI) is an artery arising between the left anterior descending artery (LAD) and the CX. Some call it a high diagonal (D) or a high obtuse marginal (OM) artery.
In this normal variant, the LMCA can trifurcate into a LAD, a LCX and a ramus intermedius. The ramus intermedius typically supplies the lateral and inferior walls, acting as a diagonal or obtuse marginal branch, while the arteries that usually supply this territory are small or absent.
Median artery
Source: Coronary anatomy and anomalies.
Robin Smithuis and Tineke Willems. Radiology department of the Rijnland Hospital Leiderdorp and the University Medical Center Groningen, the Netherlands. OB – circumflex branch of the left coronary artery, circumflex artery. Left circumflex coronary artery (LCX), circumflex artery (CX, CA).
The circumflex branch is a large branch of the LMCA; in some cases it can branch off from the left aortic sinus independently. It follows along the left atrioventricular groove, goes around the mitral valve, the left (obtuse) edge of the heart, and passes to its diaphragmatic surface. Typically, the OB gives off the left fragment of the Kugel artery, and although more often it does not reach the sinus node, in 10-12% of cases the artery of the sinus node can be formed by this branch.
VTK - branch of the obtuse edge (left marginal (marginal) branch, artery of the obtuse edge). Obtuse marginal artery, the obtuse marginals, the obtuse marginal (OM) branch, the left marginal arteries.
The obtuse margin branch is the largest branch of the OB and can arise both from the beginning of the OB and at the level of the obtuse margin. This is a very important branch involved in feeding the free wall (its anterior and posterior surfaces) of the LV along its lateral edge. Very often, the OM system is generally represented by a large VTK and unexpressed OM. There can be several branches of the obtuse edge, then they are designated by numbers as they extend from left to right: 1st, 2nd, 3rd.
Left atrial branch.
The left atrial branch may arise from the OB. It nourishes the lateral and posterior surface of the left atrium.
Posterolateral branch (left ventricular branch). Posterolateral branch (PLB).
The posterolateral branch is most often the terminal branch of the OB, but the origin of this branch, as well as the LMVA and the artery of the atrioventricular node from the OB LCA, is determined by the dominance of the right or left coronary artery.
Discussion
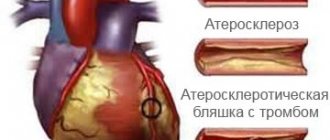
The first method of surgical treatment of atherosclerotic lesions of the arteries of the heart was coronary endarterectomy, introduced into practice by C. Bailey et al. [9] in 1957. However, the lack of a general protocol for performing the operation, indications for it and algorithms for postoperative patient management, unsatisfactory long-term and often immediate results did not allow CE to be widely introduced into the arsenal of cardiac surgeons [10, 11]. For the first time, the technique of open CE with the formation of an autovenous patch at the site of an extended arteriotomy and mammary-coronary bypass of the LAD was described by P. Fundaro` et al. [12] in 1987. But the use of an extended coronary anastomosis using the type of bypass grafting of the LAD with IAV without a venous patch was first described by N. Shapira et al. [13] in 1988. The authors noted that this technique for creating a mammary-coronary anastomosis can be used for small arterial defects. Tissue tension should be avoided as this can lead to leakage of the anastomosis or even rupture of the shunt. P. Myers et al. [14] in 2012 presented their experience of 224 EC from the LAD with plastic surgery of the arteriotomy opening with an autovenous patch (101 patients) and subsequent formation of a mammary-coronary anastomosis or using only the LVTA with an extended coronary anastomosis using the type of bypass grafting (123). There was no statistically significant difference in early postoperative outcomes. Five- and 10-year survival rates were 78.6 and 45.4% in the autovenous patch group and 87.1 and 49.4% in the bypass grafting group. The authors concluded that these techniques can be safely used for diffuse LAD lesions, but it should be noted that there is a tendency for better results when using only the IAV. In our study, in all cases, a skeletonized left internal mammary artery was used to form a mammary-coronary anastomosis using the type of bypass grafting.
FE can be performed using an open or closed method. Open endarterectomy involves dissection of the coronary artery along its entire length, removal of ASP from both the main trunk and lateral branches under visual control, followed by plastic surgery of the arteriotomy opening and bypass surgery. Closed endarterectomy begins with a small arteriotomy incision, then the plaque is carefully separated and removed from the affected vessel with gentle traction. With the closed method, one or more additional incisions may be necessary to completely remove the plaque, especially from the side branches.
Most authors believe that CE from the LAD should be performed exclusively using the open method. This is explained by the fact that the ASP of this localization has a more delicate core and a greater tendency to rupture during traction [15]. In addition, it should be remembered that the LAD gives multidirectional lateral and septal branches, the orifices of which can be blocked with inadequate K.E. The question of the best method has been analyzed by many authors. Thus, H. Nishi et al. [16] when comparing complications and mortality in 127 patients did not find statistically significant differences in the two groups. However, the best angiographic results of shunt patency were in patients with an open CE technique. In our study, all operations were performed by a single surgeon. For endarterectomy, a combined technique was used, combining the advantages of both open and closed techniques. By extending the standard arteriotomy incision by 1-2 cm, we obtained sufficient visual control, while reducing the degree of traction of the ASP, and therefore reducing the risk of rupture of the distal part of the plaque. The average length of the arteriotomy after CE was 4.3±0.6 cm, which allowed us to form an anastomosis with the LVGA without the risk of high tension.
As a result of CE, the surface of the artery is deprived of endothelium, which, upon contact with blood, leads to thrombus formation on the walls of the vessel. This process underlies the pathogenesis of shunt occlusions after endarterectomy. In order to prevent negative events in the absence of an intense rate of discharge through the drains, patients were treated with two-component antiplatelet therapy (aspirin 100 mg + clopidogrel 75 mg), as well as Clexane 0.4 for 5 days after surgery. There were no cases of reoperation due to bleeding in either group. The average postoperative blood loss through drainages was 395±285 and 214±72 ml, which coincides with the data of the available literature [17, 18]. There is still no consensus among authors regarding the use of one- or two-component therapy. A study published by M. Russo et al. [19] in 2021, showed that the immediate postoperative results are slightly better in the group of single-component therapy (aspirin 100 mg): lower hospital mortality (0 and 3.8%), the incidence of perioperative MI (15 and 11.5%). However, in the long-term period, coronary mortality and the incidence of repeated coronary interventions and MI were lower in the two-component therapy group. Recent studies show that the results of surgical treatment of patients with multiple diffuse lesions of the coronary bed are comparable to the results of myocardial revascularization in patients with local lesions of the coronary arteries and are much better than in patients undergoing conservative therapy [14, 20-23 ]. According to the findings of a number of authors, mortality after myocardial revascularization in combination with CE or SLP varies from 1.5 to 5.5%. In our study, the overall hospital mortality rate was 1.7%, and the mid-term mortality rate was 3.5%.
Types of blood supply to the heart Type of dominance (Coronary dominance)
The myocardial distribution of the coronary arteries is somewhat variable, but the right coronary artery (RCA) almost always supplies the right ventricle (RV), and the left coronary artery (LCA) supplies the anterior portion of the ventricular septum and anterior wall of the left ventricle (LV). The vessels that supply the remainder of the LV vary depending on the coronary dominance. Read More: https://www.ajronline.org/doi/10.2214/AJR.06.1295
The posterior descending artery (PDA) runs in the posterior interventricular groove and supplies the inferior wall and inferior third of the interventricular septum. The artery that supplies the PDA and a posterolateral branch determines the coronary dominance, so there can be three situations:
Right type of blood supply to the heart. Right dominant heart, RCA dominance, right-dominance, right dominant circulation.
Most hearts (approximately 70% of cases) are right dominant where the posterior descending artery (PDA) and the posterolateral branch are supplied by the right coronary artery (RCA). In this instance, the RCA supplies the inferoseptal and inferior segments of the left ventricle.
Left type of blood supply to the heart. Left dominant heart, LCA dominance, left dominant circulation.
In 10% of cases the PDA is supplied by the LAD or LCx.
Mixed type of blood supply to the heart. Codominant heart, codominance.
In 20% of cases a single or duplicated PDA and posterolateral branches are supplied by branches of both the RCA and LAD/LCx.
Dominant right coronary artery and its branches.
Right type of blood supply to the heart. Schematic structure of the right coronary artery (antero-posterior projection). AV = atrioventricular, PDA = posterior descending artery, RCA = right coronary artery, RV = right ventricular, SA = sinoatrial.
Dominant left coronary artery and its branches.
Schematic structure of the left coronary artery with the left type of blood supply to the heart (left anterior oblique projection).
AVGA = atrioventricular groove artery, PDA = posterior descending artery. Source: Sunil Kini, Kostaki G. Bis, and Leroy Weaver. Normal and Variant Coronary Arterial and Venous Anatomy on High-Resolution CT Angiography. American Journal of Roentgenology 2007 188:6, 1665-1674.
Literature and Internet resources:
- Bokeria L. A., Berishvili I. I. Surgical anatomy of the coronary arteries. M.: Publishing house NTsSSKh im. A. N. Bakuleva RAMS, 2003. + 297 pp., illustration.
- Nerantzis, C. E., Marianou, S. K., Koulouris, S. N., Agapitos, E. B., Papaioannou, J. A., & Vlahos, L. J. (2004). Kugel's artery: an anatomical and angiographic study using a new technique. Texas Heart Institute journal, 31(3), 267–270.
- Vikse, J., Henry, B. M., Roy, J., Ramakrishnan, P. K., Hsieh, W. C., Walocha, J. A., & Tomaszewski, K. A. (2016). Anatomical Variations in the Sinoatrial Nodal Artery: A Meta-Analysis and Clinical Considerations. PloS one, 11(2), e0148331. doi:10.1371/journal.pone.0148331.
- Rahalkar, A. M., & Rahalkar, M. D. (2009). Pictorial essay: Coronary artery variants and anomalies. The Indian journal of radiology & imaging, 19(1), 49–53. doi:10.4103/0971-3026.45345.
- Villa, A. D., Sammut, E., Nair, A., Rajani, R., Bonamini, R., & Chiribiri, A. (2016). Coronary artery anomalies overview: The normal and the abnormal. World journal of radiology, 8(6), 537–555. doi:10.4329/wjr.v8.i6.537.
- Sunil Kini, Kostaki G. Bis, and Leroy Weaver. Normal and Variant Coronary Arterial and Venous Anatomy on High-Resolution CT Angiography. American Journal of Roentgenology 2007 188:6, 1665-1674
- Siew Yen Ho. Structure and anatomy of the aortic root. European Journal of Echocardiography, Volume 10, Issue 1, January 2009, Pages i3–i10.
- radiopaedia.org
- Robin Smithuis and Tineke Willems. Coronary anatomy and anomalies. Radiology department of the Rijnland Hospital Leiderdorp and the University Medical Center Groningen, the Netherlands. www.radiologyassistant.nl
Copyright © 2008-2019 Medtran.ru. All rights reserved.
branches
The following are the named branches of the coronary circulation in the right dominant heart:
- Aorta Left coronary artery/Left main coronary artery (LMCA) Left circumflex artery (LCX) Obtuse marginal artery #1 (OM1)
- Obtuse marginal artery #2 (OM2)
- Diagonal artery #1
- Atrioventricular nodal branch
Rare complications:
- Myocardial infarction or heart attack.
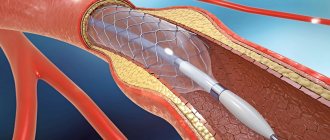
- Coronary artery rupture or dissection. Damage requires coronary artery bypass grafting or emergency stent graft implantation.
- Impaired kidney function. The contrast agent administered during stenting and angioplasty has a detrimental effect on the kidneys. If the patient already has problems with their functioning, the doctor prescribes medications for protection.
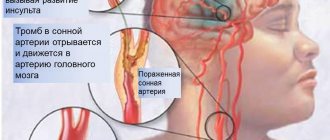
- Stroke. During surgery, blood clots may form on the catheter. If they fragment, break off and travel to the brain, the patient will suffer a stroke. To reduce the risk of developing this complication, blood thinning medications are used.
- Arrhythmia. Manifested by heart rate disturbances: bradycardia or tachycardia. This is a temporary complication that goes away after the medication is administered. A temporary pacemaker is rarely required.