- Home /
- Branches /
- CT scan /
- CT with determination of coronary calcium
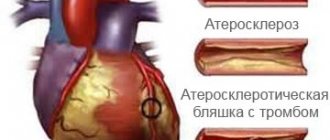
CT scan with determination of coronary calcium is a painless way to determine the risk of developing atherosclerosis. The method is based on measuring the calcium content in the plaques of the vessel walls. If a large amount of calcium is detected in the coronary arteries, this indicates the presence of atherosclerotic plaques and, as a result, ischemic changes in the heart. At the clinical center on Yauza, the test is carried out using the latest equipment, which makes it possible to predict the development of pathology and promptly select effective methods of prevention and treatment.
1.What is coronary calcium screening?
Coronary calcium screening is a special X-ray test (computed tomography) that checks for calcium deposits in the coronary arteries. Coronary calcium screening can detect heart disease early and determine its severity.
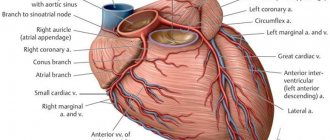
The coronary arteries deliver blood to the heart. Typically, the coronary arteries do not contain calcium. Coronary calcium may be a sign of coronary heart disease (CHD).
A CT scan allows you to take multiple layers of images of the heart. These images can be viewed on a computer or printed.
A must read! Help with treatment and hospitalization!
Material and methods
An analysis of the literature was carried out in the search engines Pubmed, GoogleScholar, Scopus and RSCI with a list of keywords “coronary artery calcification”, “coronary artery calcification score”, “coronary artery calcification”, “calcium index”.
This review included 51 papers from studies conducted from 1990 to 2021 that described the use of various scales to assess the severity of coronary artery disease, as well as studies that examined the results of direct myocardial revascularization for coronary artery calcification. Pathogenesis of coronary artery calcification
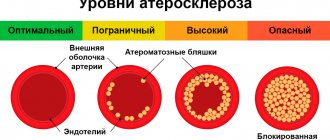
For a long time, it was believed that the mechanisms of development of CCA represent a passive degenerative process and some final stage of atherosclerosis, which was confirmed by the correlation of the degree of calcification with the age of the patient [5]. Modern researchers are inclined to believe that arterial calcification is an active process, which is based on mechanisms regulating calcium metabolism, in particular the mechanisms of growth and bone formation [6]. There is a concept that considers atherosclerosis as a chronic inflammatory process that induces osteogenic differentiation of vascular smooth muscle cells (VSMCs), which leads to CCA [7]. There is no doubt that plaque calcification begins already at the stage of formation of lipid bands and only progresses at all other stages of atherogenesis. It is currently believed that the mechanism of coronary artery calcification is similar to the process of bone tissue formation.
There are two recognized morphological types of CCA: atherosclerotic calcification with predominant damage to the intima and calcification of the medial layer of the arteries. In the first type, osteogenic differentiation of VSMCs is induced by inflammatory mediators and lipids of atherosclerotic plaques [7]. The second scenario is associated with advanced age, diabetes, and chronic kidney disease (CKD). Previously considered a benign process, medial calcification contributes to increased arterial stiffness, which increases the risk of adverse cardiovascular events [8]. Calcification of the coronary arteries in both variants leads to a decrease in the elasticity of the artery wall, pathological vasomotor responses and impaired myocardial perfusion [9].
It is known that a plaque with a calcified cap is much more stable and resistant to rupture than a “soft” plaque and even a normal vascular wall [10]. Apparently, this conclusion is applicable only in the case of homogeneous calcification. According to some studies, in patients with acute coronary syndrome, multiple small calcium inclusions, called “motley” or “spotty”, are detected, while in chronic coronary artery disease larger and uniform calcifications are determined [11]. The zone formed between the calcified cap and the non-calcified vascular wall is considered to be a potential rupture zone [12]. When performing PCI, there is a high probability of developing dissection in such a zone; Large plaques with obvious “patchy” calcification have been described to have a tendency to rupture [13].
Osteopontin, osteoprotegerin, RANKL, fetuin-A, and bone morphogenetic proteins play a role in the development of calcification. All these substances are produced in the vascular wall during the progression of atherosclerosis; their participation in the regulation of plaque calcification has been proven. A number of studies have revealed a relationship between the level of osteopontin and the level of coronary calcium measured using multislice computed tomography (MSCT) [14]; It was proposed to consider osteopontin as an independent risk factor for cardiovascular events. It has been shown that osteopontin and bone morphogenetic protein type 7 determine the differentiation of VSMCs into osteoblast-like cells and induce calcium deposition in the vascular wall, and osteoprotegerin plays an inhibitory role in vascular calcification [15-17]. Normally, there is a balance between the regulators of calcification, but CCA can develop when the balance is disturbed in favor of the inducers. The exact mechanisms of this process remain to be studied.
Prevalence of coronary artery calcification
The prevalence of CCA depends on age and gender. According to most authors, in the age group over 70 years, CCA occurs in more than 90% of men and more than 67% of women [18]. A high risk of developing CCA is observed in patients with high body mass index, high blood pressure, dyslipidemia, hyperglycemia, family history, CKD, high levels of fibrinogen and elevated levels of C-reactive protein [19], i.e., with all generally recognized risk factors for atherosclerosis .
Diagnostics
Computed tomography (CT) is the mainstay of noninvasive diagnosis of CCA; The method is able to quantify calcification and has high sensitivity and specificity. Multislice computed tomography (MSCT) is based on the measurement and computer processing of the difference in attenuation of X-ray radiation by tissue density. To quantify the degree of CCA, a calculated indicator is used - the calcium index (CI). CI correlates with the severity of coronary atherosclerosis, the presence of hemodynamically significant coronary artery stenoses and the risk of developing coronary complications [20]. The CI calculation is used according to the method proposed in 1990 by A. Agatston et al. [21]: CI is calculated by multiplying the area of calcified lesion of the coronary artery by the conditional density factor. The density factor is calculated from the peak density of the calcified zone, expressed in Hounsfield units (G. Hounsfield) - HU. It is taken as 1 unit. for calcifications with a density of 130-199 HU, for 2 units. — for calcifications with a density of 200-299 HU, for 3 units. - for calcifications with a density of 300-399 HU and for 4 units. - for calcifications with a density of 400 HU or more. So, for example, when detecting calcification with an area of 6 mm2 with a peak density of 265 HU, the CI will be 12 units. (6×2), and for calcification of the same area, but with a peak density of 432 HU - already 24 units. (6x4). Total K.I. is calculated as the sum of the indices determined on all tomographic slices. Algorithms for calculating volumetric CI and calculating the mass of calcium phosphate have also been proposed [22]. The American College of Cardiology and the American Heart Association (2010) consider it useful to non-invasively measure the degree of CAC to assess the risk of cardiovascular disease in asymptomatic patients with intermediate risk (10-year risk 10-20%); recommendation class IIa [23].
It has been shown that as the calcium index increases, sensitivity is lost and specificity in predicting coronary artery disease increases [24]. In other words, in severe coronary artery calcification and very high CI, it becomes difficult to detail the topography of the lesion and its extent. Based on this, Z. Qian et al. proposed separate methods for assessing calcification of atherosclerotic plaques (lesion-specificscore) and coronary arteries (vessel-specificscore) as an addition to the already existing Agatston scale. The use of lesion-specific and vessel-specific calcium score increases the sensitivity of the study (with 80% specificity), which is superior to the traditional Agatston score in predicting IHD [25].
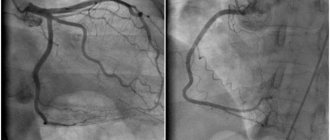
Coronary angiography has lower sensitivity compared to CT scanning in determining CCA, but at the same time has high specificity. According to angiography, CCA is assessed using the following criteria: 1) assessment of calcification of the target vessel on a 4-point scale (0 - no calcification, 1 - barely noticeable calcification, 2 - easily visualized, moderate calcification and 3 - severe calcification), 2) depth calcification after administration of contrast (superficial with calcification closer to the lumen of the vessel, deep with calcification closer to the adventitia), 3) is CCA determined reliably in two or more orthogonal projections and 4) is CCA determined in areas other than the target vessel [26] .
Intravascular ultrasound (IVUS) is a more accurate method for diagnosing coronary arteries than angiography, with high sensitivity (90-100%) and specificity (99-100%). A calcified plaque on IVUS appears as an echogenic shadow with acoustic shadowing, and the degree of calcification can be assessed by several indicators. According to the range of calcified lesions on IVUS, 4 classes are distinguished: class 1 (calcified lesion angle from 0 to 90°), class 2 (CAC angle from 91 to 180°), class 3 (CAC angle from 181 to 270°) and grade 4 (KKA angle from 271 to 360°). The location of calcium is defined as superficial (present in the intimal layer), deep (present in the medial adventitial layer) and mixed. Calcium deposits are assessed in the thickest atherosclerotic plaque [27].
Optical coherence tomography (OCT) is the optical analogue of intravascular ultrasound; it also has high sensitivity and specificity for identifying CCA. The difference in the physical principle of operation of these two methods is that with OCT, not an acoustic wave is used to study biological tissues, but infrared light radiation with a wavelength of about 1300 nm. However, the resolution of OCT (up to 10-20 µm) is approximately 10 times higher than that of IVUS (up to 100-150 µm), which makes it possible to differentiate the intima, media and adventitia. H. Yabushita et al. [28] in their analysis of OCT data described the specific features of each type of atherosclerotic plaque: fibrous plaque is characterized by a homogeneous area of high signal with low attenuation, calcified plaque is characterized by a well-defined area with low signal and clear boundaries, and lipid-rich plaque is characterized by an area of low signal and diffuse boundaries. . Despite its high resolution, OCT has a number of limitations that can pose a challenge in measuring the area of calcification and visualizing deep vascular structures. Thus, the maximum depth of signal penetration is 1-2 mm (for IVUS - up to 4-8 mm), and absorption by hemoglobin and scattering on erythrocytes lead to strong attenuation of the signal [29].
Thus, clinicians today have diagnostic tools at their disposal that allow them to assess coronary artery calcification both qualitatively and quantitatively. However, it must be recognized that convenient and non-invasive methods are more suitable for screening for coronary disease. For a detailed assessment, including the extent of calcification and involvement of the distal segments of the artery, an expensive invasive technique is required and, possibly, a comparison of its data with data obtained intraoperatively.
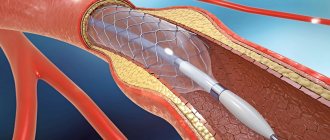
Percutaneous coronary intervention
Coronary calcification increases the likelihood of developing complications of angioplasty and is therefore often the reason for refusing to perform it [30]. The pressure exerted on the vessel wall when the balloon is inflated may be uneven due to varying degrees of calcification; this increases the risk of dissection, acute vessel occlusion, the possibility of subsequent restenosis and the development of adverse cardiovascular events [31]. Severe CCA creates difficulties during device delivery and increases the risk of vessel embolization, which in turn leads to an increase in the incidence of periprocedural MI [32].
With the introduction of bare metal stents (BMS), early and long-term survival has improved. However, incomplete stent deployment, asymmetric deployment, incorrect placement, or stent migration, observed with severe CCA, increased the risk of stent restenosis and thrombosis [33].
The use of drug-eluting stents (DES) has proven to be more effective. According to the TAXUS-IV study, in patients with calcified lesions, the rate of target vessel ischemia at 1 year was 56% lower with DES compared with BMS (5.1% vs. 11.9%, p
=0.09), however, in patients with non-calcified coronary arteries this difference was significantly greater (75% lower and 4.3% versus 15.7%,
p
<0.0001) [3].
Similar results were described in a meta-analysis by B. Zhang et al. (2015): the use of DES significantly reduces the need for repeated revascularization of target vessels compared with BMS in patients with CCA (8.5% versus 16.0%; relative risk: 0.50; 95% confidence interval: 0.38–0. 65; p
<0.00001) [34]. However, there are also studies reporting similar rates of thrombosis and restenosis of the DES and GMS in patients with CCA, with comparable rates of mortality and MI [2, 3].
Thus, a sequential study of the results of endovascular treatment of patients with coronary artery disease with calcified coronary arteries showed that the best results were obtained with implantation of drug-eluting stents. On the other hand, the results of stenting were compared with those in patients without calcification. They indicate a higher rate of restenosis and repeat revascularization in patients with CCA [35].
Potential risk factors for restenosis and repeat revascularization, such as incomplete stent deployment, damage to the drug coating of the stent due to CCA, and the use of other devices (including rotational atherectomy), may directly contribute to neointimal hyperplasia [36].
Cutting and notching balloon catheters do not remove calcium, but improve the elasticity of arterial walls by creating discrete incisions in the atherosclerotic plaque, which allows increasing the area of work on the affected parts of the artery and reducing the narrowing of the stent, preventing vessel dissection. The indication for a cutting balloon is a relatively short lesion (<20 mm). For prolonged and circular lesions, the use of such balloons is not recommended. In addition, the pressure in the cutting cylinder should not exceed 12 atm to avoid cutting the cylinder blade into the vessel wall [37].
Rotational atherectomy, unlike a cutting balloon, excises hard coronary calcium tissue to produce small particles (<10 µm) without affecting soft elastic tissue. Patients with CCA undergoing rotational atherectomy have an increased risk of thrombosis, development of the no-reflow phenomenon with an increased risk of periprocedural MI [38]. However, the use of rotational atherectomy has been found to be clinically effective in patients with CCA [39]. In order to improve the prognosis after exposure, implantation of a DES is recommended. There are a number of studies reporting favorable long-term results after DES implantation followed by rotational atherectomy [40].
Laser coronary atherectomy uses pulsed excimer or holmium laser energy to generate high-energy transient waves; There is a photoacoustic effect on resistant atherosclerotic lesions. Despite the fact that the method was introduced more than two decades ago, due to its uncertain results, as well as due to the advent of DES, laser angioplasty has lost its practical significance as an independent intervention and its use is limited to a few centers. Some studies have demonstrated potential procedural complications such as vessel dissection (especially vessels with superficial calcium), perforation, and a high risk of restenosis [41]. However, the procedure can be used in patients with CCA to destroy calcium before stent implantation in cases where there is a risk of incomplete stent deployment [42].
Orbital atherectomy, like rotational atherectomy, has a differential ablative effect on hard and soft surfaces, producing particles <2 μm in size when centrifugal force is applied to the vessel wall. The device allows operators to control the ablation depth by increasing the rotation speed (from 60,000 to 120,000 rpm). Like rotational atherectomy, orbital atherectomy improves the elasticity of arterial walls to reduce procedural complications and facilitate stent implantation. According to J. Chambers et al., the use of orbital atherectomy for severe coronary calcification not only improved stent delivery, but also improved early and 30-day clinical outcomes compared with the results of previous studies in a similar cohort of patients [43].
Thus, the evolution of endovascular revascularization and analysis of its results allow us to look optimistically at the prospects for treating patients with coronary artery disease, however, the study of immediate and delayed results shows less effectiveness of treatment if the coronary arteries are calcified.
Coronary artery bypass surgery
The fact that coronary artery calcification is a predictor of a worse prognosis after PCI leads clinicians to consider surgical revascularization as a priority treatment option in this situation. However, the question of the prognostic value of CCA for patients undergoing CABG remains unclear, and the available data do not allow us to draw solid conclusions. There are only a few studies aimed at addressing this issue. Of interest is the work of M. Castagna et al., who expressed a judgment about the more frequent development of calcification of autovenous shunts in patients with initial calcification of native coronary arteries [44].
In the analysis of K. Ertelt et al. reported on 755 patients with ACS who were included in the ACUITY study (Acute Catheterization and Urgent Intervention Triage Strategy Trial) with a follow-up period of 1 year after CABG [45]. The authors found that severe calcified lesions of the coronary artery were an independent predictor of major adverse cardiovascular events: when comparing 1-year mortality in patients with severe ( n
=103), moderate (
n
=249) and absent calcification (
n
=403) it was 11.8, 3.7 and 4.5%, respectively,
p
=0.006.
In a similar study by C. Bourantas et al. (2015) included 1545 patients (896 from the SYNTAX registry and 645 from the SYNTAX CABG registry) for 5 years of follow-up after CABG. Patients with severe calcification were compared ( n
=548) and without significant calcification of the coronary arteries (
n
=997).
Patients with severe CCA had a higher mortality rate: 17.1% versus 9.9%, p
<0.001, but the incidence of adverse nonfatal cardiovascular events was similar in the groups (26.8% versus 21.8%,
p
= 0.057 ). The higher mortality in the severe calcification group was partly explained by the presence of more severe comorbidities (renal failure, hypertension) and multifocal atherosclerosis [1]. A significant limitation of the study is the fact that the characterization of calcification was based on angiographic data without the use of MSCT or IVUS.
Drug treatment
To date, there is no generally accepted conservative treatment for CCA. The role of statins in the treatment of patients with CCA is unclear. According to numerous studies, statin therapy does not have a significant effect on arterial CCA [46]. Some researchers even express the opinion that statins can enhance the process of calcification [47]. Non-randomized studies have shown regression of CCA with the use of calcium channel blockers, hormonal therapy, and phosphate binders [48, 49].
In drug therapy, the assessment of the effect of taking calcium supplements deserves special mention. Based on the results of the large EPIC-Heidelberg study, which included 24 thousand people aged 35 to 64 years, the authors argue that the use of calcium supplements can significantly increase the risk of developing myocardial infarction [50]. According to some data, the risk of myocardial infarction increases with the use of calcium supplements at a dose of more than 800 mg/day [7]. There are also studies that have led to the opposite conclusion: dietary calcium does not have a significant effect on vascular calcification and cardiovascular events [51].
2.Why measure coronary calcium?
Coronary calcium screening is done if you are at risk for heart disease. Coronary calcium screening is not recommended for all people as a routine procedure to test for coronary artery disease (CAD).
Coronary calcium screening is not suitable for you if:
- You are not at risk for coronary heart disease (CHD);
- You have already been diagnosed with heart disease.
This test is also not suitable for men under 40 and women under 50 because... Young people do not have coronary calcium deposits.
Visit our Cardiology page
Is special training needed?
If there is a possibility of pregnancy, you should inform your doctor. Otherwise, the patient may be asked not to drink caffeinated drinks or eat anything the night before the test. Since smoking can also distort screening results, your consultation with a cardiologist may recommend that you abstain from smoking.
Under normal circumstances, this specific test is rarely ordered; a physical examination and other tests are usually sufficient to make the diagnosis. If the examination is completed and the tomography results are negative, this is regarded as a high risk of developing coronary heart disease. The patient has a chance to prevent an undesirable scenario by changing lifestyle, playing sports and establishing proper nutrition.
Experienced cardiologists will recommend other effective ways to reduce risks. The main thing is to listen to advice and think about your own health!
3.How is screening carried out?
A CT scan of the body is performed by a technologist and interpreted by a radiologist. Other doctors may also use the resulting images.
You will be asked to remove all jewelry and undress. You will be given a diaper to cover yourself during the procedure. You will need to lie down on a table to which the CT scanner is attached.
The table then slides into the CT scanner and it moves around your body to take pictures. You may hear noises during the procedure. It is important to remain still during the procedure.
If necessary, they can perform myelography, i.e. CT scan using a special dye. The contrast agent is usually injected into a vein in the arm. After this, wait some time for the dye to spread throughout the body.
The procedure takes from 30 to 60 minutes.
How to prepare for coronary calcium screening?
Before a coronary calcium screening, tell your doctor if you may or may be pregnant. You may be asked not to eat or drink anything containing caffeine for a while before the test.
About our clinic Chistye Prudy metro station Medintercom page!