Medical editor: Strokina O.A. - therapist, functional diagnostics doctor. November, 2021.
Early ventricular repolarization syndrome (EVRS) is a medical concept that includes only ECG changes without characteristic external symptoms. It is believed that SRRS is a normal variant and does not pose a threat to the patient’s life.
However, recently this syndrome has become viewed with caution. It is quite widespread and occurs in 2-8% of cases in healthy people. The older a person gets, the less likely it is that he or she will have SRR; this is due to the emergence of other cardiac problems that are similar in electrocardiographic signs as they age.
Most often, early ventricular repolarization syndrome is diagnosed in young men who are actively involved in sports, in men who lead a sedentary lifestyle, and in people with dark skin (Africans, Asians and Hispanics).
Causes
The exact causes of SRS have not been established to date. However, a number of factors have been identified that contribute to the occurrence of repolarization syndrome:
- taking certain medications, such as α2-agonists (clonidine);
- familial hyperlipidemia (high blood fats);
- connective tissue dysplasia (symptoms are more often found in people with SRGC: joint hypermobility, spider fingers, mitral valve prolapse);
- hypertrophic cardiomyopathies.
In addition, this anomaly is often diagnosed in people with congenital and acquired heart defects and in the presence of congenital pathology of the cardiac conduction system.
The genetic nature of the disease cannot be ruled out either (there are certain genes that are responsible for the occurrence of SRGC).
Syndrome of premature excitation of ventricles.
Heart rhythm disturbances are considered an important cardiac problem, since they often complicate the course and worsen the prognosis of many diseases and are one of the most common causes of sudden death.
Of particular interest to both clinicians and electrophysiologists is premature ventricular excitation syndrome (PVS), which in some cases, in the absence of clinical manifestations, can be an electrocardiographic finding, and in others it can be accompanied by life-threatening tachyarrhythmias.
Despite the successes achieved in the study of PPV, the issues of its diagnosis, patient management and treatment remain relevant today.
Definition. Classification
PPV (preexcitation syndrome, preexcitation syndrome) is an accelerated conduction of the excitation impulse from the atria to the ventricles along additional abnormal conduction pathways. As a result, part of the myocardium or all of the ventricular myocardium begins to be excited earlier than with the usual spread of excitation through the atrioventricular node, the His bundle and its branches.
According to the recommendations of the WHO expert group (1980), premature excitation of the ventricles, not accompanied by clinical symptoms, is called the “pre-excitation phenomenon,” and in the case when there are not only electrocardiographic signs of pre-excitation, but also tachyari paroxysms develop.
The anatomical substrate of PVS are bundles of specialized muscle fibers outside the conduction system of the heart, capable of conducting electrical impulses to different parts of the myocardium, causing their premature excitation and contraction.
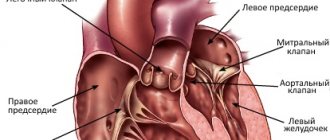
Accessory atrioventricular connections are classified according to their location relative to the annulus fibrosus of the mitral or tricuspid valves, type of conduction (decremental type - increasing slowing of conduction along the accessory pathway in response to an increase in the frequency of stimulation - or non-decremental), and also according to their ability to antegrade, retrograde or combined implementation. Typically, accessory pathways have rapid, nondecremental conduction similar to that of normal tissue of the His–Purkinje conduction system and atrial and ventricular myocardium.
Currently, several types of abnormal pathways (tracts) are known:
- atrioventricular (Kenta), connecting the myocardium of the atria and ventricles, bypassing the atrioventricular node;
- atrionodal (James), located between the sinoatrial node and the lower part of the atrioventricular node;
- nodoventricular (Maheima), connecting the atrioventricular node (or the beginning of the His bundle) with the right side of the interventricular septum or the branches of the right bundle branch;
- atriofascicular (Breschenmash), connecting the right atrium with the common trunk of the His bundle.
There are also other additional conduction pathways, including “hidden” ones, capable of conducting an electrical impulse retrogradely from the ventricles to the atria. A small (5–10%) proportion of patients have multiple abnormal conduction pathways.
In clinical practice there are:
- Wolff–Parkinson–White syndrome (WPW syndrome), caused by the presence of bundles of Kent;
- Clerk-Levy-Christesco syndrome (CLC syndrome, shortened P-Q (R) interval syndrome), caused by the presence of the James bundle.
Electrocardiographic manifestations of PPV depend on the degree of preexcitation and the persistence of conduction along additional pathways. In this regard, the following variants of the syndrome are distinguished:
- manifest PPV (the ECG constantly shows signs of pre-excitation);
- intermittent (transient) PPV (on the ECG, signs of pre-excitation are transient);
- latent PPV (the ECG is normal under normal conditions, signs of pre-excitation appear only during a paroxysm of tachycardia or during provocation - physical activity, electrophysiological study (EPI), vagal or drug tests);
- hidden (changes are not detected on a standard ECG due to the conduction of excitation along additional pathways only in a retrograde manner).
Prevalence
According to various sources, the prevalence of PPV in the general population is approximately 0.15%. At the same time, paroxysms of tachyarrhythmias occur in every second patient (in 80–85% of cases – orthodromic tachycardia, 20–30% – atrial fibrillation (AF), 5–10% – atrial flutter and antidromic tachycardia). Hidden PPV is detected in 30–35% of patients.
PPV is a congenital anomaly, but can clinically manifest at any age, spontaneously or after any disease. Typically, this syndrome manifests itself at a young age. In most cases, patients do not have any other heart pathology. However, combinations of PPV with Ebstein's anomaly, cardiomyopathies, and mitral valve prolapse are described. There is an assumption that there is a relationship between PVS and connective tissue dysplasia.
In families of patients suffering from this syndrome, an autosomal dominant type of inheritance of additional pathways was identified in relatives of the 1st, 2nd, and 3rd degrees of kinship with various clinical and electrocardiographic manifestations.
The incidence of sudden death in patients with PPV is 0.15–0.6% per year. In almost half of the cases, cardiac arrest in persons with PPV is its first manifestation.
Studies of patients with PPV who have suffered cardiac arrest have retrospectively identified a number of criteria that can be used to identify individuals at increased risk of sudden death. These include the presence of the following signs:
- shortened RR interval – less than 250 ms during spontaneous or induced AF;
- history of symptomatic (hemodynamically significant) tachycardia;
- multiple additional paths;
- Ebstein's anomalies.
Story
An ECG with a shortened PQ interval and at the same time a widened QRS complex was first described by A. Cohn and F. Fraser in 1913. Isolated similar cases were subsequently described by some other authors, but for many years the cause of this ECG pattern was considered to be blockade of the branches of the His bundle.
In 1930, L. Wolff, J. Parkinson and P. White presented a report in which electrocardiographic changes of this type were considered as the cause of paroxysmal cardiac arrhythmias. This work provided the basis for conducting comprehensive studies aimed at elucidating the pathogenesis of these changes on the ECG, which were subsequently named Wolff-Parkinson-White syndrome.
Two years later, M. Holzman and D. Scherf suggested that the basis of WPW syndrome is the spread of the excitation impulse along additional atrioventricular pathways. In 1942, F. Wood provided the first histological confirmation of the presence of a muscular connection between the right atrium and the right ventricle, identified during autopsy of a 16-year-old patient with a history of episodes of paroxysmal tachycardia.
Despite these data, an active search for alternative mechanisms for the development of the syndrome continued until the 1970s, when EPI and surgical treatments confirmed the theory of accessory pathways.
Pathogenesis
The conduction of impulses from the atria to the ventricles during PPV occurs simultaneously along the normal conduction system of the heart and along the accessory pathway. In the conduction system at the level of the atrioventricular node, there is always some slowdown in the conduction of impulses, which is not typical for the anomalous tract. As a result, depolarization of a certain area of the ventricular myocardium begins prematurely even before the impulse propagates through the normal conduction system.
The degree of preexcitation depends on the ratio of conduction velocities in the normal conduction system of the heart, primarily in the atrioventricular node, and in the accessory conduction pathway. An increase in the conduction velocity along the accessory pathway or a slowdown in the conduction velocity through the atrioventricular node leads to an increase in the degree of ventricular preexcitation. In some cases, ventricular depolarization may be entirely due to the conduction of impulses along the accessory pathway. At the same time, when the conduction of impulses through the atrioventricular node accelerates or the conduction through the accessory pathway slows down, the degree of abnormal ventricular depolarization decreases.
The main clinical significance of additional conduction pathways is that they are often included in the loop of circular motion of the excitation wave (re-entry) and thus contribute to the occurrence of supraventricular paroxysmal tachyarrhythmias.
With PPV, orthodromic reciprocal supraventricular tachycardia most often occurs, in which the impulse is conducted antegrade through the atrioventricular node, and retrograde through the accessory pathway. Paroxysm of orthodromic supraventricular tachycardia is characterized by frequent (140–250 per 1 min), devoid of signs of preexcitation, normal (narrow) QRS complexes. In some cases, inverted P waves are observed after the QRS complex, indicating retrograde activation of the atria.
With antidromic supraventricular tachycardia, the impulse circulates in the opposite direction: antegrade - along the abnormal conduction pathway, retrograde - along the atrioventricular node. Paroxysm of antidromic supraventricular tachycardia in patients with PPV is manifested on the ECG by a frequent regular rhythm (150–200 per 1 min) with ventricular complexes of the type of maximally pronounced preexcitation (QRS = 0.11 s), after which inverted P waves are sometimes detected.
In 20–30% of patients with PPV, paroxysms of AF occur, in which, as a result of antegrade conduction of a large number of atrial impulses along the accessory pathway, the ventricular contraction frequency (VFR) can exceed 300 per minute.
Clinic
In many cases, PPV is asymptomatic and is detected only by electrocardiography. 50–60% of patients complain of palpitations, shortness of breath, chest pain or discomfort, fear and fainting. Paroxysms of AF become particularly dangerous in the case of PPV, since they are accompanied by a large heart rate, hemodynamic disturbances, and can often transform into ventricular fibrillation. In such cases, patients not only experience syncope, but also have a high risk of sudden death.
Independent risk factors for the development of AF in patients with PPV are age, male gender, and a history of syncope.
Diagnostics
The main method for diagnosing PPV is ECG.
In case of WPW syndrome against the background of sinus rhythm, a shortening of the PQ interval (<0.12 s) and a D-wave (flat slope in the first 30–50 ms) are detected on the ascending part of the R wave or the descending part of the Q wave, the QRS complex is usually widened (i0, 11 s). Deviation of the ST segment and T wave in the direction opposite to the D-wave and the main direction of the QRS complex is also characteristic.
Electrocardiographic signs of CLC syndrome are shortening of the PQ (R) interval, the duration of which does not exceed 0.11 s, the absence of an additional excitation wave - D-wave - in the QRS complex, the presence of unchanged (narrow) and undeformed QRS complexes (except in cases of concomitant blockade of the legs or branches of the His bundle).
With PPV, caused by the functioning of the Maheim beam, a normal PQ interval is determined in the presence of a D wave.
The simultaneous functioning of the James and Maheim beams leads to the appearance on the ECG of signs characteristic of WPW syndrome (shortening of the PQ (R) interval and the presence of a D-wave).
In connection with the spread in recent years of surgical methods for the treatment of patients with PPV (destruction of an abnormal bundle), methods for accurately determining its localization are constantly being improved.
On the ECG, the location of the Kent beam is usually determined by the direction of the initial moment vector of ventricular depolarization (the first 0.02–0.04 s), which corresponds to the time of formation of the abnormal D-wave. In those leads whose active electrodes are located directly above the area of the myocardium that is abnormally excited by the Kent beam, a negative D-wave is recorded. This indicates the spread of early abnormal excitation away from the active electrode of this lead.
Of particular practical interest are the capabilities of the spatial vector electrocardiography method, which makes it possible to accurately determine the localization of additional conduction pathways.
More detailed, compared to ECG data, information about the location of additional conduction pathways can be obtained using magnetocardiography.
However, the most reliable and accurate methods are intracardiac EPI, in particular endocardial (preoperative) and epicardial (intraoperative) mapping. In this case, using a complex technique, the area of the earliest activation (pre-excitation) of the ventricular myocardium is determined, which corresponds to the localization of the additional abnormal bundle.
Treatment
In patients with asymptomatic PPV, treatment is usually not required. Exceptions include individuals with a family history of sudden death, athletes, and those whose work involves danger to themselves and others (for example, divers and pilots).
In the presence of paroxysms of supraventricular tachycardia, treatment consists of stopping attacks and preventing them using various medicinal and non-medicinal methods. In this case, the nature of the arrhythmia (ortho-, antidromic tachycardia, AF), its subjective and objective tolerability, heart rate, as well as the presence of concomitant organic heart diseases are important.
With orthodromic reciprocal supraventricular tachycardia, the excitation impulse is conducted antegrade in the normal way, so its treatment should be aimed at suppressing conduction and blocking impulses in the atrioventricular node. For this purpose, reflex vagal tests are used, which are most effective when applied as early as possible.
The first-line drug for stopping orthodromic reciprocal supraventricular tachycardia is considered to be adenosine, the potential disadvantage of which is a transient increase in atrial excitability, which can provoke their extrasystole and fibrillation immediately after stopping the paroxysm of such tachycardia. Verapamil is considered to be another drug of choice for stopping orthodromic tachycardia in the absence of severe arterial hypotension and severe systolic heart failure. β-blockers are usually used as second-line drugs.
If these drugs are ineffective, procainamide is used to block conduction through the accessory atrioventricular pathway. Due to its safety and effectiveness, novocainamide is the drug of choice in the treatment of tachycardia with wide QRS complexes, when the diagnosis of orthodromic reciprocal supraventricular tachycardia is in doubt.
Reserve drugs are amiodarone, sotalol and class 1C antiarrhythmic drugs (AAPs): propafenone or flecainide.
In case of antidromic reciprocal supraventricular tachycardia, the impulse is conducted retrogradely through the atrioventricular node, therefore the use of verapamil, diltiazem, lidocaine and cardiac glycosides for its relief is contraindicated due to the ability of these drugs to accelerate antegrade conduction along the accessory pathway and thereby increase heart rate. The use of these drugs, as well as adenosine, can provoke the transition of antidromic supraventricular tachycardia to AF. The drug of choice for stopping such tachycardia is procainamide; if it is ineffective, amiodarone or class 1C AAP are used.
When paroxysmal AF occurs, the main goal of drug therapy is to control the ventricular rate and slow down conduction simultaneously along the accessory tract and the AV node. The drug of choice in such cases is also novocainamide. Intravenous administration of amiodarone and class 1C AAP is also highly effective.
It should be noted that the use of verapamil, digoxin and beta-blockers in AF for the purpose of controlling heart rate in persons with PPV is contraindicated due to their ability to increase the conduction velocity along the accessory pathway. This can transfer fibrillation from the atria to the ventricles.
To prevent paroxysms of supraventricular tachyarrhythmias caused by the presence of additional conduction pathways, class IA, IC and III AAPs are used, which have the property of slowing down conduction along abnormal pathways.
Non-drug methods for stopping attacks of supraventricular tachyarrhythmias include transthoracic depolarization and atrial (transesophageal or endocardial) pacing, and for their prevention - catheter or surgical ablation of accessory pathways.
In patients with PPV, electrical cardioversion is used for all forms of tachycardia, which are accompanied by severe hemodynamic disturbances, as well as when drug therapy is ineffective and in cases where it causes a deterioration in the patient's condition.
Radiofrequency catheter ablation of accessory tracts is currently the main method of radical treatment of PPV. Indications for its implementation are a high risk of sudden death (primarily the presence of AF paroxysms), ineffectiveness or poor tolerability of drug therapy and prevention of attacks of supraventricular tachycardia, as well as the patient’s reluctance to take AAP. If a short effective refractory period of the abnormal tract is detected in individuals with rare and mild paroxysms of arrhythmia, the question of the advisability of ablation in order to prevent sudden death is decided individually.
Before catheter ablation, EPI is performed, the purpose of which is to confirm the presence of an additional conduction pathway, determine its electrophysiological characteristics and role in the formation of tachyarrhythmia.
The effectiveness of radiofrequency catheter ablation is high (reaches 95%), and the mortality associated with the procedure does not exceed 0.2%. The most common serious complications of this treatment method are complete atrioventricular block and cardiac tamponade. Recurrences of conduction along the accessory pathway occur in approximately 5–8% of cases. Repeated radiofrequency ablation usually completely eliminates conduction along additional pathways.
Currently, the scope of surgical destruction of accessory tracts has significantly narrowed. For the same indications as catheter ablation, surgical treatment is resorted to in cases where the latter is impossible to perform for technical reasons or is unsuccessful, as well as when open-heart surgery is necessary due to concomitant pathology.
Literature
- Sychev O.S. Heart rhythm disturbances // Guide to cardiology / Ed. V.N. Kovalenko. – K.: Morion, 2008. – P. 1059-1248, 1215-1218.
- ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation // Circulation. – 2006. – No. 114. – P. 257-354.
- ACC/AHA/ESC Guidelines for the management of patients with supraventricular arrhythmias – executive summary // JACC. – 2003. – No. 8. – R. 1493-1531.
- Griffin B., Topol E. Manual of Cardiovascular Medicine. – The Lippincott Williams & Wilkins, 2004. – P. 1248.
- Hanon S., Shapiro M., Schweitzer P. Early history of the preexcitation syndrome // Europace. – 2005. – No. 7. – P. 28-33.
- Keating L., Morris A., Brady W.O. Electocardiographic Features of Wolff–Parkinson–White syndrome // Emerg. Med. J. – 2003. – No. 20. – R.491-493.
N.T. Vatutin, N.V. Kalinkina, E.V. Yeshchenko.
Donetsk State Medical University. M. Gorky;
Institute of Emergency and Reconstructive Surgery named after. VC. Gusak of the Academy of Medical Sciences of Ukraine.
Ukrkardio
Kinds
There are two options for SRR:
- without damage to the cardiovascular and other systems;
- involving the cardiovascular and other systems.
From the point of view of the nature of the course, a distinction is made between transient and permanent SRGC.
Based on the localization of ECG signs, doctor A.M. Skorobogaty proposed the following classification:
- Type 1 – with a predominance of signs in leads V1-V2;
- Type 2 – with a predominance in leads V4-V6;
- Type 3 (intermediate) – without a predominance of signs in any leads.
Signs of SRS
There are no characteristic clinical signs of early ventricular repolarization syndrome. There are only specific changes on the ECG:
- ST segment and T wave changes;
- in a number of branches, the ST segment rises above the isoline by 1-2-3 mm;
- often ST segment elevation begins after the notch;
- the ST segment has a rounded shape and directly passes into a tall positive T-wave;
- the convexity of the ST segment is directed downwards;
- The base of the T wave is wide.
Depolarization and repolarization
The section "Basic principles of electrocardiography and disorders" discusses the general concept of "electrical excitation" , which means the propagation of electrical impulses through the atria and ventricles. The exact name for electrical excitation, or activation of the heart, is depolarization . The return of cardiomyocytes to a state of relaxation after excitation (depolarization) is repolarization . These terms emphasize that at rest, the myocardial cells of the atria and ventricles are polarized (their surface is electrically charged). Figure 2-1, A shows the state of polarization of a normal muscle cell of the atria or ventricles
Rice. 2-1. Processes of depolarization and repolarization A - the muscle cell of the heart is polarized at rest, i.e. the outer surface of the cell is positively charged, and the inner surface is negatively charged; B - when a cell (S) is excited, it depolarizes (the excited area is electronegative with respect to neighboring areas); B – a completely depolarized cell is charged positively inside and negatively outside; D – repolarization occurs when a cell returns from a state of excitation to a state of rest. The direction of depolarization and repolarization is indicated by arrows. Depolarization (excitation) of the atria on the ECG corresponds to the P wave, and depolarization of the ventricles corresponds to the QRS complex. Ventricular repolarization corresponds to the ST-T complex.
The outside of the cell at rest is positively , and the inside is negatively charged [about -90 mV (millivolts)]. Membrane polarization is caused by the difference in ion concentrations inside and outside the cell.
When the muscle cell of the heart is excited, it depolarizes. As a result, the outer side of the cell in the region of excitation becomes negative, and the inner side becomes positive. There is a difference in electrical voltage on the outer surface of the membrane between the depolarized area in the state of excitation and the unexcited polarized area, see Fig. 2-1, B. Then a small electric current arises, which spreads along the cell until it is completely depolarized, see fig. 2-1, V.
The direction of depolarization is shown by an arrow, see Fig. 2-1, B. Depolarization and repolarization of individual muscle cells (fibers) occurs in one direction. However, throughout the myocardium, depolarization proceeds from the inner layer (endocardial) to the outermost layer (epicardial), and repolarization occurs in the opposite direction. The mechanism for this difference is not completely clear .
| Note: Depolarization of the ventricular myocardium occurs from the endocardium to the epicardium, and repolarization occurs from the epicardium to the endocardium. This is due to the fact that the duration of TMPD in the subepicardial parts of the ventricles is 0.03-0.04 s less than in the subendocardial parts, and the repolarization process will begin earlier precisely under the epicardium. | |
The depolarizing electric current is recorded on the electrocardiogram in the form of the P wave (excitation and depolarization of the atria) and the QRS complex (excitation and depolarization of the ventricles).
After some time, the depolarized cell, completely engulfed in excitation, begins to return to a resting state. This process is called repolarization . A small area on the outside of the cell again acquires a positive charge, see fig. 2-1, D, then the process spreads along the cell until it is completely repolarized. Ventricular repolarization on the electrocardiogram corresponds to the ST segment, waves and (atrial repolarization is usually hidden by ventricular potentials).
An electrocardiogram shows the electrical activity of all cells in the atria and ventricles, rather than individual cells. In the heart, depolarization and repolarization are usually synchronized, so on the electrocardiogram these electrical flows can be recorded in the form of certain waves ( P , T , U , QRS complex , ST segment ).
| Any electrocardiogram - both normal and pathologically altered - reflects two main processes: depolarization - the propagation of an impulse throughout the myocardium - and repolarization - the return of the excited myocardium to a resting state. | |
Diagnostics
Since this syndrome is an electrocardiographic phenomenon, it can only be established with a certain examination:
- ECG;
- Ultrasound of the heart (echocardiography): stress echocardiography (for impaired ventricular contractility)
- resting echocardiography;
In addition, tests are carried out on a bicycle ergometer or treadmill: after physical activity, the heart rate increases, and the ECG signs of SIRS disappear.
A potassium test is used: after taking potassium chloride, panangin or rhythmocor at least 2 grams, the severity of ECG signs of repolarization syndrome increases.
A test with isoproterenol and atropine is not used due to severe side effects.
It is important to distinguish between SRR and myocardial infarction, pericarditis, Brugada syndrome. For this purpose, differential diagnosis is carried out.
Sudden cardiac arrest associated with early repolarization
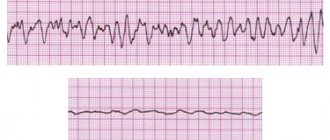
METHODS. We reviewed data from 206 patients at 22 centers who were resuscitated from cardiac arrest due to idiopathic ventricular fibrillation and assessed the prevalence of electrocardiographic early repolarization. The latter was defined as an increase in the QRS-ST junction of at least 0.1 mV from baseline in the inferior or lateral lead, manifested as a QRS wander or notch. The control group included 412 patients without heart disease who were matched for age, sex, race, and physical activity level. Follow-up data, which included implantable defibrillator monitoring results, were obtained for all patients.
RESULTS. Early repolarization was more common in subjects with idiopathic ventricular fibrillation than in control subjects (31% vs. 5%, p < 0.001). Among study subjects, those with early repolarization were more likely to be male and have a history of syncope (fainting) or sudden cardiac arrest during sleep than patients without early repolarization. In eight subjects, the origin of ectopia, which initiated ventricular arrhythmias, was located in areas corresponding to the localization of repolarization anomalies. During a mean (±SD) follow-up of 61±50 months, defibrillator monitoring showed a higher rate of recurrent ventricular fibrillation in patients with repolarization abnormality than in patients without such pathology (odds ratio, 2.1, 95% confidence interval, 1. 2 to 3.5, P = 0.008).
CONCLUSIONS. Among patients with a history of idiopathic ventricular fibrillation, there is an increased prevalence of early repolarization.
Sudden cardiac arrest remains . A major public health problem that accounts for approximately 350,000 deaths annually in the United States. Despite advances in emergency medical care, only 3-10% of patients who experience cardiac arrest outside of hospital are successfully resuscitated. Most of these sudden cardiac arrests are caused by ventricular tachyarrhythmias, which occur in 6% to 14% of cases in individuals without structural heart disease. Some of the latter cases are associated with well-recognized electrocardiographic abnormalities that affect ventricular repolarization (eg, long or short QT intervals or Brugada syndrome), while other cases with no signs during sinus rhythm are described as idiopathic ventricular fibrillation. Early repolarization is a common electrocardiographic finding that affects 1 to 5% of people. Although this condition is generally considered benign, its potential arrhythmogenicity has been suggested by experimental studies. However, there is no supporting clinical evidence. We conducted a case-control study of 206 patients with idiopathic ventricular fibrillation to assess the prevalence of early repolarization and assess its potential association with any observed arrhythmias and subsequent outcome monitored by implantable defibrillators.
Methodology.
POPULATION STUDY. Cases of the disease in people under the age of 60 were recorded in 22 territorial centers dealing with arrhythmias. All patients diagnosed with idiopathic ventricular fibrillation in this age group were selected from databases of patients who had received an implantable defibrillator; All patients aged 60 years or older were excluded to minimize the risk of subclinical structural heart disease. Verbal informed consent was obtained from all enrolled patients.
We assessed baseline electrocardiograms for the presence of early repolarization, which was defined as an increase in the QRS-ST junction (J point) in at least two leads during defibrillator implantation. The J-point height amplitude had to be at least 1 mm (0.1 mV) above baseline, either as a QRS blur (a smooth transition from the QRS segment to the ST segment) or a notch (a positive J deflection inscribed on the S wave ) in the inferior lead (II, III and aVF), lateral lead (I, aVL and V4 to V6) or both. Anterior chest leads (V1 to V3) were excluded from the analysis to avoid inclusion of patients with right ventricular dysplasia or Brugada syndrome.
Based on published guidelines, patients were classified as having idiopathic ventricular fibrillation if they had no structural heart disease as demonstrated by normal echocardiographic biventricular dimensions and function, no evidence of ischemic coronary artery disease on coronary angiography or stress testing, and no evidence of any repolarization anomalies. Patients were excluded if they had a heart rate-corrected QT interval (QTc) of less than 340 ms (short QT interval) or more than 440 ms (long QT interval) at baseline and before the arrhythmia. Patients with Brugada syndrome, defined by right branch block and ST segment elevation (>0.2 mV) in precordial leads V1-V3 without intervention or after sodium channel blocker (antiarrhythmic) infusion, were also excluded. In addition, patients with catecholaminergic arrhythmias, defined as arrhythmias during catecholamine infusion or exercise testing, were excluded.
We assessed the prevalence and amplitude of early repolarization in a control group of 412 subjects. This group was composed of health care professionals with normal echocardiographic biventricular dimensions and function and no history of syncope (fainting). The following factors were used for matching (age, gender, race, and physical activity level).
Data collection.
We collected the following clinical data: history of syncope, sudden cardiac arrest, family history of sudden death (age <60 years), physical activity level (>10 hours or ≤10 hours of activity per week), averaged by electrocardiographic signals (both standard amplification and high amplification) and the results of pharmacological testing and invasive electrophysiological testing. Electrocardiographic parameters were measured using automated online software and verified manually. The QTc interval was calculated after heart rate correction using the Bazett formula.
ELECTROPHYSIOLOGICAL STUDY. We performed electrophysiological studies using multielectrode catheters inserted percutaneously through the femoral vessels. Programmed ventricular pacing was performed using a maximum of two or three ventricular extrastimulations from two separate ventricular sites. Ventricular fibrillation was considered inducible if it lasted more than 30 seconds or required electrical cardioversion. No patient had inducible monomorphic ventricular tachycardia.
For subjects with recurrent ventricular fibrillation despite administration of antiarrhythmic drugs, catheter ablation targeting the initiating ventricular ectopy was performed as previously described. Such ectopia was localized by mapping the earliest electrical activity, either Purkinje or myocardial, relative to the onset of the QRS complex. Ablation was performed using radiofrequency energy.
THERAPY AND FOLLOW-UP. All patients with episodes received an implantable defibrillator, which provided accurate information about the recurrence of ventricular fibrillation. Patients were followed regularly every 6–12 months for clinical examination and device testing or, as needed, in the event of symptoms or device battery depletion. In subjects with recurrent arrhythmias, the choice of antiarrhythmic drugs was made by the treating physicians.
STATISTICAL ANALYSIS. Continuous variables were reported as mean ± SD or median (with 25th and 75th percentiles), as appropriate. Comparisons between the two groups were performed with the Student's t test or nonparametric Wilcoxon test, as appropriate, and with the Student t test for paired data. Categorical variables were compared with Fisher's exact test. The prevalence of early repolarization was compared between study subjects and controls using logistic regression analysis (reported as odds ratios with 95% confidence intervals) and adjusted for matching variables. The number of recurrences of ventricular fibrillation was compared using the Wilcokinson test, and the recurrence rate was estimated using actuarial curves. Hazard ratios from Cox proportional hazard models were used to estimate the relative risk associated with early repolarization. All tests were two-sided, and a P value of less than 0.05 was considered statistically significant.
RESULTS.
EARLY REPOLARIZATION.
In the group of patients with idiopathic ventricular fibrillation, there were 123 men and 83 women with a mean age of 36 ± 11 years. The control group included 412 subjects who were matched for age (36 ± 12 years), sex (270 men and 142 women), race (380 whites, 27 Asians, and 5 blacks), and physical activity (44 subjects engaged in more than 10 hours of activity per week ).
Early repolarization occurred in 64 patients (31%) compared with 21 controls (5%, P < 0.001) and was greater in magnitude in study subjects than in control subjects (J-point Height 2.0 ± 0.9 mm vs. 1.2 ± 0.4 mm, P < 0.001). After adjusting for age, sex, race, and physical activity level, the odds ratio for the presence of early repolarization in study subjects compared with control subjects was 10.9 (95% confidence interval [CI], 6.3–18.9).
Cases with early repolarization were more likely to be male, had a history of unexplained syncope or sudden cardiac arrest during sleep, and had a shorter QTc interval than those without early repolarization.
At the initial stage, early repolarization was present in the anterior leads in 28 subjects, in the lateral leads in 6 subjects, and in the inferior and basal leads in 30 patients. This pattern was considered a repolarization rather than a late depolarization because of its slower recording, spontaneous fluctuation in morphologic pattern or amplitude before sustained QRS complexes, and amplitude varying concurrently with the ST segment. The absence of late potentials on high-amplification electrocardiography further supported the repolarization pattern. This pattern occurred in isolation or was accompanied by negative T-wave elevation or discrete ST-segment elevation (horizontal or upward curvature). Electrocardiograms that were obtained several weeks before sudden cardiac arrest were available for 22 subjects and showed a pattern of early repolarization (as described above).
Electrocardiography was performed during the arrhythmic period (including incidence of ventricular ectopy and episodes of ventricular fibrillation) in 18 subjects, all studies showing a consistent increase in early repolarization amplitude compared with baseline. J-point amplitude increased from 2.6 ± 1 mm to 4.1 ± 2 mm (P < 0.001). In most cases, ectopia had a positive QRS morphological pattern in leads V1 to V2, indicating origin from the left ventricle and a short interaction interval initiating ventricular fibrillation (mean, 326 ± 41 ms, range 260 to 400).
Exercise testing or isoproterenol infusion consistently reduced or eliminated early repolarization. Isoproterenol administered to two subjects during repeated episodes of ventricular fibrillation eliminated all arrhythmias when the sinus node heart rate was increased by more than 120 beats per minute. In contrast, beta blockers increased repolarization abnormalities. Their ineffectiveness has led to attempts at catheter ablation of ventricular premature contractions, which resulted in ventricular fibrillation in some subjects.
CORRECTION OF ECTOTIA IN EARLY REPOLLARIZATION. Mapping was performed in eight patients. In two cases, mapping of both ventricles showed no findings during ventricular depolarization that coincided with wide terminal QRS abnormalities, confirming that the latter was associated with repolarization. A total of 26 ectopic patterns were mapped to either ventricular myocardium (16 patterns) or Purkinje tissue (10 patterns). In six patients with early repolarization recorded only in the lower parts, the entire ectopia arose from the lower wall of the ventricle. In two patients with widespread early repolarization, which was recorded by both low and lateral lines, the ectopia arose from multiple regions. Catheter ablation eliminated all ectopia in five subjects and did not eliminate the condition in three subjects.
RELATIONSHIP OF SUBJECTS. Table 2 summarizes results over a mean period of 61 ± 50 months (median, 51 months; interquartile range, 19 to 90) after the initial event, with no subjects losing control. Arrhythmic relapses were more frequent in subjects with early repolarization than in subjects without such repolarization (41% vs. 23%). The hazard ratio for relapse was 2.1 (95% CI, 1.2 to 3.5; P = 0.008), even after adjustment for sex. The three patients with the highest J-point level (>5 mm) had more than 50 episodes of ventricular fibrillation, resulting in death in one case. Quinidine was prescribed to four patients with multiple episodes, which reduced repolarization abnormalities and eliminated recurrent arrhythmias.
DISCUSSION. Sudden cardiac arrest from arrhythmia can occur in individuals who do not have structural heart disease or obvious electrocardiographic abnormalities during sinus rhythm. In our study, such study subjects had a significantly higher prevalence of early repolarization than control subjects, whose prevalence was similar to that of healthy subjects in previously reported studies. In nearly a third of the patients, electrocardiograms obtained before cardiac arrest were available and showed early repolarization, which indicated that this abnormality could not be the result of trauma following sudden cardiac arrest, resuscitation, or the drugs used for resuscitation.
It is unlikely that this anomaly is more common among survivors of cardiac patients than among non-survivors, since the single most important factor determining successful resuscitation is access to rapid defibrillation. This electrocardiographic pattern has also been associated with an increased rate of recurrent ventricular arrhythmias during defibrillator monitoring.
Our results show an association between early repolarization and sudden cardiac arrest, a finding that contradicts the seemingly benign nature of this common phenomenon. First, this finding may be related to the definition of early repolarization, since we specifically included abnormalities in the inferolateral leads, whereas the broad traditional definition of early repolarization included electrocardiographic patterns of varying amplitude, configuration, and extent, most often in the right chest leads. Second, few of the study subjects in our study were from subgroups that have a high prevalence of early repolarization (eg, athletes and blacks), suggesting that cofactors influence the association with sudden cardiac arrest. Third, the beneficial nature of early repolarization is challenged by experimental evidence suggesting that a form of transmural electrical heterogeneity can be dramatically enhanced under certain conditions (use of specific drugs and varying levels of autonomic tone and electrolytes), leading to malignant arrhythmias. Potential arrhythmogenicity thus depends on defective modulation of repolarization, which corresponds to the dynamic changes temporally associated with the arrhythmias that we observed in our case.
The relationship between this electrocardiographic pattern and malignant arrhythmias is supported by increased repolarization before the onset of arrhythmia in study subjects and the occurrence of trigger contractions in the region of early repolarization. Quinidine, which has been shown to restore transmural electrical homogeneity and abolish arrhythmic activity in this condition, reduced the electrocardiographic pattern and eliminated recurrent arrhythmias in four subjects.
Finally, although to our knowledge no multicenter study has examined the association between early repolarization and sudden cardiac arrest, anecdotal reports (mostly from Southeast Asia) have described patients in whom sudden cardiac arrest was associated with abnormal J waves. A repolarization abnormality that is detected in the anterolateral region may be a marker of underlying electrical vulnerability that increases the risk of fatal arrhythmias in the conditions that need to be investigated. These conditions include the presence of genetic defects associated with cardiac ion channels, as evidenced by the fact that 10 of our patients had a family history of sudden cardiac arrest.
These results are potentially important for the evaluation of patients with syncope (fainting) or a family history of sudden death. Arrhythmias associated with repolarization abnormalities may be responsible for a proportion of unexplained deaths, predominantly in young men, as previously reported. Such arrhythmias may also be responsible for some undiagnosed causes of syncope, which have been reported to increase the risk of premature death.
The results of our study, which require confirmation by other researchers, have several limitations. Although the cohort included subjects with well-defined common features, data collection was uneven among centers. In our study population, we did not have subjects with structural heart disease and did not have many athletes or blacks, so the results may not apply to these subgroups. Most importantly, although our results suggest that early repolarization is a marker of malignant arrhythmia disorder, studies predict a favorable course for most of these patients. Further research is needed to identify factors that modulate underlying arrhythmogenicity and predict which patients are at risk.
In conclusion, this multicenter study showed a higher than expected prevalence of early repolarization in patients younger than 60 years of age who had idiopathic ventricular fibrillation that caused syncope and sudden cardiac arrest.
Treatment of early ventricular repolarization syndrome
Repolarization syndrome does not require specific treatment. The only thing that is offered to the patient is observation by a cardiologist.
However, a person with SRS should avoid alcohol consumption and intense physical activity to avoid triggering an attack of tachycardia.
In some cases, radiofrequency ablation of an additional beam is performed in an invasive way (a catheter is brought to the site of the beam and destroys it).
Sometimes energotropic therapy (B vitamins, carnitine, phosphorus and magnesium preparations, Mexidol, Kudesan), antiarrhythmic drugs (amiodarone) are used.
Important! The patient should retain all previous ECGs, which is required to exclude the diagnosis of myocardial infarction if heart pain occurs.