© Author: A. Olesya Valerievna, candidate of medical sciences, practicing physician, teacher at a medical university, especially for SosudInfo.ru (about the authors)
Adrenergic agonists constitute a large group of pharmacological drugs that have a stimulating effect on adrenergic receptors located in internal organs and vascular walls. The effect of their influence is determined by the excitation of the corresponding protein molecules, which causes changes in the metabolism and functioning of organs and systems.
Adrenergic receptors are found in all tissues of the body; they are specific protein molecules on the surface of cell membranes. The effect on adrenergic receptors of adrenaline and norepinephrine (natural catecholamines of the body) causes a variety of therapeutic and even toxic effects.
With adrenergic stimulation, both spasm and vasodilation, relaxation of smooth muscles or, conversely , contraction of striated muscles can occur. Adrenergic agonists change the secretion of mucus by glandular cells, increase the conductivity and excitability of muscle fibers, etc.
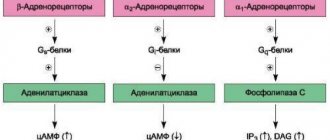
The effects mediated by adrenergic agonists are very diverse and depend on the type of receptor that is stimulated in a particular case. The body contains α-1, α-2, β-1, β-2, β-3 receptors. The influence and interaction of adrenaline and norepinephrine with each of these molecules are complex biochemical mechanisms, which we will not dwell on, specifying only the most important effects from stimulation of specific adrenergic receptors.
α1 receptors are located predominantly on small arterial-type vessels (arterioles), and their stimulation leads to vascular spasm and a decrease in the permeability of capillary walls. The result of the action of drugs that stimulate these proteins is an increase in blood pressure, a decrease in swelling and the intensity of the inflammatory reaction.
α2 receptors have a slightly different meaning. They are sensitive to both adrenaline and norepinephrine, but combining them with a mediator causes the opposite effect, that is, by binding to the receptor, adrenaline causes a decrease in its own secretion. The effect on α2 molecules leads to a decrease in blood pressure, dilation of blood vessels, and increased permeability.
The predominant localization of β1-adrenergic receptors is the heart, therefore the effect of their stimulation will be to change its work - increased contractions, increased heart rate, accelerated conduction along the nerve fibers of the myocardium. β1 stimulation will also result in an increase in blood pressure. In addition to the heart, β1 receptors are located in the kidneys.
β2-adrenergic receptors are present in the bronchi, and their activation causes expansion of the bronchial tree and relief of spasm. β3 receptors are present in adipose tissue and promote the breakdown of fat with the release of energy and heat.
There are different groups of adrenomimetics: alpha- and beta-adrenergic agonists, mixed-action drugs, selective and non-selective.
Adrenomimetics are able to bind to receptors themselves, completely reproducing the effect of endogenous mediators (adrenaline, norepinephrine) - direct-acting drugs. In other cases, the medicine acts indirectly: it enhances the production of natural mediators, prevents their destruction and reuptake, which helps to increase the concentration of the mediator at the nerve endings and enhance its effects (indirect action).
Indications for the use of adrenergic agonists may include:
- Acute heart failure, shock, sudden drop in blood pressure, cardiac arrest;
- Bronchial asthma and other diseases of the respiratory system accompanied by bronchospasm; acute inflammatory processes of the nasal and eye mucosa, glaucoma;
- Hypoglycemic coma;
- Conducting local anesthesia.
Introduction
Glaucoma is the most common cause of irreversible vision loss [1]. As ophthalmology has advanced, the understanding of the pathogenesis of glaucoma has evolved from decreased vision associated with increased intraocular pressure (IOP) to the development of optic neuropathy. According to the modern concept, apoptosis of retinal ganglion cells (RGCs) is a key factor in the pathogenesis of glaucoma. To date, the only modifiable risk factor for the development of glaucoma remains a decrease in IOP (indirect neuroprotection) [2]. However, according to a number of studies, in particular CNTGS (Collaborative Normal Tension Glaucoma Study; 160 eyes randomized into treatment and control groups), some patients with glaucoma, despite effective IOP control, continue to lose vision [3]. As a result of this fact, it is increasingly suggested that risk factors independent of IOP play an important role in the development of glaucoma. The direct drug effect on the mechanism of GCS apoptosis is direct neuroprotection.
Structural and functional damage in glaucoma
During the development of glaucoma, damage to visual functions is associated with damage to the RGC complex [4]; damage to photoreceptors in glaucoma has not been confirmed [5]. The main mechanism of death of GCS is excitotoxicity (English to excite - excite, activate) - a pathological process leading to damage and death of nerve cells under the influence of neurotransmitters that can hyperactivate NMDA and AMPA receptors (N-methyl-D-aspartate; α- amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid). Excitotoxicity was first described by Lucas in an experiment on mice in 1957 [6], and in 1996, Dreyer showed an increase in glutamate levels in the vitreous body of patients with glaucoma [7]. The excessive intake of calcium ions into the cell that occurs during excitotoxicity activates a number of enzymes (phospholipases, endonucleases, proteases) that destroy cytosolic structures and initiate apoptosis. The most prominent example of an endogenous excitotoxin is glutamate (a salt of glutamic acid), the most common excitatory neurotransmitter in the vertebrate nervous system. Increasing evidence indicates that retinal neurodegeneration due to glutamate excitotoxicity and/or glutamate-induced oxidative stress is associated with mitochondrial DNA (mtDNA) dysfunction [8–10]. However, the molecular mechanisms underlying these effects are not well understood. In the first trimester of pregnancy, RGC axons find their way to the thalamus (in primates, to the midbrain) due to the expression of certain genes [11], electrical impulses [12], and the influence of glia [13]. Axons that do not reach their target undergo apoptosis [14], while those that reach it, under the influence of neurotrophins, form a projection of their topographic relative position in the thalamus. Due to this close interaction, the death of a particular axon in glaucomatous neuropathy is accompanied by the death of neighboring RGCs [15]. Thus, the initial number of GCS is determined by the cells that survived the process of embryogenesis. A relatively larger number of GCS could delay the onset of clinical signs of glaucoma, but this advantage is offset by a more pronounced response to an increase in IOP in the large optic nerve head, associated with a larger volume of GCS [16]. The 2014 European Glaucoma Guidelines describe the dynamics of changes in the number of GCS as the main criterion for determining the principles of treatment of glaucoma: in healthy eyes without glaucoma, with a natural decrease in GCS with age, their number never decreases to a level at which significant visual deterioration occurs. With the accelerated death of GCS, characteristic of glaucoma, their critically low level is reached throughout life, leading first to visual field defects and then to blindness [17]. Changes in visual fields in patients with glaucoma are a classic example of the association of structural (sectoral lesions of the nerve fiber layer) and functional damage to the eye. Modern research methods make it possible to rethink the classical Graefe triad, more fully studying the relationship between the increase in IOP, changes in visual fields and damage to the RGC [18, 19]. A decrease in the photosensitivity of the retina and the appearance of loss of the visual field, observed on static perimetry, correlates with the thinning of the RGC and the layer of retinal nerve fibers (the coefficient of determination in regression analysis R2 is 0.303 and 0.406, respectively), verified by optical coherence tomography [20]. The relative risk (the ratio of the risk of an event in one group relative to the risk in another group) of developing visual field defects with progressive thinning of the retinal nerve fiber layer using various assessment methods is 3.95–8.44 (using Early Manifest Glaucoma Trial criteria) [21 ]. The rate of progression increases by 0.02% per year for every decibel decrease in photosensitivity [22]. Features of retinal damage in glaucoma - characteristic arcuate defects in photosensitivity in the central region and defects in the inferior nasal region (the most detrimental to the quality of life [23]) - are used in the development of test program algorithms for perimeters and programs for determining progression [19, 24]. The presence of pseudoexfoliations, older age, and higher mean IOP are associated with more rapid disease progression. However, in a sample of 362 patients with IOP dynamics over 7.8 years from 20.15 to 18.10 mm Hg. Art. a negative trend was observed in 89% of cases. At the same time, the dynamics of the decrease in photosensitivity varied from -30.4 to +1.6 decibels per year, with peaks at -0.3 and -0.7 decibels per year [25]. Changes in visual functions significantly reduce the quality of life of patients: in a survey of 3,700 patients with ophthalmological diseases, patients with glaucoma rated their condition at 62.6 points out of 100, which is noticeably lower than for other common eye diseases (78.1 points for refractive errors, 74 .4 - for cataracts, 72.7 - for retinal pathology) [26]. When tested using the SF36 questionnaire with differentiated scales, patients with glaucoma showed the lowest category of vitality, social activity and emotional state (social functioning, role-emotional functioning) [27]. Damage to binocular vision significantly affects the quality of life: a loss of binocular light sensitivity by 0.1 decibels per year increases the risk of a significant decrease in quality of life by almost a third [28]; a decrease in binocular light sensitivity by 1 decibel leads to a decrease in quality of life by approximately 2.8 points out of 100 (according to the results of testing using the NEI VFQ-25 questionnaire) [29]. When summarizing a number of studies on the quality of life in patients with glaucoma, it becomes clear that even the fact of diagnosing an incurable disease, potentially leading to blindness, can reduce the quality of life, and the ability of patients to self-care and maintain habitual daily activities directly depends on photosensitivity [30 ]. Based on the above, modern guidelines on glaucoma postulate the goal of its treatment to preserve visual functions by preventing the death of RGCs [17, 31]. The tasks that need to be addressed on the way to this goal are neuroprotective treatment (both indirect by achieving the target IOP and direct) and visual field monitoring. It is necessary to use programs for threshold study of the visual field of automatic static perimeters (30–2 or 24–2 for Humphrey, G1 or G2 for Octopus); in suprathreshold screening modes or with Goldmann kinetic perimetry, small defects often remain undetected, getting lost among the isopter boundaries [32, 33]. To establish the rate of GC loss in patients with newly diagnosed glaucoma, the European Glaucoma Guidelines recommend that static perimetry be performed at least three times in the first two years; automatic methods for assessing progression (event analysis, trend analysis) require at least five studies for calculation. Having determined the stage of the disease, rate of progression and target IOP, with further observation it is possible to perform static perimetry 2 times a year or less often.
Indications for use
β1-adrenergic agonists are used for acute heart failure associated with low cardiac output - against the background of myocardial infarction, cardiomyopathies, cardiogenic shock, and during heart surgery.
β2-adrenergic agonists are used for diseases associated with bronchospasm - bronchial asthma, chronic obstructive pulmonary disease.
Also, β2-adrenergic agonists are prescribed when there is a threat of premature birth.
β3-adrenergic agonists are used for diseases associated with increased bladder tone - overactive bladder syndrome: urinary incontinence, frequent urination.
Hypotensive effect
Brimonidine is a selective alpha-2-adrenergic agonist that combines indirect (by lowering IOP) and direct neuroprotective effects. Inhibition of adenylate cyclase by alpha-2-adrenergic receptor agonists reduces the production of cyclic AMP and, accordingly, the production of intraocular fluid by the ciliary body. Like other selective adrenergic receptor agonists, brimonidine has a rapid hypotensive effect, occurring within 20 minutes after instillation and reaching a maximum within 1–2 hours. Most likely, the rapid decrease in ophthalmotonus is due to a narrowing of the blood vessels of the ciliary body with a subsequent decrease in its volume and a decrease in secretion of intraocular fluid [34]. Subsequently, the vasoconstrictor effect of the drug gradually weakens and blood flow is restored. The long-term hypotensive effect of the drug is due to an increase in uveoscleral outflow, which develops in parallel with the restoration of blood flow and production of intraocular fluid [35]. Along with higher selectivity, brimonidine has less lipophilicity than other drugs of its pharmacological group. This property prevents its diffusion through the epithelial barrier of the cornea and conjunctiva, as well as systemic absorption through the bloodstream, which prevents the occurrence of a spectrum of side effects observed, for example, with the more lipophilic clonidine [36]. Brimonidine was patented in 1972 and introduced for widespread use in ophthalmology in 1996. During preclinical testing in rabbits, monkeys and rats, its high selectivity was revealed: binding to alpha-2 adrenergic receptors is 1000 times higher than binding to alpha-1 -adrenergic receptors, which allows minimizing its effect on the cardiovascular and respiratory systems. Its selectivity for alpha-2 receptors was 7–12 times higher than that of clonidine. In addition, unlike analogues, brimonidine does not cause mydriasis [37]. Based on the results of a study of the hypotensive effect and side effects of brimonidine at concentrations of 0.08, 0.2, and 0.5% (194 patients), the dosage of brimonidine 0.2% was considered optimal in terms of tolerability and hypotensive potential [38]. When comparing the effectiveness of brimonidine 0.2% and timolol 0.5% in two independent studies (1300 patients in total), both drugs showed satisfactory and comparable antihypertensive effectiveness and tolerability with different severity of adverse reactions - more frequent allergic manifestations when using brimonidine, and a marked decrease Heart rate in the timolol group. The use of brimonidine was accompanied by a more pronounced peak decrease in IOP, while timolol had a lower plateau phase [39, 40]. When brimonidine was prescribed to patients with decompensated IOP on the maximum hypotensive regimen, ophthalmotonus decreased by 16–32%; in 80% of the subjects, the level of IOP compensation was maintained after 6 months. with good tolerability of the drug [41]. A number of publications note the tropism of brimonidine for melanin. It has been suggested that melanin acts as a reservoir for brimonidine, storing it and then releasing it into surrounding tissues. In a comparative study of pigmented rabbits and albinos, different rates of drug accumulation were confirmed [42]. It has been established that brimonidine accumulates in the iris, ciliary body and chorioretinal complex even after a single instillation. Thus, the accumulation effect is potentiated over time, allowing the concentration of the drug in these structures to be increased by 17 times in a few weeks. This accumulation potential also suggests that topical brimonidine therapy may produce concentrations in the posterior segment of the eye that are sufficient to produce neuroprotective effects [36, 43]. An example of this effect is the results of a 2011 study in which 190 patients with normal tension glaucoma were treated with timolol or brimonidine every 4 months. Perimetry was performed. Data were obtained showing that over long periods of observation (up to 48 months), the number of patients with deterioration of visual fields was 4 times higher when using timolol monotherapy than when prescribing brimonidine [44]. Previous studies have been conducted in which patients with open-angle glaucoma showed an improvement in contrast sensitivity after 3 months. therapy with brimonidine [45], as well as improvement in retinal photosensitivity when using brimonidine [46]. Another study noted a trend towards slower visual field deterioration in patients with retinal dystrophy who received brimonidine for more than 24 months. [47].
Non-selective adrenergic agonists
Non-selective adrenergic agonists are capable of stimulating both alpha and beta receptors, causing a wide range of changes in many organs and tissues. These include adrenaline and norepinephrine.
Epinephrine activates all types of adrenergic receptors, but is considered primarily a beta agonist. Its main effects:
- Narrowing of blood vessels in the skin, mucous membranes, abdominal organs and enlargement of the lumens of blood vessels in the brain, heart and muscles;
- Increased myocardial contractility and heart rate;
- Expansion of the lumens of the bronchi, reduction of mucus production by the bronchial glands, reduction of edema.
Adrenaline is used mainly for the purpose of providing first aid and emergency care for acute allergic reactions, including anaphylactic shock, cardiac arrest (intracardiac), hypoglycemic coma. Adrenaline is added to anesthetic drugs to increase their duration of action.
The effects of norepinephrine are in many ways similar to adrenaline, but less pronounced. Both drugs have the same effect on the smooth muscles of internal organs and metabolism. Norepinephrine increases myocardial contractility, constricts blood vessels and increases blood pressure, but the heart rate may even decrease, due to the activation of other heart cell receptors.
The main use of norepinephrine is limited to the need to raise blood pressure in cases of shock, injury, or poisoning. However, caution should be exercised due to the risk of hypotension, renal failure if dosing is inadequate, and skin necrosis at the injection site due to narrowing of small microvasculature vessels.