During the diagnostic process, a Chiari or Hiari network was discovered in the right atrium, although I did not feel any special symptoms. What is it, is it dangerous to health, will there be problems in the future and what should be done?
Chiari vasculature (Chiari)
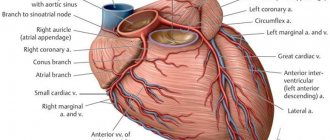
Hello. The Chiari network in the right atrium is a non-pathological, mobile, thin mesh-like structure in the right atrium that is visible on an ultrasound monitor from any position where the inferior vena cava enters the atrium. That is, the mesh consists of remnants of the embryonic coronary sinus valve, which are attached to the Eustachian valve of the inferior vena cava on one side, and on the other to parts of the right atrium (RA). It is discovered truly by chance when diagnosed in 1-2% of the population.
To be more precise, this congenital abnormal structure of the heart in the form of a reticular plate with holes - the atrial septum - begins at the valve of the coronary sinus (Tebesian valve) and the valve of the inferior vena cava (Eustachian valve). It can block the atrium to some extent.
The plate can have different positions, but is generally attached slightly anterior to the Lower (intervenous) tubercle. The Chiari network appears due to insufficient reduction of the right (less often left) sinus valve or due to a false septum. An extended flap of the Eustachian valve and the Chiari network are considered a normal variant.
Diagnostics
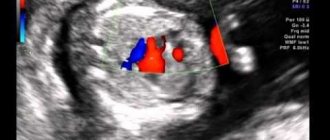
To visualize the Chiari network, echocardiography is used. In this case, positions are used in which the PP is simultaneously examined: apical 4CP, modified parasternal biccaval position and short axis at the level of AOC from the left parasternal approach, but this can only be performed by professional diagnosticians.
Subcostal positions are also used: 4KP, short axis at the level of the Aoc and long axis of the vena cava from below. To assess the insertion site of the Chiari network, it is necessary to perform a polypositional study and include intermediate attachment positions on the eustachian and coronary sinus valve.
In other possible locations in the same plane, the network may not be visualized. Then a 3D EchoCG study is used. The best way to assess the mouth of the coronary sinus is with a sensor from the esophagus, or more precisely, from its middle sections.
Snapshot of the Chiari network
Functions and participation in blood circulation
Features of the location and structure of the walls of the PP regulate the performance of the chamber functions:
- Control of the heart rate, which is realized by a conglomerate of pacemaker cells located between the mouth of the superior PV and the right ear.
- Blood sampling from the entire body through the superior and inferior vena cava systems. There are no valves at their openings, so the RA fills even at low venous pressure.
- Regulation of blood pressure due to:
- reflexes from baroreceptors (nerve endings that respond to a decrease in blood pressure in the half of the RA): the signal transmitted to the hypothalamus stimulates the production of vasopressin, fluid retention in the body and stabilization of indicators;
- natriuretic peptide, which dilates peripheral vessels and reduces the volume of circulating fluid (through diuresis) in arterial hypertension.
- Blood deposition (reservoir function) is provided by the right ear when the RA is overloaded (excess fluid stretches the walls of the structure).
The role of the right atrium in systemic hemodynamics is determined by:
- collection of venous blood (PP - the functional end of a large circle of hemodynamics);
- filling of the right ventricle;
- formation and control of the tricuspid valve, pathologies of which cause disorders in the small and large circle of hemodynamics.
Severe dystrophic damage to the walls of the RA leads to arrhythmias, stagnation of blood in peripheral vessels (swelling of the legs, enlarged liver, fluid in the abdomen, chest cavity) and systemic failure.
Clinic
The Chiari network is highly associated with the OOO (patent foramen ovale). 82% of patients undergoing endovascular PFO closure have a Chiari network or Eustachian valve. If there is a PFO, then right-to-left shunting of the blood is more often detected than in the absence of a network. The network, like the valve, prevents the closure of the patent foramen ovale, and then an atrial septal aneurysm (ASA) and paradoxical embolism may develop.
In this case, the blood flow will be directed from the inferior vena cava to the MPP. With an aneurysm of the atrium, the wall between the atria protrudes in an S-shape (bend in both directions), to the right or to the left. Symptoms do not appear, and the pathology disappears after a while.
In some cases, when the Chiari network is present, patients experience migraine attacks. If patients develop infective endocarditis, the disease can affect the network tissue. They may have the appearance of a tumor formation, then a false diagnosis cannot be ruled out.
If we consider Chiari networks as thromboembolic complications, then their role is twofold:
- the network promotes the formation of blood clots in situ;
- is a trap for thromboembolism and protects the patient from fatal pulmonary embolism.
There have been cases of fragments of network tissue tearing off and migrating into the lungs. The arrhythmogenicity of the Chiari networks may occur due to the close relationship of the SAS (sympathetic-adrenal system) with the venous sinus in some areas, as well as due to the presence of myocardial fibers in the reticular structure of the network tissues. Research has not confirmed that Chiari networks interfere with blood flow. But there have been cases where these atrial formations made it difficult to pass catheters into the coronary sinus or right side of the heart.
Thrombosis of the heart chambers after surgical interventions under artificial circulation (CPB) is a rare and little-studied pathology. Most often, these conditions occur with antiphospholipid syndrome (APS) and other thrombophilias.
The main predisposing factors for thrombus formation in the cavities of the heart are the presence of an obstruction to blood flow, septal defects (congenital and acquired defects of the heart and great vessels), heart rhythm disturbances (atrial fibrillation, atrial flutter), the presence of an intravenous catheter, endocardial electrodes, changes in the blood coagulation system, including including transient ones. In a number of observations, the cause remains unidentified [5]. One of the mechanical causes of thrombus formation and pathological thromboembolism includes a developed Chiari network (incompletely reduced remnants of the venous sinus valve in the right atrium (RA) in the area of the mouth of the inferior vena cava). It can serve as a “trap” for thromboembolism and subsequently as a site for thrombus formation [6].
Various authors [1—3, 5] propose the use of conservative (thrombolytic, antiplatelet and anticoagulant) therapy with varying degrees of effectiveness, as well as surgical correction, for the treatment of patients with thrombosis of the cardiac cavities.
In the available literature [3, 4], we found descriptions of 4 observations of RA thrombosis with the site of thrombus fixation to the Chiari network, which occurred in the delayed period after correction of a congenital heart defect - atrial septal defect (ASD) under CPB.
We present our own description of a rare observation of RA thrombosis in the area of the Chiari network after correction of a congenital heart defect.
Patient K., 14 years old, was admitted to the Federal Research Center for Transplantology and Artificial Organs with a diagnosis of congenital heart disease - MPP defect. She complained of weakness and fatigue during physical activity.
According to X-ray data, the heart is enlarged in volume due to the right sections, signs of hypervolemia of the pulmonary circulation.
The ECG shows sinus rhythm, heart rate up to 80 beats/min, normal position of the electrical axis of the heart, incomplete blockade of the right bundle branch.
According to echocardiography (body weight 53 kg, height 160 cm, body surface area 1.54 m2): end-diastolic size (EDD) of the right ventricle (RV) 3.3 cm; PP 3.7×3.6 cm; Left ventricular (LV) EDV 4.3 cm, end systolic dimension (ESD) 2.8 cm, ejection fraction (EF) 66%; thickness of the interventricular septum (IVS) 0.8 cm; thickness of the posterior wall of the left ventricle (PLW) 0.8 cm; aortic diameter at the level of the fibrous ring is 1.7 cm; pulmonary artery (PA) pressure 30 mm Hg; blood flow speed in the aorta 0.96 m/s; blood flow speed in the LA is 1.36 m/s. In the area of the MR at its lower edge, color Doppler mapping reveals a turbulent oblique flow into the RA cavity, up to 2.1 cm in diameter, with blood discharge from left to right.
Coagulogram before surgery: activated partial thromboplastin time (aPTT) 32 s, prothrombin index 86%, fibrinogen 2600 mg/l, soluble fibrin-monomer complexes (SFMC) 5 mg%. Platelet aggregation with adrenaline is 97% versus 72%, with ADP - 99% versus 87%.
General blood test, general urinalysis, biochemical blood test without any features.
The defect was hemodynamically significant and was subject to surgical correction.
On January 10, 2013, an operation was performed - plastic surgery of the IV defect with a xenopericardium patch under IR conditions. Intraoperatively, during revision, there was an IV defect without a lower edge, measuring 1.8×3.0 cm. In the area of the mouth of the inferior vena cava (IVC), the septum was 5 mm wide, thinned, with multiple fenestrations. Excision of the perforated part of the septum was performed. The defect is closed with a patch of xenopericardium, fixed with four U-shaped sutures (Prolene 4/0) along the posterior semicircle of the IVC and a continuous wrapping suture along the remaining perimeter. When checked, the MPP is sealed.
The postoperative period proceeded without complications. Antibacterial, anti-inflammatory, and desensitizing therapy was carried out. On the 8th day, in satisfactory condition, the patient was discharged under the supervision of a pediatrician and cardiologist at her place of residence. According to the control examination: the ECG shows a sinus rhythm, regular, heart rate 91 beats/min, incomplete blockade of the right bundle branch. With echocardiography, RV EDR is 1.8 cm; PP 2.9×3.2 cm; LV ESD 4.3 cm, LV ESD 2.8 cm; FI 66%; IVS 0.8 cm; PA pressure 25 mm Hg; blood flow speed in the aorta 1.1 m/s; blood flow speed in the LA is 1.12 m/s. Pathological flows in the MPP area are not determined. A consultation at the clinic after 6 months is recommended.
At home, 2 months after discharge, episodes of fever up to 37.3 °C were observed for 2 days against the background of catarrhal symptoms. Symptomatic therapy was carried out with a positive effect.
During a planned control echocardiography 6 months after surgery, a RV ECD of 1.8 cm, RA 4.4×3.8 cm, FI 67% was revealed, the MSP area was intact, a mobile space-occupying RA pedunculated formation was visualized (2.5×1.7 cm), prolapsing into the tricuspid valve (Fig. 1).
Figure 1. Transthoracic echocardiography of the heart of patient K. A mass formation is visualized in the cavity of the right atrium.
The patient was urgently hospitalized. Upon admission, the condition was satisfactory, there were no complaints. According to X-ray multislice computed tomography and magnetic resonance imaging of the chest organs, an additional soft tissue formation of an irregular “grape-shaped” shape measuring 45x30x25 mm, movable, hanging over the mouth of the IVC and prolapsing into the tricuspid valve is determined in the RA cavity (Fig. 2) .
Figure 2. Magnetic resonance computed tomography of patient K. On the left is a mass formation in the cavity of the right atrium, on the right is a mass lesion protruding through the tricuspid valve into the right ventricle.
A differential diagnosis was made between a thrombus and a neoplasm of the PP.
Coagulogram: aPTT 32 s, prothrombin index 82%, fibrinogen 3090 mg/l, RKFM 22 mg%, platelet aggregation with adrenaline 88% versus 44%, with ADP - 86% versus 57%, with ristomycin - 94% versus 72%, antithrombin-3 97% (normal 75-125%), plasminogen 99% (normal 75-150%), D-dimer 523 ng/ml (normal ≤500 ng/ml), protein C 94% (normal 70-130% ).
An increase in D-dimer levels could indicate the possible presence of an organized thrombus in the body in the lysis stage. The formation was regarded as a floating PP thrombus, and the patient underwent surgery for vital indications (due to possible pulmonary embolism).
Intraoperatively: due to the risk of thrombus destruction and pulmonary embolism, IVC cannulation was performed under visual control through the opened RA. The intervention was performed on a beating heart (CPB duration 17 min) and normothermia. When revising the PP in the area of the IVC mouth, the Chiari network is visible, to which a multi-lobular formation (4.2×2.9×1.2 cm) of dense consistency, shaped like a cauliflower, is fixed (see Fig. 3 on the color insert).
Figure 3. Macroscopic specimen of an extracted thrombus attached to a Chiari network (on tweezers). In the area of plastic surgery, the IVS is intact, the patch is endothelialized, and there is no connection between the patch and the neoplasm. No other formations were identified in the RA cavity. Excision of the Chiari network was performed en bloc with the formation.
According to the pathomorphological examination, the formation excised during the operation had a variegated appearance on the section from pink to gray-white with a thin strand of the Chiari network extending from it.
Histological examination (see Fig. 4 on color insert)
Figure 4. Microscopic examination of a remote mass. a — erythrocyte thrombus, Masson staining, uv. 400; b - area of white (leukocyte) thrombus, Masson staining, uv. 400; c — areas of fibrin thrombus at the stage of organization, stained with hematoxylin and eosin, UV. 400; d - close connection of the thrombus (left, red color) with the Chiari network (right, blue color), Masson stain, uv. 400; d — organization of a thrombus in the area of the Chiari network, Masson staining, uv. 400; e — Chiari network, Masson staining, uv. 100. It was established that the removed formation was a thrombus of mixed structure: erythrocyte, leukocyte, fibrin, the degree of maturity of which was different, from a fibrin network to homogeneous eosinophilic clumps. The base of the thrombus was attached to a Chiari network. In these areas, the thrombus was in a state of organization, proliferation of connective tissue cells and the formation of collagen fibers were noted. Thus, there was recurrent Chiari network thrombosis. The Chiari network itself was a connective tissue fibrous structure covered with endothelium.
The postoperative period proceeded without complications. On the 2nd day after surgery, prophylactic anticoagulant (Fragmin) and antiplatelet (Cardiomagnyl) therapy was started, and on the 4th day, warfarin was started. When the target international normalized ratio (INR) of 2.0-2.5 fragmin is reached, the treatment is cancelled. On the 7th day, the central venous catheter was removed and the sutures were removed.
The patient was examined to identify APS and hereditary thrombophilias. Antibody titers to phospholipids IgG, phospholipids IgM, cardiolipin, IgG, cardiolipin, IgM, b2 glycoprotein I, IgG, b2 glycoprotein I, IgM, annexin V, IgG, annexin V, IgM were below the reference values, which made it possible to exclude APS. Data confirming genetic pathology (Leiden mutation, prothrombin gene mutation) were not obtained.
Ultrasound examination of the veins of the lower extremities and pelvis did not reveal any evidence of thrombosis.
On the 10th day, the patient was discharged from the hospital in satisfactory condition under the supervision of a cardiologist and hematologist at her place of residence. At discharge, aPTT was 34 s, prothrombin index 59%, fibrinogen 3102 mg/l, EKFM 28 mg%, platelet aggregation with adrenaline 25% versus 20%, with ADP - 78% versus 40%, D-dimer 1082 ng/ml (< 500), INR 2.79.
During routine examinations 1, 3 and 6 months after surgery, no data were obtained confirming a neoplasm of the heart and great vessels. The patient's condition is satisfactory, there are no complaints. Antiplatelet (cardiomagnyl) and anticoagulant (warfarin) therapy continues, INR is maintained within 2.0-2.5.
Discussion
Our observation should obviously be considered as spontaneous thrombosis of the Chiari network after correction of congenital heart disease under cardiopulmonary bypass, in the absence of pathological prerequisites (APS, thrombophilia). It is impossible to exclude the fixation of a microembolus in the Chiari network as a trigger for thrombus formation, although there were no vascular catheters in the veins of the lower extremities.
To prevent such complications, all patients with a relevant history (venous thrombosis, recurrent miscarriage) require careful additional examination in order to identify coagulopathies, thrombophilias or APS. The threat of thrombosis of the cardiac cavities in the postoperative period is the reason for in-depth preoperative examination and appropriate therapy after surgery.
The question of the advisability of preventive resection of the Chiari network when it is detected during the primary operation as a measure to prevent postoperative RA thrombosis is controversial [6].
Clinical significance
Isolated network is less common than in combination with communications between the atria. If it is detected, then this gives grounds to look for LLC and AMSA (atrial septal aneurysm) by polypositional study, using standard and intermediate positions.
Clinically, due to the pathology, thromboembolic complications (thrombus in situ), infective endocarditis, difficulties with differential diagnosis due to various intra-atrial formations (tumors, blood clots, cysts) are possible, and difficulties are possible when conducting endovascular approaches to the heart.
Echocardiography
Echocardiography shows the Chiari network (CN):
- in panel A, the tricuspid valve (TV) is displaced in the apical 4-chamber view;
- in panel B, it is in the inflow of the right ventricle;
- Panel C shows laminar flow through an atrial septal defect.
Lamina reticulum fibers
In the video in this article you can see the work of the heart with the presence of the Chiari network.
Chiari malformation (Arnold-Chiari syndrome)
Chiari malformation type I is the most common type in clinical practice. It is manifested by liquor-hypertension, cerebellobulbar and syringomyelic syndromes, as well as damage to the cranial nerves. Typically, Chiari I malformation manifests itself during puberty or in adulthood.
CSF hypertensive syndrome, which is accompanied by Chiari I malformation, is characterized by headache in the back of the head and cervical region, aggravated by sneezing, coughing, straining or straining the neck muscles. Vomiting may occur, regardless of food intake and its nature. When examining patients with Chiari malformation, increased neck muscle tone is revealed. Cerebellar disorders include speech impairment (dysarthria), nystagmus, and cerebellar ataxia.
Damage to the brain stem, the nuclei of the cranial nerves and their roots located in it is manifested by decreased visual acuity, diplopia, swallowing disorder, hearing loss of the type of cochlear neuritis, systemic dizziness with the illusion of rotation of surrounding objects, tinnitus, sleep apnea syndrome, repeated short-term losses consciousness, orthostatic collapse. Patients who have a Chiari malformation report increased dizziness and tinnitus when turning their head. Turning the head in such patients can provoke fainting. There may be atrophic changes in half of the tongue and paresis of the larynx, accompanied by hoarseness and difficulty breathing. Tetraparesis is possible with a greater decrease in muscle strength in the upper extremities than in the lower extremities.
In cases where Chiari I malformation is combined with syringomyelia, syringomyelic syndrome is observed: dissociated sensory disturbances, numbness, muscle wasting, pelvic disorders, neuroarthropathy, disappearance of abdominal reflexes. At the same time, some authors point out the discrepancy between the size and location of the syringomyelic cyst, the prevalence of sensitivity disorders, the severity of paresis and muscle wasting.
Chiari II and Chiari III malformations have similar clinical manifestations, which become noticeable from the first minutes of a child’s life. Chiari II malformation is accompanied by noisy breathing (congenital stridor), periods of short-term respiratory arrest, bilateral neuropathic paresis of the larynx, and impaired swallowing with reflux of liquid food into the nose. In newborns, Chiari II malformation is also manifested by nystagmus, increased muscle tone in the upper extremities, and cyanosis of the skin that occurs during feeding. Movement disorders can be expressed to varying degrees and progress up to tetraplegia. Chiari III malformation has a more severe course and is often a developmental disorder of the fetus that is incompatible with life.
Conclusion
The network itself is not clinically significant, but it is often associated with pathologies: patent foramen ovale (PFO), intraatrial thrombosis, or atrial arrhythmias. The Chiari mesh is complicated by thrombosis followed by thromboembolism, but no treatment is prescribed because it blocks the progression of the thrombus and is a rudiment of the coronary sinus valve.
A reticulate plate with holes has no practical significance. But to detect it, an unusual diagnosis is made, which does not exclude a medical error. Therefore, it is important to perform a thorough echocardiography, which during cardiovascular interventions will make it easier to overcome the difficulties when performing catheterization in the coronary sinus or right heart.
Echocardiographic diagnosis of the Chiari network
The Chiari network is a mobile, mesh-like structure that is sometimes (2% of the time) seen in the right atrium, attaching to the Eustachian valve and other intra-atrial structures. This network embryologically represents a remnant of the right valve of the sinus venosus and usually has no significant clinical significance. However, sometimes the Chiari network can become a site for the formation of blood clots and tumors or interfere with the advancement of emboli and the passage of the catheter. The article presents a review of the literature on this problem and clinical observations of two patients with a Chiari network, which was diagnosed during an echocardiographic examination. These cases are notable for the rather large size of the Chiari network, as well as the fact that on some images the Chiari network gives the impression of the presence of an additional space-occupying lesion in the cavity of the right atrium. In one case, this circumstance even led to the hospitalization of the patient. Identification of the Chiari network can be important as it can be mistaken for a number of pathological formations such as thrombi, vegetations, tumors or avulsed tricuspid valve chords.
Keywords:
echocardiography, Chiari's network, coronary sinus, and right atrium
Literature:
1.Loukas M., Sullivan A., Tubbs RS et al. Chiari's network: review of the literature // Surg. Radiol. Anat. 2010. V. 32. No. 10. P. 895-901. 2.Bhatnagar KP, Nettleton GS, Campbell FR et al. Chiari anomalies in the human right atrium // Clin. Anat. 2006. V. 19. No. 6. P. 510-516. 3. McMahon CJ, Nihill MR, Kovalchin JP, Lewin MB Echocardiographic features of Chiari's network in association with tricuspid atresia // Tex. Heart Inst. J. 2000. V. 27. No. 3. P. 312-313. 4.Helwig FC The frequency of anomalous reticula in the right atrium of the human heart “Chiari network”. Report of eight cases // Am. J. Pathol. 1932. V. 8. No. 1. P. 73-79. 5.Schneider B., Hofmann T., Justen M.H., Meinertz T. Chiari's network: normal anatomic variant or risk factor for arterial embolic events // J. Am. Coll. Cardiol. 1995. V. 26. No. 1. P. 203-210. 6.Yater WM Variations and anomalies of the venous valves of the right atrium of the human heart // Arch. Pathol. 1929. V. 7. P. 418-441. 7. Orbison JL Thrombosis of anomalous chordae in the right atrium (Chiari's network) // Am. Heart J. 1949. V. 37. No. 1. P. 119-122. 8.Teo EY, Ittleman F., Hamlin MP A Chiari network and difficult cannulation of the coronary sinus for retrograde perfusion // Anesth. Analg. 2010. V. 111. No. 1. P. 79-81. 9. Goldschlager A., Goldschlager N., Brewster H., Kaplan J. Catheter entrapment in a Chiari network involving an atrial septal defect // Chest. 1972. V. 62. No. 3. P. 345-346. 10.Salmeron O., Zarco P., Nunez L. Unusual complication of right catheterization: hooking of the catheter in Chiari's network // Arch. Inst. Cardiol. Mex. 1966. V. 36. No. 4. P. 387-390. 11. Maruyama T., Kurogouchi F. Entrapment of a tinned lead by the Chiari network with preserved atrial sensing ability in a patient with atrioventricular block: a case report // J. Cardiol. 2007. V. 44. No. 6. P. 251-254. 12.Latif F., Peyton M., Laszik Z., Sivaram CA Infectious endocarditis of a papillary fibroelastoma on Chiari network of right atrium: a case report // J. Am. Soc. Echocardiogr. 2008. V. 21. No. 2. P. 188.e3-188.e4. 13. Mousavi N., Bhagirath K., Ariyarajah V. et al. Chiari network endocarditis: not just an innocent bystander // Echocardiography. 2008. V. 25. No. 6. P. 642-645. 14. Yuen ST, Dickens P. Sudden death from secondary massive pulmonary embolism derived from marrow emboli trapped by Chiari's network // Forensic Sci. Int. 1992. V. 52. No. 2. P. 211-214. 15. Powell ED, Mullaney JM The Chiari network and the valve of the inferior vena cava // Brit. Heart J. 1960. V. 22. P. 579-584. 16. Koz C., Yokusoglu M., Baysan O., Uzun M. Giant Chiari network mimics intracardiac tumor in a case of neurofibromatosis // Int. J. Cardiol. 2008. V. 130. No. 3. P. 488-489. 17. Alekhin M.N., Vaniev S.B., Pavlov A.V. and others. Thromboembolism of the Chiari network and pulmonary artery // Cardiology. 2014. No. 11. P. 54-57. 18. Clements J., Sobotka-Plojhar M., Exalto N., van Geijn HP A connective tissue membrane in the right atrium (Chiari's network) as a cause of fetal cardiac arrhythmia // Am. J. Obstet. Gynecol. 1982. V. 142. No. 6. P. 709-712. 19. Benbow EW, Love EM, Love HG, MacCallum PK Massive right atrial thrombus associated with a Chiari network and a Hickman catheter // Am. J. Clin. Pathol. 1987. V. 88. No. 2. P. 243-248. 20.Jongbloed MR, Schalij MJ, Poelmann RE et al. Embryonic conduction tissue: a spatial correlation with adult arrhythmogenic areas // J. Cardiovasc. Electrophysiol. 2004. V. 15. No. 3. P. 349-355. 21. Haissaguerre M., Jais P., Shah DC et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins // N. Eng. J. Med. 1998. V. 339. No. 10. P. 659-666. 22. Kalman JM, Olgin JE, Karch MR et al. Cristal tachycardias: origin of right atrial tachycardias from the crista terminalis identified by intracardiac echocardiography // J. Am. Coll. Cardiol. 1998. V. 31. No. 2. P. 451-459. 23.Tsai CF, Tai CT, Hsieh MH et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation // Circulation. 2000. V. 102. No. 1. P. 67-74. 24.Prajapat L., Ariyarajah V., Spodick DH Abnormal atrial depolarization associated with Chiari network? // Cardiology. 2007. V. 108. No. 3. P. 214-216. 25. Pellett AA The Chiari network in an echocardiography student // Echocardiography. 2004. V. 21. No. 1. P. 91-93.
Methods for diagnosing disorders
Comprehensive diagnosis of right atrium dysfunction includes:
- X-ray of the chest organs (diagnosed as a shift in the boundaries or an increase in the size of the heart);
- electrocardiography (bioelectric characteristics of the myocardium, state of the cardiac conduction system);
- ultrasound examination (EchoCG);
- Doppler diagnostics to study the speed, volume and presence of obstacles to blood flow.
Functional methods that assess the body's response to stress tests have become widespread. For example, dosed walking (treadmill) or bicycle ergometry are used for ECG load.