Express diagnosis of myocardial infarction
ICA studies:
05.09.2018
Shibanov A.N., Gervaziev Yu.V.
Myocardial infarction (MI) is one of the main causes of death in civilized countries. According to data presented at the Board of the Ministry of Health and Social Development in 2008, in the structure of causes of mortality in Russia, MI accounts for 20% and ranks second, only slightly inferior to cerebrovascular diseases.
Over the past 20 years, world medicine has made great strides in the treatment of cardiovascular diseases, including MI. Methods such as thrombolytic therapy, reperfusion of the heart muscle, and balloon angioplasty in many cases can restore blood circulation in the heart muscle and save the patient’s life. The effectiveness of these methods depends critically on the time between the onset of acute heart failure and the start of treatment. Every hour of delay significantly reduces the likelihood of a positive outcome. Therefore, timely diagnosis plays an important role in the successful treatment of MI.
Diagnosis of MI is based on 3 groups of criteria:
- history and physical examination;
- instrumental research data;
- determination of cardiac markers in the blood: proteins specific to the infarction state.
History and physical examination data are essentially subjective and cannot serve as criteria for diagnosing myocardial infarction. The purpose of the physical examination is, first of all, to exclude non-cardiac causes of chest pain (pleurisy, pneumothorax, myositis, inflammatory diseases of the musculoskeletal system, chest trauma, etc.). In addition, physical examination reveals heart diseases not associated with damage to the coronary arteries (pericarditis, heart defects), and also assesses hemodynamic stability and the severity of circulatory failure [1].
The key method of instrumental diagnostics is electrocardiography (ECG). The most reliable electrocardiographic signs of MI are the dynamics of the ST segment and changes in the T wave. However, up to 25% of all MI do not cause any changes on the ECG, and from 20% to 30% of all cases proceed without a painful attack, especially in the elderly, as well as in patients diabetes and hypertension.
Therefore, the determination of biochemical markers of myocardial necrosis is a necessary component of the comprehensive diagnosis of MI [1].
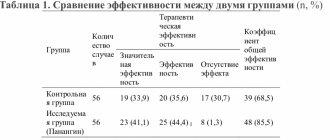
The National Academy of Clinical Biochemistry Laboratory Practice of the United States offers a differential diagnosis scheme for acute coronary syndrome, presented in Figure 1 [2].
Figure 1. Diagram of differential diagnosis of acute coronary syndrome.
MI marker proteins
During myocardial necrosis, the contents of the dead cell enter the general bloodstream and can be determined in blood samples.
With the correct selection of these proteins (markers or, more precisely, cardiac markers) based on information about their concentration in the blood, a correct diagnosis can be made. The choice of markers of myocardial necrosis is determined, first of all, by their specificity for a given disease. Also important are such characteristics as the time of appearance in diagnostically significant concentrations in the blood and the time during which their concentration remains elevated. Biomarkers of myocardial necrosis include cardiac troponins I and T (cTnI and cTnT), cardiac fraction of creatine kinase (CK-MB), myoglobin (Myo), lactate dehydrogenase-1 isoenzyme, transaminases and a number of other molecules [3]. Figure 2. Structure of Troponin I.
Troponin I is a protein localized in the heart muscle and involved in its contraction. In the muscle cell there are three forms of troponins - I, T and C, in a ratio of 1:1:1. They are part of the troponin complex, which is associated with the protein tropomyosin. The latter, together with actin, forms thin filaments of myocytes - the most important component of the contractile apparatus of striated muscle cells. All three troponins are involved in the calcium-dependent regulation of contraction-relaxation.
- TnI is the inhibitory subunit of this complex, binding actin during the period of relaxation and inhibiting the ATPase activity of actomyosin, thus preventing muscle contraction in the absence of calcium ions.
- TnT is a regulatory subunit that attaches the troponin complex to thin filaments and thereby participates in the calcium-regulated act of contraction.
- TnS is a calcium-binding subunit and contains four calcium receptor sites.
- TnI and TnT exist in three isoforms, unique in structure for each type of striated muscle (fast, slow and cardiac).
The cardiac isoform of TnI differs significantly from the TnI isoforms localized in skeletal muscle: TnI contains an additional N-terminal polypeptide consisting of thirty-one amino acid residues. Thus, TnI is an absolutely specific myocardial protein. The molecular weight of TnI is about 24,000 daltons.
Cardiac isoforms of both TnI and TnT are used in the diagnosis of myocardial infarction. They can be differentiated from similar skeletal muscle proteins immunologically, using monoclonal antibodies, which is used in methods for their immunotesting.
Cardiac TnS, in contrast to TnI and TnT, is completely identical in structure to muscle TnS and, therefore, is not a cardiac-specific protein.
Figure 3. Structure of creatine kinase.
Creatine kinase is an enzyme that converts phosphocreatine with the formation of creatine and ATP (resynthesis of ATP in the transphosphorylation reaction of ADP and creatine phosphate), which is necessary for muscle contraction.
The total activity of creatine kinase consists of the activity of the enzyme isoforms - CK-MM, CK-BB and CK-MB, where the M is the muscle subunit of the enzyme (muscle) and the B-brain subunit. The CK-BB isoform is mainly present in brain tissue, lungs, and stomach. The CK-MM isoenzyme is characteristic of muscle tissue, and the CK-MB isoform is concentrated in heart tissue. When the heart muscle is damaged, it is this isoform that exits the heart cells into the bloodstream, which is accompanied by an increase in the activity of the isoenzyme in the blood. Figure 4. Structure of myoglobin.
Myoglobin is an iron-containing protein in muscle cells that is responsible for oxygen transport in skeletal muscles and in the heart muscle. An increase in protein levels in the blood is observed 2 hours after the onset of pain during MI. Myoglobin levels are the first of all cardiac markers to increase; the degree of increase depends on the area of myocardial damage. This is the shortest-lived marker of MI - normalization of the indicator usually occurs within 24 hours. This is where its unique diagnostic value lies. If the myoglobin level remains elevated after an acute attack of myocardial infarction, this is evidence of an expansion of the infarction zone. Repeated increases in the level of myoglobin in the blood against the background of normalization that has already begun indicate the formation of new necrotic foci. Thus, myoglobin is very important for diagnosing recurrent myocardial infarction. A significant disadvantage of this marker is its low specificity - it also appears in the blood when skeletal muscles are damaged.
Other potential cardiac markers (lactate dehydrogenase-1 isoenzyme, transaminases, fatty acid binding protein) are practically not used for diagnosing MI for various reasons.
The properties of cardiac markers can be summarized as follows:
| Marker | Molecular weight | Cardio-specificity | Advantages | Flaws | First increase in concentration | Duration of maintaining the increased level |
| CTnT | 37000 | ++++ | High specificity for cardiac tissue. Detection of MI within two weeks. | It is not an early marker of myocardial necrosis. Biphasic kinetics of release into the bloodstream makes it difficult to diagnose recurrent myocardial infarction. | 3-4 hours | 10-14 days |
| CTnI | 23500 | ++++ | High specificity for cardiac tissue. Detection of MI within 7 days. | It is not an early marker of myocardial necrosis. | 4-6 hours | 4-7 days |
| CK-MB | 85000 | +++ | Extensive experience in clinical use. Former “gold standard” for detecting MI. | Reduced specificity for skeletal muscle injury. | 3-4 hours | 24-36 hours |
| Myo | 18000 | Absent | Possibility of excluding MI in the early stages. Possibility of diagnosing recurrent infarctions. | Low specificity for skeletal muscle damage and renal failure. Rapid elimination from the body. | 1-3 hours | 12-24 hours |
Patients with suspected acute myocardial infarction should undergo simultaneous determination of three cardiac markers - troponin, CK-MB and myoglobin.
In urgent situations, in particular in emergency situations, it is preferable to determine cardiac markers using the express method, since the time factor for making a diagnosis is critical.
Currently, the only method that allows rapid determination of troponin, creatine kinase and myglobin is the immunochromatography method (ICA). It allows you to identify disease markers outside of laboratory conditions and within 15 minutes.
Principle of immunochromatographic analysis
The apparatus of the immunochromatographic test is shown in Figure 5.
Figure 5. Schematic representation of the immunochromatographic test.
It consists of the following elements: a filter, a membrane with a conjugate, a chromatographic membrane containing one or more immune complex capture zones and a control capture zone, and an absorption membrane. This entire device is placed in a plastic case, which has a receiving window. The disassembled appearance of the immunochromatographic test is shown in Figure 6.
Figure 6. View of the immunochromatographic test with the body removed.
Visible are a pre-filter, a membrane with a conjugate, a chromatographic membrane, and an absorption membrane. The method is based on thin layer chromatography technology (Figure 7). Blood in a volume of 100 μl (5-6 drops) is applied through a special receiving window onto the sample substrate. Blood plasma, having passed through the filter, under the action of capillary forces, impregnates the strip, where the marker proteins present in the blood plasma react with monoclonal antibodies labeled with colloidal gold, forming antigen-antibody complexes. Colloidal gold is a special dye that is visible even in ultra-low concentrations.
Figure 7. Principle of the immunochromatographic method.
Further, under the action of capillary forces, these complexes move along the chromatographic membrane and react with immobilized antibodies in the corresponding zones against the same proteins. In this case, if the target marker protein is present in sufficient quantity, the colored conjugate associated with the protein accumulates in the zone of immobilization of antibodies against this protein. A kind of “sandwich” is formed (picture). The free conjugate moves across the chromatographic membrane and is captured in the control band by immobilized secondary antibodies. If a sufficient amount of immune complexes accumulates in the capture zones, the stripes, thanks to colloidal gold particles, acquire a characteristic burgundy hue. The control zone is always colored.
If a clear color band does not appear in the control zone, the test result is incorrect, in which case the sample must be retested. In this case, a new test device must be used.
If the capture zones do not contain a single bright color band, and the control zone shows such a band, then the test result is negative.
The test is positive if colored stripes appear in the areas of immune complex capture within 15 minutes. Diagnosticums are designed in such a way that the barely visible presence of a colored band already indicates that the concentration of the marker protein exceeds the threshold level.
Figure 8 shows several clinical cases.
The studies were carried out in the SklifLab laboratory of the Research Institute for Emergency Medicine named after. N.V. Sklifosovsky, where serum samples from patients with suspected myocardial infarction were received from May 18 to May 22, 2009. The three-component cardiotest “ImmunTech” produced by YD Diagnostics, South Korea was used as a test system. Figure 8. Examples of cardiac marker analysis results using an immunochromatographic test.
Explanations in the text. On test A we see only a bright control line; the test does not detect any of the cardiac markers. This analysis result indicates that the likelihood of myocardial infarction in this patient is low. However, it should be pointed out here that a diagnosis cannot be made based on the results of immunochromatographic analysis alone. It is necessary to take into account the entire range of diagnostic information. If there is a clinical picture characteristic of myocardial infarction, the study of cardiac markers should be repeated after 4 and 8 hours.
Test B shows the presence of myoglobin in the patient's blood with a concentration much higher than the detection threshold, very faint coloring of the CK-MB zone is visible, and troponin I is not detected. This test result indicates that the patient's risk of myocardial infarction is quite high. The absence of troponin may be due to the fact that little time has passed since the onset of the process of cardiomyocyte necrosis and troponin has not yet had time to enter the patient’s blood.
Test B shows a characteristic picture of myocardial infarction - all three cardiac markers are present.
On test D we see the presence of staining of the troponin I zone, very weak staining of the creatine kinase MB zone (at the detection threshold) and the absence of myoglobin. The presence of troponin in the patient’s blood indicates that the process of cardiomyocyte necrosis has taken place. The absence of myoglobin and the detection of creatine kinase MB at the sensitivity threshold suggests that, apparently, myocardial infarction occurred more than 96 hours ago.
From the examples given, we see how the efficiency of diagnosing myocardial infarction increases when, along with traditional diagnostic methods, we use the method of immunochromatographic analysis of cardiac markers. This is especially effective when three cardiac markers are determined at once and in dynamics.
In addition to high-quality non-instrumental ICA diagnostics, systems for quantitative instrumental immunochromatographic analysis have been developed. The idea is that these tests use a fluorescent tag instead of a visual dye (colloidal gold), which increases the sensitivity of the test, but requires the use of a special instrument that can quantify the presence of markers. These systems can also work on whole blood, the analysis time is 15-20 minutes. The described systems make it possible to carry out diagnostics directly in the department, at the patient’s bedside. However, the cost of diagnostics is extremely high: up to a thousand rubles for one analysis. The most well-known diagnostic tool for quantitative ICA detection in Russia is the Triage system from Biosite (USA).
The widespread use of immunochromatographic methods for rapid diagnosis of cardiac markers will significantly increase the effectiveness of treatment of myocardial infarction and reduce mortality. Rapid tests must be used in all medical institutions, and not just in cardiology departments of hospitals. In case of any ailment, a person first of all goes to the clinic. If the patient does not have a clearly defined clinical picture of myocardial infarction and there are no clear signs in the cardiogram, not every doctor will decide on urgent hospitalization of the patient. Determining cardiac markers in a clinic will, in some cases, reduce the likelihood of a diagnostic error and allow the doctor to make the right decision. For the same reasons, it is necessary to have rapid tests for diagnosing myocardial infarction in medical and obstetric centers. A positive test result will allow you to reasonably call an ambulance or air ambulance team from the nearest regional center. The small size and weight of immunochromatographic tests allow them to be used by a local doctor or general practitioner when visiting a patient at home.
The relevance of using immunochromatographic rapid tests in emergency medicine is especially high.
It is obvious that the use of such diagnostic methods as computed tomography, ultrasound, various invasive techniques and “classical” laboratory tests in this area is extremely difficult, since the vast majority of emergency cases do not occur in a hospital setting, but in “street” or “home” conditions . In addition, these methods often require preliminary preparation of the patient, which is simply not feasible in an emergency situation. In the Russian Federation, an emergency physician, responding to a call related to any emergency situation, can only rely on the data of a physical examination and the results of electrocardiography, which is not informative in all cases of acute myocardial infarction.
Currently, the requirements for diagnostic methods in emergency medicine, and these are, first of all, quick and accurate results, are most fully met by ICA express diagnostics. This diagnosis can be carried out directly at the point of care for the patient. And given that the time it takes for a patient to be transported by ambulance to the hospital is usually at least 30 minutes, it can be argued that rapid diagnostics will help save the lives of many patients, not to mention reducing the overall cost of treatment.
However, the use of ICA is recommended not only in field conditions. In 2006, American doctors compared the economic indicators of treating patients in a hospital if cardiac markers were determined using immunochromatographic rapid diagnostics and if they were determined in the laboratory. It turned out that despite the relative high cost of express diagnostics, the overall costs of treatment have decreased due to a decrease in the time from blood sampling to obtaining the result. Thanks to this, the costs of medical procedures and pharmacological drugs have decreased, and the length of stay of patients in the hospital has decreased.
Thus, immunochromatographic rapid diagnostics can be recommended for diagnosing MI both in medical institutions and in emergency medicine.
Bibliography.
- Recommendations for the treatment of acute coronary syndrome without persistent ST segment elevation on the ECG. Approved at the Russian National Congress of Cardiologists on October 11, 2001 // https://www.cardiosite.ru/medical/recom-ostcorsin.asp
- Morrow DA, Cannon CP, Jessa RL et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical Characteristics and Utilization of Biochemical Markers in Acute Coronary Syndromes //Clinical Chemistry 2007. V.53(4). P.552-574.
- Saprygin DB. Current status of the use of myocardial biomarkers of necrosis in acute coronary syndrome. // Laboratory medicine 2009. T.10 P.35-38.
- Apple FS, Chung AY, Kogut ME et al. Decreased patient charges following implementation of point-of-care cardiac troponin monitoring in acute coronary syndrome patients in a community hospital cardiology unit. //Clin Chim Acta. 2006. V.370(1-2). P.191-195.
Tags: Cardiotest, Diagnostics, Prevention of heart attack, Shibanov A.N., Troponins, Three-component Cardiotest "ImmunTech"
« Back to article list Share on
Differential diagnosis of angina and other cardiovascular diseases
Cardiologist of the Department of Cardiology No. 1 Verbitsky V.L.
In some patients with a typical clinical picture of angina pectoris and positive stress tests (according to objective criteria), coronary angiography does not find any changes in the coronary arteries; Such patients have no signs of spontaneous angina. In these cases, we can talk about coronary artery disease with unchanged coronary arteries. In the English-language literature, this type of pathology is called “X syndrome.” Special studies show that in these patients the ability of the coronary arteries to dilate is significantly reduced, which is detected when assessing coronary blood flow using argon or rubidium radionuclides under the conditions of a dipyridamole test. When performing a myocardial biopsy in these patients, electron microscopy reveals degenerative changes in cardiomyocytes. Thus, both the decrease in coronary reserve and biopsy data allow us to think of “syndrome X” as the initial manifestations of dilated cardiomyopathy. This diagnosis becomes even more reliable if patients have persistent or transient (appearing under load) blockade of the left branch of the His bundle.
With mitral valve prolapse syndrome, there is a pressing or burning pain in the third or fourth intercostal space to the left of the sternum. Much less often the pain is localized behind the sternum or xiphoid process. Mild pain can last for hours, intensifying after physical and emotional stress. When intensified, the pain can cover the entire heart area. In some patients, the pain is relieved by nitroglycerin. The pain is often combined with cardiac arrhythmias (extrasystole, atrial fibrillation, atrioventricular block). It is believed that mitral valve prolapse predisposes to spasm of the coronary arteries. This syndrome is more common in patients with an asthenic build with a flat chest - with a reduced anteroposterior size. In patients with mitral valve prolapse, abnormalities of the ST segment and T wave are often detected, especially when performing an exercise test. On FCG and during auxultation in patients, a mesosystolic murmur is detected at the apex, which is often preceded by a mesosystolic click. The diagnosis of mitral valve prolapse has improved significantly due to the widespread use of ultrasound methods of cardiac examination in the clinic. It allows you to detect prolapse of one or both mitral valve leaflets into the atrium. Valuable diagnostic data can be obtained by ventriculography. Establishing a diagnosis of mitral valve prolapse does not exclude the simultaneous presence of stenosing coronary atherosclerosis in the patient.
Valvular stenosis of the aortic mouth, like arterial hypertension, leads to overload and hypertrophy of the left ventricle, increasing the myocardial oxygen demand. The shortening of the period of diastolic filling of the left ventricle observed with a defect of this type contributes to a decrease in coronary blood flow. These patients often complain of pain in the heart area. In the early stages of the disease, they have the character of cardialgia, and with severe aortic stenosis, typical attacks of angina occur. If a systolic murmur is heard on the aorta in patients with angina, then it is necessary to carry out all available diagnostic studies aimed at identifying aortic stenosis. The diagnosis of aortic stenosis is made on the basis of the characteristic systolic murmur (diamond-shaped murmur on a phonocardiogram), physical, radiological and electrocardiographic signs of left ventricular hypertrophy. X-rays often reveal calcification of the aortic valve. Echocardiographic data are of great differential diagnostic importance. Detection of aortic stenosis does not exclude the simultaneous presence of atherosclerosis of the coronary arteries. Aortic stenosis in a patient with angina pectoris, combined with attacks of cardiac asthma, has a dire prognosis.
With mitral heart disease, patients often complain of pain in the heart area, in some cases indistinguishable from angina. The cause of this pain may be pulmonary hypertension, characteristic of mitral stenosis, pathogenetically associated with insufficient blood supply to the hypertrophied right ventricle. Sometimes pain sensations have a neurotic basis, but typical attacks of angina pectoris are highly likely to indicate a concomitant stenotic lesion of the coronary arteries of the heart, which is confirmed by coronary angiography data. When carrying out a differential diagnosis of angina pectoris, it is necessary to exclude aortalgia - pain accompanying inflammatory and degenerative diseases of the aorta. With syphilitic aortitis, which is rare in our time, aortic insufficiency occurs; the pathological process also affects the mouths of the coronary arteries of the heart, which can be accompanied by typical attacks of angina. With nonspecific aortoarteritis, typical angina is rarely observed; prolonged pain in the heart area without irradiation is characteristic.
With an aortic arch aneurysm, numerous accompanying symptoms caused by compression of neighboring organs (cough, dysphagia, hoarseness, visual disturbances, fainting, asymmetrical pulse, compression of the superior vena cava) help to make a correct diagnosis. With aortic dissection, the pain is of maximum intensity from the very beginning. The widest irradiation is characteristic: pain, starting behind the sternum, then radiates to the neck, back, abdominal area, along the spine and even to the legs. Spider fingers and other signs of Marfan syndrome may lead the doctor to believe that chest pain is related to aortic dissection, to which these patients are predisposed.
Pain in the heart area is the most common and one of the early symptoms of myocarditis . They are varied, unlike angina pectoris, they last for hours and days. In the acute period of the disease, the intensity of pain may vary, but the pain is almost constant. When diagnosing, it is necessary to take into account the connection with a recent infection, fever, leukocytosis, and enlargement of both ventricles of the heart. With a more or less prolonged course, cardiomegaly and heart failure become obligatory symptoms of myocarditis. Differential diagnostic difficulties more often arise with mild heart damage, when after a sore throat, flu or other infection, unpleasant sensations appear in the heart area, accompanied by changes in the final part of the ventricular ECG complex. Patients have a tendency to tachycardia and shortness of breath, and a systolic murmur is heard. More often, these symptoms are manifestations of infectious-allergic myocarditis; they gradually undergo a reverse development.
Pain in the heart area is a common occurrence in people suffering from alcoholism. In this case, a diagnosis of coronary artery disease is often made, and pain is regarded as angina pectoris. Difficulties in diagnosis are associated with the fact that patients hide alcohol abuse. The ECG often reveals nonspecific changes in the ST segment and T wave. A bicycle ergometer test may be positive. X-rays reveal an expansion of the borders of the heart. An echocardiogram reveals dilatation of the left ventricle. Dynamic observation of patients shows worsening myocardial damage and the appearance of severe arrhythmias provoked by large amounts of alcohol. Diagnosis of alcoholic cardiopathy is facilitated by simultaneous signs of liver damage. Many cardiovascular disorders in these patients are associated with autonomic disorders, which manifest themselves very early, even before the development of severe myocardial damage. Coronary angiography, as a rule, does not reveal stenotic lesions of the coronary arteries.
is often mistaken for ischemic heart disease in patients with neurocirculatory dystonia (we used one of many designations for the disease). Cardiac syndrome with neurocirculatory dystonia is long-lasting and persistent. The pain is mostly stabbing or aching, localized mainly in the apex of the heart or in the second to fourth intercostal space to the left of the sternum. The pain is relieved or reduced by taking valocordin, validol, sedatives, and using mustard plasters.
Vegetative-dystonic syndrome in neurocirculatory dystonia is manifested by lability of pulse and blood pressure, peripheral vascular disorders, and vegetative-vascular paroxysms of predominantly sympathetic-adrenal origin. Asthenic syndrome is characterized by both physical and intellectual exhaustion and a significant decrease in performance. Weakness (of a neurogenic nature) manifests itself primarily in the morning. During the day, the feeling of weakness and fatigue may increase, forcing patients to go to bed. Neurocirculatory dystonia is characterized by a syndrome of neurotic respiratory disorders: a feeling of lack of air, dissatisfaction with inhalation and yawning, the need to periodically take deep breaths. Sometimes the feeling of suffocation or a lump in the throat comes first. Periods of “paroxysmal shortness of breath of a neurotic” are less common. Eggs with neurocirculatory dystonia never feel completely healthy; they always exhibit some syndrome or a combination of several. The onset or exacerbation of the disease is usually associated with a stressful situation (mental and physical stress), less often with infectious exposure or hormonal changes (pregnancy, abortion, dysvariant disorders, menopause).
The long-term existence of cardiovascular disorders without clear organic heart pathology favors the diagnosis of neurocirculatory dystonia. ECG changes concern only the final part of the ventricular complex. With normal heart sizes and normal position of the electrical axis, biphasic or negative T waves are recorded, especially in the chest leads. To more accurately interpret ECG abnormalities, a number of functional and pharmacological tests have been proposed. Hyperventilation and orthostatic tests lead to the appearance or deepening of isoelectric or negative T waves in the chest leads. After stopping the test, the ECG approaches the initial level. Tests with propranolol and potassium chloride, with positive results, are characterized by the transition of a negative or biphasic T wave to a positive one. Positive test results are more often observed in neurocirculatory dystonia, indicating that changes in the final part of the ventricular complex are associated with functional disorders. However, the differential diagnostic value of these tests should not be overestimated. A positive test with propranolol rather indicates increased activity of the sympathetic-adrenal system, which also happens with coronary pathology. The bicycle ergometer test is negative in most patients. Patients often refuse to undergo it until diagnostic criteria are reached due to fear or fatigue. In these cases, other stress tests become more valuable. The dipyridamole test in these patients is usually negative. The isoproterenol test is occasionally false-positive in hypersympathicotonia. Transesophageal electrical stimulation of the atria has great diagnostic value.
In patients in this category, it is extremely important to conduct the entire complex of stress tests. If all tests are negative, then the diagnosis of IHD is confidently removed. If some of the samples are positive, then patients need to undergo coronary angiography. Excluding coronary pathology allows you to more clearly formulate the diagnosis and choose treatment.
When establishing a diagnosis of neurocirculatory dystonia, it is necessary to take into account possible variants of the course of the disease (from mild to severe). The lung is characterized by relative monosymptomatics, spontaneous disappearance of symptoms, and preservation of tube ability are possible. The ECG is usually slightly changed. Drug therapy is often not needed. Moderately severe disease is long-term, with an abundance of symptoms, decrease or temporary loss of disability; patients require drug therapy. A severe course is characterized by persistence and multiplicity of pathological symptoms without a tendency to disappear, and the ability to work is reduced.
The severity of the clinical manifestations of neurocirculatory dystonia is determined mainly by the severity of tachycardial and asthenic syndromes, as well as the presence of vegetative-vascular paroxysms, the addition of cardiophobia and other severe psycho-emotional disorders. Among the many complaints of a patient with neurocirculatory dystonia, it is necessary to highlight the meager, but quite identifiable symptoms of angina pectoris. It is necessary to take into account the possibility of a combination of ischemic heart disease and neurocirculatory dystrophy, which makes it possible to make both diagnoses simultaneously. Usually in such cases the doctor relies on coronary angiography data.