Indications for use of the drug Actovegin injection solution
Metabolic and circulatory disorders of the central nervous system - ischemic stroke, residual effects of hemorrhagic stroke, traumatic brain injury, encephalopathy of various origins. Disturbances of peripheral arterial or venous circulation, angiopathy, including diabetic origin. I-III degree burns (chemical, thermal, solar, radiation). For wound healing (ulcers of various etiologies, trophic disorders, bedsores, impaired wound healing processes); radiation damage to the skin, mucous membranes, radiation neuropathy.
Use of the drug Actovegin injection solution
It is used intravenously (stream, drip), intravenously, 5–20 ml per day. Injected intramuscularly slowly, no more than 5 ml per day. For intravenous administration, the drug is diluted in 0.9% sodium chloride solution or 5% glucose solution. The permissible final concentration of Actovegin is up to 2000 mg of dry matter per 250 ml of solution. The usual recommended regimen: initially 5-10 ml IV or IM, then 5 ml IV or slowly IM daily or several times a week. In severe conditions, intravenous drip administration of Actovegin is indicated at a dose of 20–50 ml/day for several days until a pronounced clinical effect is achieved. For conditions of moderate severity or exacerbation of chronic diseases, it is administered intravenously or intramuscularly at a dose of 5–20 ml per day for 14–17 days. When carrying out a planned course of treatment, 2–5 ml per day is prescribed intravenously or intramuscularly for 4–6 weeks. The frequency of administration is from 1 to 3 times, depending on the severity of the disease. The drug is used to treat children. Newborns are prescribed at a dose of 0.4–0.5 ml/kg 1 time per day intramuscularly or intravenously, children aged 1–3 years — at a dose of 0.4–0.5 ml/kg 1 time per day IM or IV, 3–6 years — 0.25–0.4 ml/kg 1 time per day IM or IV.
List of used literature
- Karpov Andrey Vladimirovich “Complex treatment of patients with critical ischemia of the lower extremities.” Abstract for the degree of Doctor of Medical Sciences. St. Petersburg, 2007;
- Morozov Mikhail Yurievich “Evaluation of the effectiveness of mydocalm, emoxipin and actovegin in the complex treatment of patients with obliterating atherosclerosis of the lower extremities.” Abstract for the academic degree of Candidate of Medical Sciences. Saransk, 2007;
- Mainugin S. V., Fomin A. A., Krasavin V. A., Krasavin V. G., Averin S. V., Kamensky S. Yu., Khudoyarov T. A. “First experience in using laser Doppler imaging technology when choosing the level of amputation of the lower limb in patients with critical ischemia // Thrombosis, hemostasis and rheology”. – 2012, No. 3;
- Fomin A. A., Mainugin S. V., Krasavin V. A., Kazmiruk N. A., Kamensky S. Yu., Khudoyarov T. A. “Laser Doppler visualization in the practice of the department of purulent surgery // Thrombosis, hemostasis and rheology" - 2012, No. 4;
- Brecht M., de Grool H. “Protection from hypoxic injury in cultured hepatocytes hy glycine, alanine and serine. Amino Acids." 1994. Vol.6. P. 25-35.
diabetes ischemia lower limb ischemia surgery
Special instructions for the use of the drug Actovegin solution for injection
It is advisable to administer no more than 5 ml of the solution intramuscularly, since it is hypertonic. Due to the possibility of anaphylactic reactions, it is recommended to carry out a test injection (2 ml of the solution is administered intramuscularly to assess the local and general reaction). The injection solution has a yellowish tint, the intensity of which depends on the batch number and the starting material, but the color of the solution does not affect the effectiveness and sensitivity to it. The use of the drug Actovegin during pregnancy is allowed only for health reasons.
Recent decades have been characterized by significant advances in the prevention and treatment of cardiovascular diseases (CVD): arterial hypertension (AH); various forms of coronary heart disease (CHD) – acute coronary syndrome (ACS), myocardial infarction (MI); chronic heart failure (CHF). These achievements are due to the introduction into clinical practice of modern high-tech methods for diagnosing and treating left ventricular failure, which are based on a clear understanding of the mechanisms of development of ischemia and death of cardiomyocytes (structural and functional units of the myocardium), and adaptive changes in central hemodynamics.
IHD is widespread throughout the world, especially in economically developed countries, and occupies a leading place in the structure of disability and mortality from CVD among a socially significant age group of the population. In most European countries, the prevalence of IHD is 20–40 thousand per 1 million population. Mortality from diseases of the circulatory system in the Russian Federation, according to medical statistics for 2010, amounted to 56.5% of total mortality; Of these, more than half account for ischemic heart disease as the cause of death.
IHD as an “independent disease” was identified by the World Health Organization (WHO) only in 1965 due to the increasing frequency of this pathology, its dominant participation in the progression of cardiac pumping disorders in CHF, and was included in the International Statistical Classification of Diseases, Injuries and Causes of Death.
In IHD, there is a discrepancy between the level of oxygen consumption by the myocardium and the volume of its delivery by the coronary bloodstream. Adequate energy supply for the pumping activity of the heart in a wide range of its activity - from rest to the level of maximum load (corresponding to the level of basal metabolism of the whole organism) depends on the state of the coronary reserve. Coronary reserve is the ability of the coronary vascular bed to increase coronary blood flow many times due to dilatation of the coronary vessels adequately to the oxygen needs of the myocardium.
Oxygen is a key component of oxidative phosphorylation in the synthesis of ATP, the “fuel” that ensures the functioning of cardiomyocytes and the pumping activity of the heart in general. Energy metabolism in the myocardium represents interconnected mechanisms of O2 delivery and its utilization by the subcellular structures of the cardiomyocyte - mitochondria [1, 2, 4].
To provide energy for its activity, the heart “utilizes” various biological substrates: carbohydrates (glucose, glycogen, lactate), free fatty acids (FFA), and, to a lesser extent, amino acids (proteins). Regardless of the energy substrate, in the final stage of the breakdown of biological substrates, acetyl coenzyme A is formed, which enters the tricarboxylic acid cycle (Krebs cycle), and with the participation of O2 in the mitochondria, the energy substrate ATP is formed.
Under physiological conditions, 10% of ATP is formed during oxidative phosphorylation in mitochondria due to aerobic glycolysis (the breakdown of glucose to pyruvate). The amount of ATP generated as a result of aerobic glycolysis is not enough to ensure the operation of ion channels of the sarcolemma, in particular for the calcium pump of the sarcoplasmic reticulum (SR), which consumes up to 50% of the synthesized energy to ensure diastolic relaxation. Replenishment of the remaining amount of phosphate energy for the functioning of the cardiomyocyte as a whole, with normal oxygen supply, occurs due to the oxidation of FFA. The metabolism of FAs during oxidative phosphorylation provides up to 80% of ATP synthesis. However, FFA oxidation, compared to glycolysis, is a less efficient source of ATP: “fuel” for the heart pump. When oxidizing FFAs, the production of the same amount of ATP requires approximately 10% more oxygen than during glycolysis [1, 4].
FFAs penetrate into mitochondria through active transport, for which the carnitine palmitine enzyme complex is responsible, then β-oxidation of FFAs occurs in mitochondria. This process is strictly controlled and depends mainly on the intensity of FFA translocation into mitochondria. In the case of moderate ischemia, aerobic oxidation of FFA and glucose decreases and anaerobic glycolysis becomes the main source of ATP. Under these conditions, glycogen reserves are mobilized to support glycolysis.
With the development of varying degrees of ischemia (partial or complete occlusion of the coronary artery), anaerobic glycolysis remains the only source of limited ATP formation. As O2 delivery decreases, the activity of oxidative metabolism decreases, producing a limited amount of ATP. A pronounced imbalance between the oxygen demand during the oxidation of glucose and FFA towards the latter leads to the fact that during ischemia in the mitochondria of cardiomyocytes, ATP synthesis switches to β-oxidation of FA with the accumulation of many under-oxidized active forms of FA acyl-coenzyme-A (Acyl-CoA) and acylcarnitine (AcCar). ), which further exacerbates the uncoupling of oxidative phosphorylation (Fig. 1). Underoxidized active forms of FA, in particular AcCar and Acyl-CoA as metabolites, block the transport of ATP from the site of synthesis in mitochondria to the site of their intracellular consumption, have a destructive effect on the membrane - the sarcolemma, increasing the energy deficit necessary for the life of cardiomyocytes [2, 4, 6, 10].
In parallel, under conditions of severe ischemia (lack of blood flow), lactate and H+ accumulate in cardiomyocytes, i.e., against the background of anaerobic metabolism, protons (H+, Na+) accumulate and “acidification” of the cytoplasm occurs. H+ and Na+ ions are exchanged for other cations (mainly Ca2+), as a result of which the cardiomyocytes are “overloaded” with Ca with the formation of incomplete diastole - myocardial contracture (Fig. 2).
Modern advances in the study of cell function (in particular, endothelium) of various organs indicate the key role of oxidative stress - excessive formation of reactive oxygen species (ROS - O2) in the formation of CVD through lipid peroxidation (LPO) of the cell membrane. The main source of ROS in cells is mitochondria, during the normal functioning of which 98% of the supplied oxygen is used for the oxidation of substrates with the formation of ATP (the main energy substrate of cells) and 2% for the synthesis of ROS, which can increase significantly in various pathological conditions (Fig. 3) .
A decrease or cessation of O2 delivery to the heart muscle can be caused by various mechanisms: from spasm to total blockage of the coronary artery. After restoration of coronary blood flow, damaged mitochondria are not able to completely utilize the “surging” supply of oxygen, part of which is used by other oxidative systems of cells and is accompanied by the formation of an increased amount of ROS. The activity of one of the powerful oxidative enzymes, xanthine oxidase, is at a low level under conditions of aerobic metabolism, but increases sharply under hypoxia, in addition, with the conversion of Fe3+ to Fe2+. The combination of these two factors contributes to the excessive formation of ROS [8]. Excessive formation and release of free radicals (ROS) activate lipid peroxidation (LPO) with damage to cell membranes, which consist of phospholipids, cholesterol and protein inclusions that act as ion channels or receptors.
All of the above is an incentive for clinicians in the treatment and prevention of possible complications in the conditions of ischemic episodes in various regions (heart, central nervous system): the use of drugs with antioxidant and antihypoxic pharmacological orientation, which have pleiotropic effects (cardio-, neurocytoprotection), restoration of aerobic intracellular metabolism. A typical representative of drugs with similar pharmacokinetic and pharmacodynamic properties is Actovegina.
Actovegin is a highly purified hemodialysate from the blood of calves, obtained by ultrafiltration, does not contain endotoxins and antigens and consists of biologically active physiological components with high biological activity: amino acids, oligopeptides, nucleosides, products of carbohydrate and fat metabolism. The pharmacological composition of Actovegin is formed by 2-stage ultrafiltration using filters to isolate molecules of different sizes. The molecular weight of the final filtered product does not exceed 5000 Daltons. The composition of Actovegin was tested using modern analytical techniques, including gas liquid chromatography combined with mass spectrometry. Data from quantitative methods for analyzing possible metabolites showed that Actovegin is a combination of more than 200 bioactive molecules [6, 7, 10].
The atomic emission spectrometry method showed the presence in Actovegin of macro-electrolytes (Mg, Na, Ca, P, K) and microelements (Si, Cu), which are included in the prostatic groups of antioxidant enzymes (superoxide dismutase, glutathione peroxidase, catalase). The antioxidant effect of Actovegin is due to superoxide dismutase activity [8]. Magnesium, which is included in Actovegin, is a component of cardiopeptide fragments and enzymes and functions as a catalytic center that provides control and launch of enzymobiochemical intracellular processes.
The anti-ischemic effect of Actovegin at the cellular level is carried out due to the transfer of cell energy metabolism towards aerobic glycolysis with inhibition of β-oxidation of fatty acids. Actovegin®, selectively inhibiting 3-ketaocetyl-CoA catalase, slows down the ß-oxidation of fatty acids, while competitively restoring the coupling between glycolysis and oxidative decarboxylation, which overall leads to an increase in the amount of ATP, which underlies the anti-ischemic protection of cardiomyocytes by Actovegin (Fig. 4) .
Experimental studies at the cellular level have shown that Actovegin® supports the energy metabolism of the heart. The cardioprotective effect of Actovegin is due to its ability to maintain the physiological level of creatine phosphate (the main carrier of energy inside the cell) and ATP under conditions of ischemia, stabilize the pH inside the cell (prevents the development of intracellular metabolic acidosis), and reduce damage to the membrane - sarcolemma - by lipid peroxidation caused by free radicals. Normalization of metabolic balance leads to limiting the accumulation of inorganic phosphate, Na and Ca inside the cell while maintaining normal K concentration. At the same time, Actovegin® reduces the level of migration and infiltration of polynuclear neutrophils (inhibition of chemotaxis) in ischemic and reperfused heart tissues, which reduces autoimmune damage to the myocardium without causing influence on central hemodynamics [4, 5, 9].
Oxidative stress causes breakage of 1 strand of DNA, which leads to activation of the nuclear enzyme poly-ADP-ribose polymerase (PARP). Excessive activation of PARP has negative consequences in the form of triggering successive cellular processes that ultimately stop glycolysis and the process of mitochondrial respiration (oxidative phosphorylation - Krebs cycle), which leads to cell death due to energy depletion and activation of oxidative stress [8].
Further studies have confirmed the role of PARP metabolism as an important mechanism in the development of endothelial dysfunction in cardiovascular pathologies caused by impaired carbohydrate metabolism - diabetes, and it has recently been confirmed that PARP may be involved in the development of diabetic polyneuropathy. Summarizing these data, we can make an assumption about the important role of PARP in ischemic heart disease, cerebrovascular diseases and diabetes [3, 5, 7, 8].
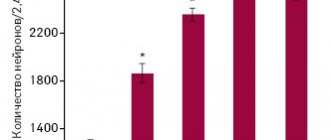
MW Elmlinger et al., using brain cell cultures (primary hippocampal neurons), in their study showed the inhibitory effect of Actovegin on oxidative stress processes. In neurons treated with increasing concentrations of tert-butyl hydroperoxide (> 0.2 mM), an increase in intracellular ROS levels was found (p < 0.001), but in the case of Actovegin in cultured neurons, a dose-dependent decrease in the severity of oxidative stress was noted after 10 days (p < 0.001 at concentrations > 0.3 µg/ml) [8]. In in vivo studies, the effect of Actovegin on the analyzed parameters in experimental diabetic polyneuropathy corresponded to the results obtained in this in vitro study [6, 7].
Actovegin® has a multifaceted effect by normalizing the consumption and use of oxygen, increasing the entry of glucose into cells, thereby restoring cellular metabolism [6, 9, 10]. Actovegin® enhances oxidative processes, shifting the balance of redox reactions towards oxidation, which helps to increase the content of high-energy phosphates, such as ATP and creatine phosphate. K. Schwabe showed that Actovegin® activates intracellular oxidative processes and accelerates not only energy, but also reserve metabolism, which in the case of heart disease is accompanied by increased accumulation of glycogen and potassium. These data were one of the first observations showing a direct positive effect of the drug Actovegin® on the metabolism of the brain and myocardium [5, 8].
Previous studies document that Actovegin® has an insulin-like effect through the activation of GLUT 1–4, stimulating cellular metabolism, increasing oxygen consumption and energy production. One of the constituent parts of Actovegin fractions is Inositol-Phosphate-Oligosaccharide (IFO-fraction), which, through the activation of cAMP and adenylate cyclase, promotes intracellular glucose utilization, stimulates the efficiency of O2 consumption and reduces the formation of lactates.
The effect of Actovegin on glucose transport into the cardiomyocyte is insulin-independent (does not affect insulin receptors), since it is realized through the direct activation of GLUT 1–4, so its effect persists even against the background of insulin resistance in patients with type 2 diabetes. At the same time, the IFO fraction, in synergy with superoxide dismutase and magnesium, promotes the inhibition of lipid peroxidation of cell membranes (membrane stabilizing effect) [4, 6–8, 10].
A number of clinical studies have shown that the use of the drug Actovegin® has a positive effect on cognitive functions in cardiovascular encephalopathies, improves psychological and behavioral reactions, and is most effective in mild and moderate cognitive impairment [3, 6].
Thanks to the development and introduction into clinical practice of new medical technologies, in particular positron emission tomography (PET), there is now a real possibility of quantitative assessment of myocardial perfusion in vivo, oxygen uptake, glucose utilization, FA, and contractility. PET allows a non-invasive way to study oxygen uptake, glucose and FA metabolism with the calculation of quantitative parameters in absolute values. [18F]-2-fluoro-2-deoxyD-glucose ([18F]FDG), a glucose analogue labeled with 18-fluorine, which is not metabolized and remains unchanged in the cell cytosol, is used as a natural marker of glucose uptake and metabolism in PET. . To quantify the metabolism of FFAs in the human myocardium, [18F]-thia-hepta-decanoic acid ([18F]TDA), a long-chain false FA labeled with 18-fluorine, the accumulation of which indicates β-oxidation of FFAs, is currently used as a natural marker. in the myocardium as the main source of energy in the myocardium. To assess oxidative metabolism in PET, a model was developed using [1-11C]-acetate as a marker of myocardial oxygen uptake [1, 4].
In Fig. Figure 5 shows examples of quantitative assessment of O2 utilization of glucose metabolism (Fig. 5A) and free fatty acids (Fig. 5B) before and after intravenous infusion of 1000 mg Actovegin. Actovegin promotes a 3-fold increase in O2 utilization with a simultaneous 6-7-fold increase in glucose uptake and a similar decrease in FFA metabolism. Such dynamics of oxidative metabolism indicate Actovegin’s stimulation of aerobic oxidation, the most beneficial source of macroenergy phosphates [4].
There is evidence that the effect of Actovegin on patients with coronary artery disease complicated by acute coronary insufficiency is multicomponent, in addition to improving myocardial metabolism, it has a positive effect on the rheological properties of blood: it reduces the aggregation activity of platelets, increases the mobility of erythrocytes, and reduces blood viscosity (through a hypoglycemic effect). At the same time, Actovegin promotes angiogenesis – the development of collateral circulation [9].
According to a number of authors, the use of Actovegin by patients on the first day of development helps restore the contractile function of the myocardium of the left ventricle of MI through improving the metabolism of cardiomyocytes, eliminates electrical heterogeneity, which overall manifests itself in a reduction in the frequency of complications and early hospital mortality [4, 5].
In our studies, the use of Actovegin (from 800 to 1200 mg intravenously) in the acute phase of MI in 49 patients against the background of thrombolysis and standard therapy contributed to a more effective prevention of the development of “reperfusion” syndrome (progression of pain syndrome, increase in episodes of ventricular arrhythmias, spread of the MI zone, increase in HF ). The comparison group consisted of 67 patients with AMI who underwent artificial thrombolysis without prophylactic intravenous administration of Actovegin. Analyzes of the clinical status and studies of the pumping activity of the heart before and after treatment by group are presented in the table.
As can be seen from the table, the reduction in the incidence of reperfusion syndrome to 18.4% in the study group of AMI patients compared to the control group of AMI patients (67 AMI patients) - 34%, was mediated by improving effective diastole (elimination of “contracture” of ischemic myocardium) . All parameters of blood flow through the mitral valve, characterizing the diastolic relaxation of the left ventricular myocardium, improved statistically significantly, which contributed to an effective increase in the ejection fraction of the left ventricle - an integral indicator of the pumping activity of the heart. EF in the study group during the administration of Actovegin increased statistically significantly (p < 0.05) by 8.4% (from 39.2 ± 5.5% to 46.6 ± 2.1%) compared with the control group of patients with AMI (from 39.1 ± 2.9% to 42.7 ± 3.1%).
More effective restoration of the metabolism of ischemic myocardium during the administration of Actovegin helps to minimize electrical heterogeneity, which is clinically manifested by a decrease in the incidence of ventricular arrhythmias: in the study group after treatment they occurred in 22.4% of cases, in the comparison group - in 32.8 %.
Similar results were obtained in other clinical observations [5].
Actovegin, as an antihypoxant and secondary antioxidant, when used in clinical practice, activates aerobic respiration of cells in a state of ischemia and metabolic failure, and has a systemic effect on the body (Fig. 6). The main pharmacological actions of Actovegin are increasing the efficiency of oxygen absorption and activating glucose transport, in particular in the cardiomyocyte. Activation of aerobic oxidation processes increases the energy potential of the myocardial cell. The listed effects of Actovegin are most pronounced in the hypoxic status of the heart muscle.
Thus, the development of cardiovascular disorders during ischemic episodes is accompanied by a set of pathophysiological events, the elimination of which requires an integrated pharmacological approach, rather than a simplified unidirectional effect. Multiple cardio- and neurotropic effects suggest a simultaneous modulating effect on various damaging pathological mechanisms (inflammation, apoptosis, oxidative stress, and many others).
As a biological agent with pleiotropic effects, Actovegin in its mechanisms of action (antioxidant, antihypoxic) corresponds to the concept of an integrative therapeutic approach.
The presented review and analysis of our own experience shows that Actovegin takes an active part in restoring the balance of cellular metabolism by correcting a number of pathophysiological processes that occur during the development of IHD. Actovegin has a cardioprotective effect on the cardiovascular functional block due to its anti-apoptotic and antioxidant effects, activates the mechanisms of glucose and oxygen utilization with the normalization of intracellular energy balance, which helps improve the pumping activity of the heart.