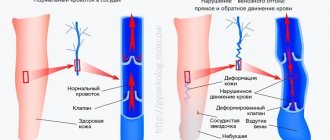
Normally, the pulmonary veins carry arterial blood from the lungs and drain into the left atrium. From there, the blood enters the left ventricle and carries oxygen throughout the body through the aorta and arteries.
If the pulmonary veins drain abnormally, they may drain into the right atrium or into the vena cava, which carries venous blood into the right atrium. This defect can be complete or partial.
- With complete anomalous pulmonary venous drainage, there is no direct communication between the pulmonary veins (pulmonary circulation) and the left atrium (systemic circulation). This state is incompatible with life. But if the child has other developmental defects (the presence of an atrial or ventricular septal defect, an unclosed foramen ovale), blood can still enter the left side of the heart and treatment is possible.
- With partial anomalous drainage of the pulmonary veins, one vein may flow into the left atrium and then the condition of the newborn depends on the volume of abnormal blood discharge. Complete anomalous pulmonary venous drainage occurs in approximately 3% of all congenital heart defects.
What you need to know about complete anomalous pulmonary venous drainage
- Accounts for 1-3% of all congenital heart defects.
- Complete anomalous drainage of the pulmonary veins consists of their flow into the large veins of the systemic circulation
- It is associated with other defects such as atrial septal defect, patent foramen ovale, and congenital cystic adenomatous transformation.
Complete anomalous drainage of the pulmonary veins :
type I: supracardial drainage (50% of cases) into the innominate vein;
Type II: cardiac drainage (28% of cases) into the coronary sinus or right atrium;
Type I III: subphrenic drainage (17% of cases) into the portal venous system or inferior vena cava through the open ductus venosus;
Type IV: mixed form (5% of cases) includes a combination of NI types.
Partial anomalous drainage of the pulmonary veins:
- Right-sided volume overload shunt
- Only some pulmonary veins drain into the systemic circulation
- The remaining pulmonary veins drain normally into the left atrium
- Combined with asplenia syndrome (right isomerism).
Congenital left atrial aneurysm
A congenital defect manifested by aneurysmal dilatation of the left atrium without diseases of the left ventricle or mitral valve (Fig. 90).
Fig.90.
Congenital aneurysm of the left atrium.
EchoCG criteria
One-dimensional echocardiography:
- Marked dilatation of the anteroposterior size of the left atrium.
- Normal echocardiogram pattern of the mitral valve (may be mitral valve prolapse).
Two-dimensional echocardiography:
- Large mass formation associated with the left atrium.
- There may be compression of the wall of the left ventricle by the aneurysmal body.
- Changes in the position of the chambers of the heart due to their displacement by a space-occupying formation.
Doppler EchoCG:
- Assessment of the obturator function of the mitral valve.
- Assessment of pulmonary hemodynamics.
Differential diagnosis:
- Congenital mitral valve insufficiency.
- Mitral stenosis.
- Restrictive cardiomyopathy.
- Hypertrophic cardiomyopathy with a small cavity of the left ventricle.
Which method of diagnosing abnormal drainage to choose: MRI, CT, ultrasound, angiography
What will a chest x-ray show?
- “Snowman” figure (expansion of the superior mediastinum due to dilatation of the superior vena cava and left vertical pulmonary vein (type I)
- Cardiomegaly (types I and II)
- Small heart size and pulmonary edema (type III)
- Narrowing of the mediastinum (types II and III)
- Increased vascularization of the lungs.
What will a heart ultrasound show for pulmonary vein anomalies?
- Lack of drainage of the pulmonary veins into the left atrium.
Is MSCT of pulmonary vessels informative in case of abnormal drainage?
- Contrast study with an adequately low dose
- Direct visualization of pulmonary veins
- Thickening of interlobar septa
- Thickening of the walls of the bronchi
- Glassy opacification of the lungs.
When is an MRI prescribed?
- Axial and coronal projections, balanced pulse FFE mode (SSFP)
- Direct visualization of the anatomy of the venous system
- Coronal contrast-enhanced MR angiography provides images with high spatial and temporal resolution using pulsed GE T1-weighted images with gradient filters
- Phase contrast angiography.
In what cases is angiography prescribed?
- Rarely shown
- May be useful in the postoperative period for visualization of the pulmonary veins and interventional procedures for venous stenosis.
Congenital aortic stenosis
Fig.94.
Valvular aortic stenosis. Poststenotic dilatation of the ascending aorta (scheme). Aortic stenosis is divided into the following forms:
- Valvular congenital stenosis (Fig. 94).
- Subvalvular aortic stenosis
- A. discrete membranous stenosis.
- B. discrete fibromuscular subaortic stenosis.
- B. tunnel subaortic stenosis.
- Supravalvular aortic stenosis.
- Bicuspid aortic valve with stenosis.
In 20% of cases, congenital aortic stenosis is accompanied by PDA, coarctation of the aorta, VSD, and pulmonary stenosis.
Congenital valvular stenosis (see aortic valvular stenosis)
Note:
if all 3 semilunar leaflets are fused, then a narrow central opening is determined; when 2 leaflets are fused (more common), the opening in the aortic lumen is located asymmetrically.
Subvalvular aortic stenosis discrete membranous
Immediately below the aortic valve there is a thin fibrous membrane (Fig. 95).
Fig.95.
EchoCG criteria
One-dimensional echocardiography:
- Partial early systolic closure of the aortic valve.
- Systolic flutter of the aortic valve.
- The presence of additional signals in the left ventricular outflow tract.
- Narrowing of the left ventricular outflow tract.
- Anterior systolic movement of the anterior mitral leaflet.
- Left ventricular hypertrophy.
- Delayed opening of the mitral valve.
Two-dimensional echocardiography:
- A fibrous membrane just below the aortic valve prolapses into the left ventricular outflow tract in diastole (Fig. 96).
- Narrow left ventricular outflow tract.
Doppler EchoCG:
- Increase in peak systolic flow in the ascending aorta over 1.5 m/s.
- The presence of a systolic gradient (over 5 mm Hg).
Fig.96.
Subvalvular perimembranous aortic stenosis.
Differential diagnosis:
- Asymmetric hypertrophic cardiomyopathy with obstruction of the left ventricular outflow tract (idiopathic hypertrophic subaortic stenosis): with discrete stenosis, partial early systolic closure of the aortic valve is usually observed (on average after 0.05 s), with IGSS - mid-systolic (on average after 0.14 s from the moment opening of the aortic valve)
Subvalvular aortic stenosis discrete fibromuscular
Formed by a fibromuscular ring, it is usually located more distal to the aortic valve than in discrete membranous stenosis, approximately 1 cm or more.
Clinical manifestations
Typical symptoms:
Pulmonary vein obstruction:
- acute emergency situation in the first few hours or days of life
- Cyanosis
- Dyspnea
- Pulmonary hypertension.
Without pulmonary vein obstruction:
- Increased pulmonary blood flow
- Pulmonary hypertension
- Tachypnea
- Signs of heart failure
- Dystrophy.
A slight increase in pulmonary artery pressure may cause only a minimal number of symptoms; Cyanosis may be absent or mildly expressed.
Aortopulmonary septal defect
With this defect, there is a communication between the ascending aorta and the trunk of the pulmonary artery (Fig. 93).
Fig.93.
Aortopulmonary septal defect (diagram).
EchoCG criteria
One-dimensional echocardiography:
- Dilatation of the left ventricle.
- Volume overload of the left ventricle.
- Dilatation of the left atrium (increase in the ratio of the diameter of the left atrium to the diameter of the aorta).
- Volume overload of the left atrium.
Two-dimensional echocardiography:
- Direct visualization of the interruption of the echo signal from the aortopulmonary septum from the suprasternal or high parasternal approach in the long axis view.
- Detection of associated anomalies (observed in 50% of cases): PDA, VSD, coarctation of the aorta, subvalvular aortic stenosis.
- Identification of complications of the defect: pulmonary hypertension.
Doppler EchoCG:
- Diastolic turbulent flow in the pulmonary artery.
- Reversal of diastolic flow in the descending aorta.
Differential diagnosis:
- Patent ductus arteriosus.
- Ventricular septal defect with aortic valve insufficiency.
- Systemic arteriovenous fistula.
- Arteriovenous fistula of the coronary vessels.
- Rupture of the sinus of Valsalva into the right atrium or ventricle.
- Anomalous origin of the left coronary artery from the pulmonary artery.
Complications
- Obstruction of anastomoses (5-10% of cases)
Complete anomalous drainage of the pulmonary veins into the right atrium (type II, cardiac), combined with an atrial septal defect. X-ray of the chest in direct projection. Enlargement of the right heart and increased vascularization of the central parts of the lung. MRI with contrast enhancement of the chest. Partial anomalous drainage of the pulmonary veins, the left pulmonary vein drains through the vertical vein (arrow) into the innominate vein (*).
Patent ductus arteriosus
The duct departs from the aorta at the level of the left subclavian artery and flows into the trunk of the pulmonary artery at the site of its division into two branches (Fig. 91).
Fig.91.
Patent ductus arteriosus (diagram).
EchoCG criteria
One-dimensional echocardiography:
- Dilatation of the left ventricle.
- Volume overload of the left ventricle.
- Dilatation of the left atrium (increase in the ratio of the diameter of the left atrium to the diameter of the aorta).
- Volume overload of the left atrium.
Two-dimensional echocardiography:
Direct visualization of the ductus from the suprasternal approach as an echo-free space between the descending aorta and the pulmonary artery.
Fig.92.
Patent ductus arteriosus.
Doppler EchoCG:
- Diastolic turbulent flow in the pulmonary artery.
- Reversal of diastolic flow in the descending aorta.
Differential diagnosis:
- Aortopulmonary septal defect.
- Ventricular septal defect with aortic valve insufficiency.
- Systemic arteriovenous fistula.
- Arteriovenous fistula of the coronary vessels.
- Rupture of the sinus of Valsalva into the right atrium or right ventricle.
- Anomalous origin of the left coronary artery from the pulmonary artery.
What diseases have symptoms similar to abnormal drainage of arteries in the lungs
Scimitar syndrome
— hypoplasia of the right lung;
- dextroposition of the heart;
— hypoplasia of the right pulmonary artery;
- blood supply to the right lower lobe is provided by the branches of the abdominal aorta;
- venous outflow from the right lung into the inferior vena cava (the vein has a typical scimitar shape in the right paracardial region).
Atrial septal defect
- it is impossible to differentiate the acquired defect using conventional x-ray methods.
Triatrial heart
- separation of the entry of the pulmonary veins into the left atrium by a septum (with fenestration).
"Riding" tricuspid valve
An obligatory component of the defect is a VSD and displacement of the valvular apparatus of the tricuspid valve into the cavity of the left ventricle.
EchoCG criteria
One-dimensional echocardiography:
- Small size of the right ventricle.
- The anterior leaflet of the tricuspid valve crosses the IVS during diastole.
- Visualization of echo signals from the valve apparatus of the tricuspid valve in the cavity of the left ventricle.
- Interruption of the echo signal from the interventricular septum.
- Dilation of the left ventricle and left atrium.
Two-dimensional echocardiography:
- Hypoplasia of the right ventricular cavity.
- Displacement of the right atrioventricular ring and subvalvular apparatus into the left ventricle.
- Direct visualization of a ventricular septal defect.
- Detection of associated anomalies.
Doppler echocardiography:
- Assessment of the state of pulmonary blood flow.
- Estimation of the magnitude of the systolic gradient between the ventricles.
Differential diagnosis:
Open common atrioventricular canal.
Symptoms
Signs of ADLV make themselves known immediately after the birth of the child. With a total defect, which is not accompanied by the presence of defects in interatrial communications, blood circulation between the small and large circles becomes completely impossible, and the newborn quickly dies. In such cases, only an emergency cardiac surgery using the Rashkind method, such as balloon endovascular atrioseptostomy, can save the child’s life in such cases.
In other cases, the severity of clinical manifestations of ADLV is determined by its anatomical variant, the size of atrial septal defects and the nature of hemodynamic disorders. Typically, parents of children with such a congenital defect notice the following manifestations of this pathology:
- fast fatiguability;
- the appearance of shortness of breath after physical activity (feeding, crying, etc.);
- pain in the heart area (the child sleeps poorly, is restless, cries loudly and stretches his legs);
- pale skin;
- mild cyanosis;
- cough;
- nausea and vomiting;
- slow weight gain;
- frequent ARVI and pneumonia.
By palpating the pulse, the doctor can detect its rapidity and arrhythmia. Upon examination, deformation of the chest and an increased heartbeat are determined.
Mild cyanosis that occurs in the first weeks of life may disappear or become less pronounced over time. As a rule, cyanosis is more pronounced during physical activity.
When listening to heart sounds, specific signs of this defect are not detected. Can be heard:
- loud I tone over the heart;
- splitting of the second tone with increased pulmonary component;
- heard III sound above the apex of the heart (in many patients);
- soft systolic murmur over the pulmonary artery with variable duration and intensity.
The ECG determines the overload of the right parts of the heart - high voltage of the P wave in the right leads and deviation of the electrical axis to the right in standard leads. Overload of the right atrium is indicated by a high P wave in the right chest and standard leads. Often there are signs of incomplete blockade of the right bundle branch.
If there is a large defect in the interatrial septum, the child may develop normally and then, at an older age, complaints arise of a significant decrease in exercise tolerance, shortness of breath, pallor and poor general health. Later, such children develop signs of right ventricular failure.
Diagnostics
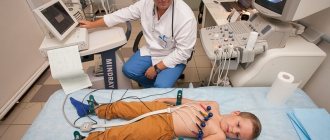
One of the methods that helps detect and verify ADLV is cardiac ultrasound.
For the first time, ADLV can be detected in the fetus during an ultrasound examination in the third trimester of pregnancy.
To clarify all the data about such a congenital heart defect, the following instrumental research methods are prescribed:
- radiography;
- ECG;
- Echo-CG (for adults and older children, transesophageal echo-CG is prescribed);
- MRI;
- probing of the heart chambers and angiocardiography.
A little history
This rare congenital defect was first described by Wilson back in 1798, but its variants were examined in more detail only after sufficient cardiac surgical experience had been accumulated in the 50-60s of the 20th century. The first successful operation to correct this pathology was performed by Miller in 1951. Subsequently, cardiac surgical techniques were improved, and in 1956, radical correction of ADLV was carried out using superficial hypothermia. In the same year, a similar intervention was performed using artificial circulation.
On the territory of the USSR, the first successful operation to eliminate such a heart defect was performed by Nikolai Mikhailovich Amosov in 1978, and one of the leading Russian cardiac surgeons, Georgy Eduardovich Falkovsky, reported on a series of successful corrections in young children in 1984.