Description
Blood calcium has 3 forms: 2 bound - with anions and albumins and 1 free. Only free form is active.
To accurately determine the proportion of free calcium in cases where there are chronic heart and kidney diseases, the content of albumin protein in the blood serum is determined in order to understand how much calcium can be associated with protein. The normal proportion of calcium compounds with protein is up to 38% of the total. The proportion of calcium bound by anions - bicarbonates, lactates, citrates - is about 10%.
Calcium is required for the following processes:
- muscle contraction;
- maintaining heart rhythm;
- transmission of nerve impulses;
- exchange of iron and phosphorus;
- maintaining the strength of bones and teeth;
- construction of the fetal skeleton;
- regulation of cell membrane permeability;
- the formation of thrombin or the main component of a blood clot, without which it is impossible to stop bleeding;
- regulation of enzymatic activity;
- improving sleep quality.
Calcium enters the body with food, is absorbed in the intestines, participates in metabolic processes, and is excreted from the blood by the kidneys. If there is not enough mineral intake from food or its absorption is impaired, then the parathyroid hormone and vitamin D3 extract it from the bones and teeth. If there is too much calcium in the blood, then calcitonin and other hormones reduce its level in the blood, depositing it in the bones and internal organs.
Many biologically active compounds take part in the regulation of calcium levels - hormones of the adrenal cortex, thyroid gland, sex hormones, as well as magnesium and phosphorus.
Both an increase and a decrease in calcium levels in the blood have a bad effect on all types of metabolism and the functioning of the body.
Read completely
Total calcium - 300 rub.
Urgent analysis - 600 rub.
Deadlines
Normal – 1 day.
Urgent – 1.5 – 2 hours.
Taking blood from a vein is paid separately - 300 rubles.
(If several tests are performed simultaneously, the service for collecting biomaterial is paid once)
Indications
- bone loss or osteoporosis, diagnosis of this condition and general population survey or screening;
- decreased muscle tone with normal peripheral nerves;
- a feeling of tingling and crawling, if there is no other reason for this;
- convulsive syndrome or involuntary twitching of the distal muscles, especially the legs;
- chronic diseases of the stomach and intestines, especially gastric and duodenal ulcers;
- passing an abnormally large amount of urine;
- heart rhythm disturbances;
- fluctuations in vascular tone;
- comprehensive examination before surgery;
- disruption of the thyroid and parathyroid glands;
- malignant breast tumors in women and lung cancer;
- kidney disease, including nephrolithiasis or urolithiasis with the presence of stones visible on an x-ray;
- bone pain in the absence of metastases of the malignant process;
- pregnancy.
Material for analysis
Blood serum.
Preparing for the study
- You should eat moderately during the day before the test. Blood is taken on an empty stomach in the morning, a break in food is from 8 to 14 hours, water is allowed. For half an hour before taking blood, you need to be at rest for half an hour and not smoke.
When there is not enough calcium in a person's bones...
As you know, there are many bones in the structure of the skeleton, each of which performs its own function. Moreover, bone is a living element of the system that requires nutrition and renewal. Moreover, the bones themselves cannot function normally if they do not receive the appropriate load.
Why is there not enough calcium in the skeletal system: what are the reasons?
Every part of the bone skeleton contains calcium, which makes bones strong and hard. If the body does not receive this chemical element in full, pathological changes may begin in the bones, and serious changes do not require much time. If a person breaks his leg and walks in a cast for even thirty days, we can already talk about the beginning of disorders in the bone tissue.
But not only bone injury leads to calcium deficiency. Scientists have already proven that human aging is accompanied by a sharp leaching of calcium from the body. This is why bones in older people become less strong and brittle. A disease such as osteoporosis appears. He can accompany any elderly person. This disease begins much earlier. This calcium deficiency is considered normal and cannot be avoided. If you have osteoporosis, treatment should only be carried out under the supervision of specialists.
Decalcification of the skeletal system can be caused by various diseases:
- Diseases of the gastrointestinal tract, when the body is not able to obtain enough calcium from the foods it eats.
- In case of kidney disease, when a huge amount of calcium is washed out along with the urine.
- In women expecting the birth of a baby, the fetus requires calcium in large quantities for the proper formation of the skeleton. But, in fact, it is produced in such a large amount that it leads to damage to bone tissue and makes the bones completely fragile.
- If a person takes a large number of medications that are hormonal drugs. In this case, the balance of calcium in the skeletal system is also disturbed, since these medications capture free calcium and remove it from the body.
How can you determine that there is a calcium deficiency in the body?
If bone tissue lacks an element such as calcium, the bones begin to lose hardness, become less strong and brittle. It is for this reason that older people get broken bones when they fall.
Sometimes there is not even a fall, but the bones break: this is due to bone deformations, most often the vertebral bodies are deformed. If the cartilage and bone endplate breaks, part of the nucleus pulposus is displaced. It is embedded in the spongy substance. Due to such an injury, an intervertebral hernia appears.
Elderly people often experience fractures:
- In the area of the femoral neck.
- The vertebral bodies themselves.
- Spongy bones can have microfractures, after which microcalluses subsequently form.
What you need to know about the treatment of diseases associated with calcium deficiency?
Decalcification is a serious process, sometimes leading to irreparable consequences. The fight against such a disease is never easy.
If a person does not have problems with the intestines or kidneys, and the metabolism is not impaired, then maintaining the functioning and structure of the skeletal system is not so difficult. It is enough to adhere to a healthy lifestyle. Dealing with decalcification is often very difficult. This is an effective method that has a beneficial effect on the cells responsible for the structure of bone tissue.
But in our fast-moving times, it is not always possible to find the opportunity to visit the gym. You can purchase a Swing Machine, with which you can not only load your spine physically, but also receive the necessary dose of massage.
In order for the metabolism in the body not to be disturbed, it is necessary to treat nutrition correctly; products should be healthy and properly balanced. Food should contain enough calcium-rich foods. We should not forget that in addition to this element, other minerals and vitamins must be supplied to the body in the required quantities.
A lot of calcium is found in a number of foods that you need to have in your diet:
- Milk and dairy products, including cheese. You need to eat it every day.
- Fish.
- Don't shun cow butter and eggs.
- Spinach, legumes, cabbage.
Complete absorption of calcium is possible only with the presence of vitamin D, so milk, margarine, and liver should be present in your diet.
Author: K.M.N., Academician of the Russian Academy of Medical Sciences M.A. Bobyr
Increasing values
- increased production of parathyroid hormones due to its benign growth or malignant tumor;
- bone metastases in kidney, lung, and breast cancer;
- a special type of malignant tumor - carcinoma, which produces parathyroid hormone, while the primary cancer can be in the uterus, ovaries, thyroid gland or kidneys;
- injuries and illnesses in which a person is forced to remain motionless for a long time, causing calcium from the bones to move into the blood - bone fractures, spinal tuberculosis, Paget's disease or damage to bone tissue with improper formation and resorption, congenital hip dislocation;
- increasing vitamin D levels;
- adrenal disease, especially insufficiency;
- hemoblastoses or tumor diseases of the blood and lymph - especially myeloma, accompanied by destruction of bone tissue;
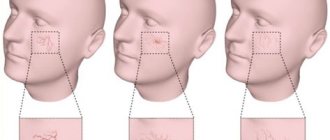
- Williams syndrome or “elf face” is a genetic disorder where several genes are missing;
- kidney failure;
- granulomatous diseases, especially sarcoidosis;
- excessive intake of medications containing calcium;
- milk-base or Burnett syndrome, when there is a lot of calcium in the blood, and the acid-base balance is shifted to the alkaline side;
- overdose of diuretics containing thiazide, or substances that potently remove potassium and sodium.
Blood Ca levels
The daily intake of macronutrients depends on age and gender.
Adults:
- men and women under 65 years of age – 800–1000 mg;
- from 65 years – 1200 mg;
- lactating and pregnant women – 1500–2000 mg.
Children:
- up to 6 months – 400 mg;
- up to 3 years – 600 mg;
- from 3 to 10 years – 800 mg;
- from 10 to 14 years – 1000 mg;
- up to 18 years – 1200 mg.
Increased amounts of calcium are required by athletes and people engaged in heavy physical labor. Also, its consumption should be increased in the presence of injuries and bone fractures. The exact daily dose is determined by the attending physician.
Decrease values:
- DiGeorge syndrome, or a genetic disorder in which the parathyroid and thymus glands are underdeveloped or absent;
- dysfunction of the parathyroid glands due to its autoimmune lesion or surgical intervention;
- decreased magnesium levels;
- decreased vitamin D levels;
- liver and kidney damage, which reduces albumin levels;
- damage to pancreatic tissue or pancreatic necrosis;
- long-term use of certain medications;
- massive blood transfusion.
How does deficiency manifest itself?
Calcium is necessary for healthy teeth and bone tissue, growth and beautiful appearance of nails and hair. The mineral also participates in metabolic processes. In the case of prolonged calcium deficiency, various diseases occur: osteoporosis, rickets (together with a lack of vitamin D), hypertension, cataracts, growth retardation in children, scoliosis.
The main signs of mineral deficiency in the blood:
- fatigue, constant weakness, drowsiness, loss of strength;
- depressed mood, depression, irritability;
- fragility and weakness of nails, their separation;
- leg cramps (especially at night);
- hair loss;
- unhealthy complexion, pallor;
- dryness and flaking of the skin;
- tooth decay, frequent caries;
- decreased immunity;
- spontaneous formation of bruises on the body;
- weak tooth enamel;
- bleeding gums;
- fragility of bones (especially in childhood and old age);
- frequent colds;
- severe form of PMS in women.
To find out for sure whether there is a lack of calcium in the bones of the skeleton, you need to conduct a biochemical blood test. The following mineral content is considered normal:
- 2.10–2.55 mmol/l – in adults;
- 2.20–2.70 mmol/l – in children.
Interpretation of results.
Calcium levels are assessed over time by a doctor in combination with other laboratory parameters and clinical data. Independent, isolated assessment leads to incorrect conclusions.
All laboratory services
Make an appointment through the application or by calling +7 +7 We work every day:
- Monday—Friday: 8.00—20.00
- Saturday: 8.00–18.00
- Sunday is a day off
The nearest metro and MCC stations to the clinic:
- Highway of Enthusiasts or Perovo
- Partisan
- Enthusiast Highway
Driving directions
Treatment of hypercalcemia
In certain cases, drug treatment is not prescribed. This applies to cases where the calcium level does not greatly exceed the permissible normal limits. In such a situation, it is enough to discuss the correction of the diet, including the list of prohibited foods, which include:
- milk;
- cheese;
- cottage cheese;
- yogurt;
- kefir;
- nuts.
After a certain period of time, the patient will be scheduled for a repeat blood test. It will show whether its composition has returned to normal or not.
In more severe situations, diuretics may be prescribed, provided that kidney function is not impaired and the body is not at risk of dehydration. It is important to strictly adhere to the dosage, because such drugs lead to a sharp decrease in blood pressure.
If the excess calcium is caused by a serious health problem, the patient will be hospitalized for medical attention.
Biochemical blood test: normal indicators
| Analysis: | Men: | Women: |
| Total protein | 64-84 g/l. | 64-84 g/l. |
| Hemoglobin | 130-160 g/l | 120-150 g/l. |
| Haptoglobin | 150-2000 mg/l | 150-2000 mg/l |
| Glucose | 3.30-5.50 mmol/l. | 3.30-5.50 mmol/l. |
| Urea | 2.5-8.3 mmol/l. | 2.5-8.3 mmol/l. |
| Creatinine | 62-115 µmol/l | 53-97 µmol/l. |
| Cholesterol | 3.5-6.5 mmol/l. | 3.5-6.5 mmol/l. |
| Bilirubin | 5-20 µmol/l. | 5-20 µmol/l. |
| AlAT (ALT) | up to 45 units/l. | up to 31 units/l. |
| ASAT (AST) | up to 45 units/l. | up to 31 units/l. |
| Lipase | 0-190 units/l. | 0-190 units/l. |
| Alpha amylase | 28-100 units/l. | 28-100 units/l. |
| Pancreatic amylase | 0-50 units/l. | 0-50 units/l. |
2. Reasons
The definition of “nutritional” (food) implies and reflects the main cause of calcium deficiency: insufficient dietary intake. The largest amount of calcium available for absorption is found in dairy products (especially cottage cheese and butter), seafood, eggs, and vegetables. Since calcium is biochemically related to vitamin D, a lack of the latter most often leads to calcium deficiency; Thus, the reasons should also include insufficient exposure to the open sun.
The most significant risk factors include smoking, situations of chronic stress, long-term use of certain medications (cytostatics, antiparoxysmal drugs, etc.).
Visit our Therapy page
Determination of electrolytes in the blood (sodium, potassium, ionized calcium in complex)
Short description:
Sodium, potassium and ionized calcium are the body's main electrolytes. Electrolytes are mineral compounds that are capable of conducting an electrical charge. Being in tissues and blood in the form of salt solutions, they help move nutrients into cells and remove metabolic products from cells, maintain water balance and the required level of acidity in them. Synonyms (rus): Serum electrolytes. Synonyms (eng): Electrolyte Panel, Sodium, Potassium, Chloride, CMP, BMP.
Method: ion selective electrodes.
Units: mmol/l (millimoles per liter)
Preparing for the study:
• Do not eat for 12 hours before the test. • Avoid physical and emotional stress 30 minutes before the test. • Do not smoke for 30 minutes before donating blood.
Type of biomaterial: Venous blood.
Tube type: vacuum tube with lithium heparin (with green cap).
Service price: 458 rub.
Completion time: one business day.
Reference values:
Electrolyte Reference values
Potassium (K+) 3.4 – 5.1 mmol/l Sodium (NA+) 136 – 146 mmol/l Ionized calcium (Ca+) 1.15-1.29 mmol/l
Increased potassium levels (hyperkalemia): • excessive intake of potassium into the body: rapid infusion of potassium solutions; • release of K+ from cells into extracellular fluid: with massive hemolysis, rhabdomyolysis, tumor disintegration, severe tissue damage, deep burns, malignant hyperpyrexia, acidosis; • reduced release of K+ by the kidneys: acute renal failure with oligo- and anuria, acidosis, end-stage chronic renal failure with oliguria, Addison's disease, pseudohypoaldosteronism, hypofunction of the renin-angiotensin-aldosterone system, shock conditions, tissue ischemia; • decrease in the volume of extracellular fluid - dehydration; • taking medications such as amiloride, spironolactone, triamterene, aminocaproic acid, antitumor agents, digoxin, non-steroidal anti-inflammatory drugs, trimethoprim-sulfamethoxazole.
Decreased potassium levels (hypokalemia): • insufficient intake of potassium into the body: with chronic fasting, diet poor in potassium; • loss of potassium by the body with intestinal secretions with frequent vomiting, profuse diarrhea, intestinal villous adenoma, intestinal fistulas, suction of stomach contents through a nasogastric tube; • loss of potassium in the urine due to renal tubular acidosis, renal tubular failure, Fanconi syndrome, Conn's syndrome (primary aldosteronism), secondary aldosteronism, Cushing's syndrome, osmotic diuresis (diabetes mellitus), alkalosis, administration of ACTH, cortisone, aldosterone; • redistribution of potassium in the body (increased entry of potassium into cells): during treatment with glucose and insulin, familial periodic paralysis, alkalosis; • sweat loss in cystic fibrosis; • treatment of megaloblastic anemia with vitamin B12 or folic acid; • hypothermia; • taking corticosteroids, diuretics (except potassium-sparing), beta-blockers, antibiotics; • administration of large amounts of fluid with low potassium content; • VIPoma (pancreatic islet cell tumor that secretes vasoactive intestinal polypeptide - VIP); • Magnesium deficiency.
Increased sodium levels (hypernatremia): • hypertensive dehydration: a) loss of fluid through the skin due to severe sweating; b) loss of fluid through the lungs during prolonged shortness of breath; c) loss of fluid through the gastrointestinal tract with frequent vomiting and severe diarrhea11356433 d) with high fever (typhoid fever, paratyphoid fever, typhus, etc.); • insufficient intake of water into the body; • sodium retention in the kidneys (reduced urinary excretion) with primary and secondary hyperaldosteronism, Cushing's syndrome (excess of corticosteroids); • excessive administration of sodium salts, for example, hypertonic sodium chloride solution; • taking drugs such as ACTH, anabolic steroids, androgens, corticosteroids, estrogens, methyldopa, oral contraceptives, sodium bicarbate.
Reduced sodium levels (hyponatremia): • insufficient sodium intake into the body; • loss of sodium through vomiting, diarrhea, severe sweating with adequate water and inadequate salt replacement; • overdose of diuretics; • adrenal insufficiency; • acute renal failure (polyuric stage); • osmotic diuresis; • hypotonic overhydration: a) excessive parenteral fluid administration; b) reduced excretion of water in renal failure, increased secretion of vasopressin, deficiency of corticosteroids; • dilution hyponatremia with edema and ascites in chronic heart failure, liver cirrhosis, liver cirrhosis, liver failure, nephrotic syndrome; • taking medications such as furosemide, aminoglycosides, hypertonic glucose solution, non-steroidal anti-inflammatory drugs, amitriptyline, haloperidol; • hypothyroidism.
Increased calcium levels (hypercalcemia): • Primary hyperparathyroidism; • Ectopic tumors that produce parathyroid hormone; • Excessive intake of vitamin D. • Malignant tumors (an increase in ionized calcium can occur with normal values of total calcium) and metastases; • Acidosis; • Taking medications: hydrochlorothiazide (long-term use), lithium, androgens.
Decreased calcium levels (hypocalcemia): • Primary hypoparathyroidism, • pseudohypoparathyroidism; • Vitamin D deficiency; • Sepsis; • Acute pancreatitis; • Kidney failure; • Severe damage to skeletal muscles; • Hemodialysis with low calcium concentration in the dialysate; • After blood transfusions containing calcium complexing anions (citrate); • After extensive injuries, surgical interventions; • Burns; • Multiple organ failure; • Magnesium deficiency; • Alkalosis; • Hypernatremia; • Atrophic gastritis; • Substances that bind calcium (citrate, oxalate, EDTA, heparin); • Medicines (anticonvulsants, danazol, foscarnet, furosemide initial action), • alcohol.
Code B03.016.011
1.General information
Probably, any modern person, when asked “Why is calcium needed in the body?” will answer without hesitation: “For the bones.” To a large extent, this is true: almost the entire volume of calcium contained in the body is located in the bone tissues of skeletal structures, and the main processes of its absorption and processing are carried out there. However, it is needed not only “for the bones”.
The role of calcium as a biochemical reagent is much broader and more important: this macroelement (not to be confused with microelements, the required concentration of which is orders of magnitude lower) is involved in the regulation of the processes of excitation-inhibition of the nervous system, desensitization and immune response (it is no coincidence that calcium salts are prescribed for allergic reactions), cellular nutrition, contractile activity of muscle fibers, peristalsis, differentiation of stem cells, secretion of enzymes and hormones, hair and tooth growth.
Nutritional deficiency of calcium, as well as deficiency of other key macro- and microelements (for example, iodine, magnesium, potassium, etc.) at this stage of development of civilization is becoming an increasingly serious problem, since the intake of these substances into the body depends critically on lifestyle and diet, and both are becoming less and less natural for humans. An additional complication is the fact that a deficiency of such elements is most often not the first diagnosis: patients present with symptoms of inflammatory, infectious, metabolic, allergic, gastroenterological and many other disorders - and are treated specifically for them - while the underlying painful condition there lies a deficiency of one or another biologically active substance, which can clearly manifest itself only many years after the first noticeable disturbances appear.
A must read! Help with treatment and hospitalization!