Congenital heart disease in newborns - what is it?
The disease congenital heart disease implies an anatomical pathology or defect (impairment) in the development of the heart and/or large vessels, as well as the valve apparatus, which leads to cardiac overload, myocardial insufficiency of the heart chambers and changes in blood flow.
Congenital heart defects in children are called when pathologies formed before the birth of the child, during the intrauterine period of fetal development.
Most often, congenital heart defects in future newborns develop between the second and eighth weeks of pregnancy.
A disease such as congenital heart disease is relatively rare. Out of every thousand babies born, six to eight children are given a similar diagnosis.
Features of pregnancy management with heart defects
Pregnancy management in women with heart defects, as mentioned above, is carried out with the participation of several specialists. It requires not only the coordinated activities of specialists at the antenatal clinic, but also the disciplined behavior of the woman herself: early registration with the antenatal clinic, timely visits to doctors and tests, a full examination, and timely comprehensive treatment.
If possible, then, of course, it is better to entrust your health to a large medical center specializing in this problem. This could be a department for women with cardiovascular pathology at an institute or a specialized department at a large maternity hospital, where competent specialists with experience in managing patients with such pathology can effectively help.
The course of pregnancy in women with heart defects has its own characteristics. Often there are complications such as gestosis (complications of pregnancy, manifested by the appearance of edema, protein in the urine, increased blood pressure), which are characterized by a latent course and are difficult to treat. Pregnancy in such patients is often complicated by the threat of termination - the number of spontaneous abortions and premature births significantly exceeds the average. In addition, the course of pregnancy can be complicated by impaired uteroplacental blood flow, which leads to hypoxia (oxygen starvation) or intrauterine growth retardation. The risk of placental abruption is also high. The accumulation of blood clots in the placenta leads to the exclusion of part of the placenta from the bloodstream and increased oxygen starvation of the fetus.
For all the above reasons, women with heart defects and other pathologies of the cardiovascular system must be hospitalized at least three times during pregnancy:
The first hospitalization is at the 8-10th week of pregnancy to clarify the diagnosis and decide on the possibility of continuing the pregnancy. The issue of termination of pregnancy before 12 weeks is decided depending on the severity of the defect, the functional state of the circulatory system and the degree of activity of the rheumatic process.
The second hospitalization is at the 28-29th week of pregnancy to monitor the state of the cardiovascular system and, if necessary, to maintain heart function during the period of maximum physiological stress. This is due to the fact that it is during this period that the load on the heart normally increases significantly (one of the periods of maximum physiological stress) - the so-called cardiac output increases by almost a third, mainly due to an increase in heart rate.
The third hospitalization is at the 37-38th week to prepare for childbirth and choose a method of delivery, drawing up a birth plan.
If signs of circulatory failure, exacerbation of rheumatism, the occurrence of atrial fibrillation (frequent irregular heartbeat), gestosis or severe anemia (decreased hemoglobin) and other complications occur, hospitalization is necessary, regardless of the stage of pregnancy.
The issue of termination of pregnancy at a later stage is quite complex. Doctors often have to decide what is less dangerous for the patient: to terminate the pregnancy or allow it to develop further. In any case, if signs of circulatory failure or any concomitant diseases appear, the patient should be hospitalized, subjected to a thorough examination and treatment.
If the situation does not require such drastic measures, the pregnant woman should exercise maximum caution. First of all, you need to take care of sufficient rest and long, 10-12-hour sleep. A 1-2 hour nap during the day is beneficial. Quite effective means of treatment and prevention are physical therapy, morning exercises, and walks in the fresh air. The set of morning exercises should be the simplest, not leading to excessive strain or fatigue.
The diet must be made as varied and nutritious as possible, with a high content of protein products (up to 1.5 g/kg body weight). You need to take multivitamins. In addition, the doctor may prescribe sessions of hyperbaric oxygenation (sessions in a pressure chamber where air with a high oxygen content is supplied under pressure), general ultraviolet irradiation.
Classification of congenital heart defects in children:
- With unchanged or slightly changed pulmonary blood flow (anomalies of the location of the heart, pathologies of the aortic arch, aortic stenosis, atresia of the aortic valve, insufficiency of the pulmonary valve, stenosis, atresia and insufficiency of the mitral valve, heart with three atria, pathologies of the arteries and conduction system).
- With hypervolemia of the pulmonary circulation: Without early cyanosis (open duct in the arteries, defective septa between the ventricles or atria, aortopulmonary fistula, Lutambashe syndrome).
- With cyanosis (atresia with a septal defect between the ventricles, open ductus in the arteries with pulmonary hypertension and blood flow into the aorta from the pulmonary trunk).
- Without cyanosis (isolated pulmonary stenosis).
There is also a classification of congenital heart defects in newborns, which is more understandable for the common man:
- So-called “blue” defects (accompanied by early cyanosis (blue skin); among them are tetralogy of Fallot, pulmonary atresia, transposition of the great vessels).
- So-called “white” or “pale” defects (may be accompanied by pallor of the skin or occur without pronounced symptoms and are detected randomly during a preventive examination of the child; among them are defects of the septa between the ventricles and atria).
Congenital heart defects are a common group of fetal congenital anomalies and the most common cause of neonatal mortality. Today, the birth rate of children with congenital heart defects is 0.7-1.7%. Every year in Russia, about 35 thousand children are born with congenital anomalies of the cardiovascular system. In recent years, this figure has been growing (mainly due to improved diagnostics and, accordingly, an increase in the frequency of recognition cases) [1]. Diseases can be incompatible with life, and there are those that can lead to lifelong disability. The complex task of providing medical care is early diagnosis and treatment of this pathology [2-4]. There is a large group of fetal developmental anomalies, including cardiac malformations, which cause irreversible disorders that complicate surgical correction after the birth of the child. In such cases, intrauterine intervention becomes the optimal solution [5–7].
The purpose of this work is to study intrauterine surgical correction of cardiac malformations.
In preparation for publication, a literature search was carried out, as well as systematization and analysis of information from scientific literature sources located in the PubMed, eLIBRARY.RU, CyberLeninka databases.
To perform intrauterine operations on the fetal heart, it is necessary to take into account detailed information about the features of the topographic anatomy of the heart in general and the intracardiac structures of the fetus in particular.
The fetal heart occupies most of the chest cavity. Features of the syntopy of the human heart in prenatal ontogenesis include the presence around the heart of non-functioning prenatal lungs, a large thymus, the esophagus and descending aorta adjacent to the heart, as well as the close proximity to the vagus and phrenic nerves passing through the chest cavity. Considering the heart as a whole, it can be noted that it is located for the most part in front of the frontal plane of the body. In relation to the median sagittal plane of the body, the heart is located as follows: the fifth part, represented mainly by the right atrium, is to the right of it, and the rest of the volume is to the left of the median sagittal plane. In this case, part of the right, the entire left ventricle and the left atrium are located on the left. The heart is farthest from the posterior and right walls of the chest cavity [8, 9].
The use of certain radiation diagnostic methods during pregnancy made it possible to determine intrauterine pathology of the fetus with a high degree of probability. In modern clinical practice, in addition to ultrasound, magnetic resonance imaging, computed tomography, and fetal echocardiography are used. In addition, fetoscopy plays an important role in the field of prenatal diagnosis [10–13].
Fetal heart defects are often accompanied by chromosomal abnormalities, and damage to the left side of the heart is more common [14-17]. In this regard, when identifying the presence of abnormalities in cardiac development, additional genetic research is required, which is carried out today in many developed countries of the world [18, 19].
With a high degree of probability, congenital malformations of the cardiovascular system can be detected at the 16th week of pregnancy, and at the 21-22nd week, in almost 100% of cases the type of congenital defect can be identified [20-23]. It is possible to carry out intrauterine correction mainly at 22-32 weeks, depending on the type of defect [4, 10, 24, 25].
Heart defects amenable to fetal intervention are distinguished by the fact that they can progress rapidly from mild to severe during pregnancy. This results in significant irreversible myocardial damage and vascular and valvular hypoplasia developing during birth. In this unique group of cardiac lesions, there is usually a time-limited window for intrauterine intervention when the negative effects on cardiac growth and function are considered potentially reversible [26–28].
Currently, intrauterine cardiac interventions are mainly performed for critical aortic stenosis, pulmonary atresia, critical pulmonary stenosis with an intact interventricular septum, and hypoplasia of the left or right heart [29–32]. These diseases lead to postplacental hypoxia and, as a consequence, to intrauterine fetal death or spontaneous abortion. One of the goals of intrauterine interventions is to prevent irreversible changes in the left and right ventricles, as well as other cardiac structures, which ultimately leads to improved postnatal outcomes [33–36].
There are two main methods of surgical access: open (dissection of the anterior abdominal wall and uterus) and fetoscopic (access to the fetal heart is carried out using an endoscope). Today, preference is given to the fetoscopic method because of its low invasiveness and minimization of the risk of premature birth and physiological stress in the fetus [20, 35, 37, 38].
With open access, surgery is performed under general anesthesia. Access to the uterus is carried out by wide laparotomy: the incision on the anterior abdominal wall is 10-12 cm, the incision on the uterus is 5-7 cm. The edges of the wound on the uterus are clipped to prevent bleeding. The fetus is partially exposed and brought out into the wound, adhering to the rule “as much as possible inside the uterus, as little as possible outside,” and the necessary manipulations are performed. During the operation, comprehensive monitoring of the condition of not only the mother, but also the fetus is carried out. After the operation, the contractile activity of the uterus is inhibited by administering tocolytics [38–40].
With fetoscopy, the operation can be performed under local, regional or general anesthesia. Optimal fetal position plays an important role in successful intervention. Premedication of the fetus is carried out through the umbilical cord with a solution of fentanyl, atropine and pancuronium to immobilize it. 1.5-2 cm incisions are made through which a trocar is inserted into the abdominal cavity and uterus of a pregnant woman and appropriate manipulations are performed with its help. The insertion site of the trocars is determined under ultrasound control. The principle of the fetoscopic method: through the umbilical vessels, a special conductor (hollow tube) is inserted into the fetal circulatory system, which, after passing through a number of vessels, reaches its heart. Subsequently, miniature instruments are inserted through this guide, with the help of which the operation is performed [14, 15, 41, 42]. Valvular interventions require access through the apex of the heart ventricle. After passing into the left or right ventricle, a conductor with a catheter and a balloon is inserted, which is installed in the stenotic valve. Then the balloon is inflated and the narrowed valve opening is expanded. In addition, if necessary, a stent can be installed [43, 44].
The first indication for intrauterine correction is critical aortic stenosis and developing hypoplastic left heart syndrome, which leads to minimal or complete absence of blood flow to the aortic valve, resulting in left ventricular dysfunction, dilation, right-left shunt through an atrial septal defect or patent foramen ovale. , as well as retrograde blood flow in the aortic arch. As myocardial perfusion decreases, ischemia, necrotization, and scar formation occur. Left ventricular growth slows and eventually stops. All this leads to the fact that the left-sided structures of the heart cannot support systemic circulation [45-47]. Left ventricular hypoplasia is well tolerated in utero, since the ductus arteriosus fully supports the perfusion of fetal organs and tissues. However, after delivery, closure of the duct leads to systemic hypoperfusion, acidemia, and death, usually within a few days. A way out of this situation may be to perform balloon valvuloplasty of the aortic valve. A potential benefit of fetal cardiac intervention for hypoplastic left heart syndrome is the reduction of left ventricular workload to prevent progressive left heart dysfunction and hypoplasia [48–51].
The basic principle of fetal aortic valve valvuloplasty is identical to that in infants, children and adults, but it also has significant features. The differences are largely due to access to the fetus and the associated risks to the mother and fetus.
To gain access to the fetus, it is necessary to position it in such a way that the chest is directed anteriorly with its left side. This position can be achieved as follows: firstly, the mother should take a position on her left side before starting the procedure, secondly, it is necessary to gently manipulate the fetus through the maternal abdominal wall so as not to disturb its position, and thirdly, stabilization of the fetus in an ideal position using a 19-gauge needle is necessary. Next, the fetus is given an intramuscular paralytic drug, which ensures the cessation of spontaneous movement [52-55].
Percutaneous access to the fetal heart is carried out under ultrasound guidance. A cannula with a catheter located inside it crosses the following structures: the maternal abdominal wall, the uterine wall, amniotic fluid, the fetal chest wall and its heart, and then passes to the aortic valve. Placenta should be avoided if possible, but transplacental access can be used if necessary. Once the cannula reaches the fetal chest wall, its tip is directed to the apex of the left ventricle, which is punctured in a quick motion to minimize damage to the heart. A catheter with a balloon located at its end is exposed near the aortic valve. When carefully moved, the wire passes over the valve and is fixed in the ascending aorta. The balloon is then sequentially inflated to expand the lumen of the aortic valve. Antegrade flow is usually restored immediately and is often accompanied by a rapid, marked improvement in left ventricular function [55–58].
Postnatal outcomes are highly variable and depend largely on the extent of left ventricular damage before intervention. A retrospective study conducted at Stanford University found that mid-pregnancy valvuloplasty can effectively improve fetal left ventricular function [59–61]. The Austrian surgeon W. Arzt and his assistants performed 24 procedures on 23 fetuses at 21–26 gestational weeks with aortic stenosis [10]. Technical success was achieved in 67% of cases, intrauterine fetal death - in 13%. Restoration of biventricular blood flow was observed in 30% of cases. It should be noted that in 50% of cases, bradycardia and/or right ventricular dysfunction occur after surgery. To prevent bradycardia, epinephrine and sodium bicarbonate are administered through a balloon catheter during surgery. Although biventricular flow is not achieved in all cases, a functioning left ventricle can support systemic circulation for further postpartum operations. Thus, the success of intrauterine valvuloplasty for critical aortic valve stenosis depends on the patient's cardiac structural pathology, the sophistication of the equipment used during the procedure, and the experience of the operating team [36, 43, 62, 63].
The second indication for intrauterine intervention is pulmonary atresia or critical pulmonary stenosis in a fetus with an intact interventricular septum and developing hypoplastic right ventricular syndrome. This pathology is characterized by fibromuscular atresia of the right ventricular outflow tract, severe right ventricular hypoplasia and coronary anomaly. Blood flow is determined from left to right through the patent ductus arteriosus into the pulmonary artery system [64-67]. Pulmonary valvuloplasty may be a solution, but this procedure is extremely technically complex. Fetal pulmonary valvuloplasty can lead to regrowth of the right ventricle, restoration of antegrade blood flow through the right heart, increased growth of energy sources, and achievement of biventricular circulation [68, 69].
Pulmonary valvuloplasty is performed under local or general anesthesia. During ultrasound guidance, a needle is inserted into the amniotic sac through which a 0.014-inch diameter guide wire is guided and passed through the pulmonary valve. A balloon catheter is then inserted and inflated to dilate the stenotic valve. Studies have been carried out in which a coronary balloon catheter was used: it was directed into the left pulmonary artery and then removed through a perforated valve followed by inflation. As surgeon S. Yuan reports in his work, citing the results of a study by surgeon Polat and his team, of 27 newborn infants who underwent intrauterine pulmonary valvuloplasty at the 25th gestational week, 8 patients had non-biventricular outcomes, and 19 patients had biventricular circulation , which corresponds to 70.4% [36, 70, 71]. Successful pulmonary valve perforations allow significant growth of the right ventricle as well as the tricuspid and pulmonary valves. Intrauterine pulmonary valvuloplasty for pulmonary atresia facilitates antegrade blood flow of the right ventricle, causing proper cardiac growth and improving postpartum survival with biventricular circulation [36, 72, 73]. It should be noted that fetuses with significant myocardiopathy due to chronic right ventricular hypertrophy are not good candidates for this procedure because they do not have the ventricular reserve to respond favorably to valvuloplasty. However, transplacental administration of terbutaline may help improve cardiac function in such cases [74, 75].
The third indication for intrauterine cardiac intervention is hypoplastic left heart syndrome with an intact atrial septum or a restrictive atrial septal defect [76]. This pathology is a variant of hypoplastic left heart syndrome, during which blood flow through the oval window is limited or absent due to abnormal closure of the orifice or abnormal thickening of the atrial septum. A septal defect accompanies hypoplastic left heart syndrome in approximately 22% of cases [68, 77]. In the presence of an intact or restrictive atrial septum in hypoplastic left heart syndrome, there is intrauterine atrial hypertension, leading to permanent damage to the pulmonary vasculature, lung parenchyma, and lymphatic system. After childbirth, vascular resistance in the lungs increases and blood flow in the pulmonary vessels slows, which leads to the fact that oxygenated pulmonary venous blood does not penetrate the systemic circulation. At the same time, the pressure in the left atrium increases sharply, causing pulmonary venous overload and respiratory failure. Ultimately, this leads to hypoxemia, respiratory and metabolic acidosis and, as a consequence, the rapid death of the child. Correction of atrial septal defects in utero may interrupt or alleviate lung injury and provide a more favorable postnatal prognosis [78–81].
With the described heart damage, it is possible to perform balloon valvuloplasty, as well as enlargement of the oval window, creation or expansion of an atrial septal defect (septostomy) to facilitate the discharge of oxygenated blood from left to right and improve blood flow through the pulmonary veins, which will lead to normalization of oxygenation of the body [38, 82].
For fetal atrial septostomy, ultrasound-guided transatrial access is used [83, 84]. A cannula with an 18- or 19-gauge catheter inside is advanced in a similar way: it passes through the maternal abdominal wall, uterine wall, amniotic fluid, fetal chest wall and fetal heart. The right atrium is punctured, where a 0.014-inch wire with a balloon at the end is inserted through the interatrial septum into the left atrium or pulmonary vein. To create a defect in the septum, after the balloon exits, the entire system is pulled back until the balloon stretches the interatrial septum. Then it is inflated several times until the required diameter is achieved; if necessary, a stent can be installed into the defect [68, 83, 85].
Thus, atrial septostomy for hypoplastic left heart syndrome with an intact atrial septum or restrictive atrial septal defect may protect the fetus from damage to the lung parenchyma. In addition, intrauterine atrial septostomy can significantly reduce the need for postpartum interventions [61, 86–88].
Another type of intrauterine intervention is cordocentesis. This is an invasive procedure in which a thin needle is used to puncture the umbilical cord to obtain blood for laboratory tests or to administer medications to the fetus, including to treat heart disease. The administration of drugs to the fetus using the cordocentesis technique is called fetotherapy [89–91]. An example of fetotherapy is the transplacental administration of digoxin, which improves fetal heart function and supports a long and successful pregnancy. The indication for the use of digoxin is persistent fetal tachyarrhythmia, which can lead to congestive heart failure and, as a consequence, premature death. Digoxin restores myocardial function and sinus rhythm normalizes. A report by the team of surgeon K. Zhou reported that during a 14-month follow-up there was no recurrence of arrhythmia, and there were no neurological deficits, slow growth and development [13, 92-94].
One of the main problems when performing endoscopic operations is poor visibility due to insufficient transparency of the amniotic fluid. This problem was partially solved by German doctors from the Medical University of Bonn: they proposed pumping carbon dioxide, which is harmless to the fetus, into the amniotic fluid. Thanks to this approach, there is no need to replace amniotic fluid [35, 95]. Modern imaging techniques are also capable of fully providing a complete overview for the surgeon.
Today, the level of availability of correction of coronary heart disease, congenital and acquired heart defects has reached certain heights [36, 96, 97], however, intrauterine treatment of defects in Russia is performed only in St. Petersburg at the Federal Center of Heart, Blood and Endocrinology named after. V.A. Almazov (single operations), in Irkutsk and Yekaterinburg [14].
Thus, intrauterine heart surgery is the most promising area of prenatal surgery. They improve fetal heart function, in most cases normalizing postnatal biventricular blood flow, minimizing fetal mortality, thereby increasing neonatal survival. At the same time, this type of operation is extremely complex. To improve the technical success of such interventions and their results, a well-trained multidisciplinary team with sophisticated equipment is required, so such operations are performed only in specialized large medical institutions. Further developments in intrauterine surgery may significantly improve the rates of subsequent surgical interventions in patients with congenital heart defects.
Information about authors
Skorobogachev R.V. — student of the Federal State Budgetary Educational Institution of Higher Education “South Ural State Medical University” of the Ministry of Health of Russia; https://orcid.org/0000-0002-1229-7011
Belekhova D.A. — student of the Federal State Budgetary Educational Institution of Higher Education “South Ural State Medical University” of the Ministry of Health of Russia; https://orcid.org/0000-0002-8662-1216
Belova E.A. — methodologist at the Federal State Budgetary Educational Institution “Federal Center for Cardiovascular Surgery” of the Ministry of Health of Russia; https://orcid.org/0000-0003-0270-1153
Belov D.V. — Assistant at the Department of Hospital Surgery at the South Ural State Medical University of the Russian Ministry of Health, cardiovascular surgeon at the Federal Center for Cardiovascular Surgery of the Russian Ministry of Health; https://orcid.org/0000-0003-4985-9716
Peshikov O.V. — Candidate of Medical Sciences, Associate Professor of the Department of Anatomy and Operative Surgery of the Federal State Budgetary Educational Institution of Higher Education “South Ural State Medical University” of the Ministry of Health of Russia; https://orcid.org/0000-0001-8906-2133
Author responsible
for correspondence:
Peshikov Oleg Valentinovich - https://orcid.org/0000-0001-8906-2133; e-mail
How to quote:
Skorobogachev R.V., Belekhova D.A., Belova E.A., Belov D.V., Peshikov O.V. Indications and methods of intrauterine intervention for surgical correction of cardiac malformations. Operative surgery and clinical anatomy (Pirogov scientific journal).
2019;3(2):25-33. https://doi.org/10.17116/operhirurg2019302125
Symptoms of congenital heart defects in newborns
The clinical picture of the manifestations of the disease depends on the type of congenital heart defect in newborns, the types of hemodynamic and circulatory disorders.
Most often, immediately after the birth of a child (or soon after that - in the first days, weeks, months of life) “blue” defects begin to appear, which are impossible without the manifestation of the main symptom - cyanosis (blue skin). However, “white” defects may also have similar symptoms. Both are serious congenital heart defects in newborns that must be treated (including congenital heart surgery) to avoid further complications or even death.
Symptoms of congenital heart disease in a newborn that should not be ignored:
- A bluish or bluish tint to the skin, lips, and ears (cyanosis), which can appear both at rest and during feeding and crying.
- On the contrary, pallor, unhealthy white skin color, and cold extremities are possible.
- Dyspnea.
- Swelling (around the eyes, stomach and legs).
- Low weight gain (caused by the baby's shortness of breath, which does not allow him to eat properly during breastfeeding).
- Tachycardia (rapid heartbeat).
- Heart murmurs (this symptom can only be detected by doctors when listening to the child’s heart).
How do heart valve diseases manifest?
Most often, patient complaints are nonspecific: shortness of breath, rapid pulse, arrhythmia, fatigue, cyanosis, dizziness.
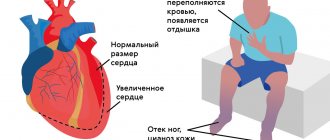
The severity and nature of symptoms depend on the location of the affected valve. With valve defects of the left half of the heart (mitral and aortic), the lungs are primarily affected, because Blood stagnates in their vessels, which manifests itself as shortness of breath. There are also signs of insufficient blood supply to all organs and systems, primarily the brain and the heart itself. Dizziness, fainting, and angina occur. If the functioning of the valves of the right half of the heart (tricuspid and pulmonary valves) is disrupted, blood stagnates in the vessels of the systemic circulation, i.e. all organs except the lungs are affected. Swelling of the legs and feet, ascites (fluid in the abdominal cavity), enlargement of the liver, etc. develop (LIVER INCREASES, ETC.).
Heart valve defects are dangerous due to their complications and impact on the body, so the main prevention of pathological conditions is regular examinations and treatment of diseases leading to the formation of valve defects.
Causes of congenital heart disease in newborns
Scientists still cannot name the exact causes of congenital heart disease.
However, the good news is that most of them can be corrected surgically. Surgery on a congenital heart defect gives the child the opportunity for normal development and growth.
Thanks to a series of studies, scientists were able to identify a number of factors that increase the risk of congenital heart disease in newborns:
- Viral (if the child was conceived or carried for the first three months of pregnancy during a viral epidemic; influenza and rubella viruses are especially dangerous).
- Ecological (parents living in environmentally disadvantaged areas).
- Exposure of the fetus to ionizing radiation.
- Genetic (hereditary predisposition).
- Bad habits of parents (alcoholism and drug addiction of mothers are especially terrible).
- Autoimmune or severe chronic diseases in parents (the most common negative effect is systemic lupus erythematosus or diabetes in the expectant mother).
- Taking certain medications during pregnancy (containing ethanol, thalidomide, amphetamines, anticonvulsants, trimethadione, lithium, progestogens).
- The mother's age is over 35-37 years.
Possible complications of congenital heart disease in newborns
Sometimes there are cases when, when diagnosed with congenital heart disease, a newborn does not show any signs of the disease.
The absence of symptoms may continue until primary school age. At this age, any congenital heart defect in children manifests itself. The child begins to lag behind his peers in physical development, suffocates during exercise, and his skin turns pale or blue. Not only poor physical performance, but also learning problems, emotional disorders - all this can be caused by one disease.
Unfortunately, a child with congenital heart disease subsequently needs lifelong monitoring of his health due to the possible development of complications and concomitant diseases. Even if surgery was performed at an early age, it is not a panacea for life. In addition, strict dosing of physical activity will be necessary throughout your life.
Congenital heart defects in children can be complicated by other diseases.
Among them:
- Heart failure
- Early prolonged pneumonia
- High pulmonary hypertension
- Endocarditis
- Syncope (loss of consciousness)
- Angina pectoris
- Myocardial infarction
- Attacks of shortness of breath and blueness
Self-diagnosis
To independently diagnose the state of the cardiovascular system, functional tests are carried out: Martinet-Kushelevsky, Kotov-Deshin, Rufier, with a 15-second run at the fastest pace, and others. You can familiarize yourself with the sampling methodology in the relevant articles on the Internet.
These are simple tests to roughly assess the adaptation of the heart and blood vessels to stress. In schools and universities, they are carried out in special medical physical education groups to monitor the well-being and fitness of students.
Diagnosis of congenital heart disease in newborns
Naturally, the earlier a congenital heart defect in newborns is detected and timely treatment begins, the more favorable the prognosis. However, due to the absence of clearly defined symptoms and, accordingly, no treatment at all, there are cases of death among children under 1 year of age (occasionally even older).
Usually, during preventive examinations, the doctor must listen to the baby’s heart. If murmurs are heard there, this is a reason for a detailed examination of the heart (although not necessarily a symptom of congenital heart disease).
For this purpose, parents and children are referred to specialists with a narrower profile - a cardiologist and a cardiac surgeon.
If you suspect a heart defect, the baby’s parents do not need to immediately turn to Internet search engines with the request “congenital heart defect surgery.” Not all defects require radical surgical intervention. And the main thing is to first establish the correct diagnosis.
Changes characteristic of congenital heart disease in a newborn are revealed by the following research methods:
- X-ray of the chest (as well as ventriculography - x-ray with contrast injection).
- Echocardiography (using ultrasound to examine the condition of the heart muscle, valves, blood flow in the heart cavities).
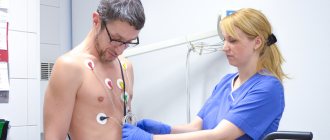
- Electrocardiogram – ECG (or methods based on it: stress ECG (treadmill test, bicycle ergometry), ECG Holter monitoring).
If the above examination methods reveal the presence of a serious disease - congenital heart disease, then further diagnostics are carried out in the cardiac surgery department on an inpatient basis. Surgeons, if necessary, conduct examinations using procedures such as angiocardiography and probing of the heart chambers.
Modern high-tech medical equipment allows you to conduct a full comprehensive examination of the heart and blood vessels in order to establish the most accurate diagnosis and choose the necessary treatment tactics.
Diagnostics
At each appointment, the pediatrician conducts auscultation (listening with a phonendoscope) and examination of the chest to check the functioning of the child’s heart, even if the patient came in for ARVI. Pathological murmur is easily auscultated by a cardiologist. It indicates heart problems, even if the patient is not worried about anything or does not attach importance to some symptoms (in addition, children are sometimes afraid to report their problems and worries).
Basic diagnostic methods in cardiology:
- ECG (assessment of the conduction of electrical impulses through the heart muscle);
- Daily ECG monitoring;
- Ultrasound of the heart;
- Echo-CG (study of the morphology and functional state of the heart);
- Doppler echo-CG (study of blood flow in the chambers of the heart and great vessels);
- Plain radiography of the chest (determining the size and location of the cardiac shadow);
- Coronary angiography (study of coronary vessels);
- CT;
- MRI.
It is important to identify the disease in time: the earlier treatment is started, the better the effect. However, most violations can only be detected using special equipment. In addition, the baby is not able to voice complaints, so instrumental diagnostics in pediatrics cannot be avoided.
Treatment for the diagnosis of congenital heart disease in newborns
Treatment methods depend on the type of heart defect:
- Minimally invasive procedures
- Radical surgery
- Heart transplant
- Drug therapy
- Long-term therapy
If the heart defects are not very serious, minimally invasive procedures can be used. Their essence is that a thin catheter is inserted into the heart through the femoral vein, and the necessary instruments are delivered to the defect inside the catheter.
The most radical but effective method of treating congenital heart disease is surgery. This is a traditional open intervention with opening of the chest and the heart itself. Most of these operations are performed using a heart-lung machine. After heart surgery, a child faces a long and difficult recovery period, but sometimes it is the only possible way to cure.
Heart transplantation is used in the most severe cases, in which traditional surgery is helpless in case of severe congenital heart disease.
Drug therapy is used mainly for auxiliary purposes, improving the functioning of the heart and blood flow.
Long-term therapy involves several consecutive operations on congenital heart disease.
Management of childbirth with heart disease
The issue of medical tactics during childbirth is of particular importance. The best choice is early hospitalization at 36-37 weeks of pregnancy. The delivery plan is drawn up in consultation with the participation of an obstetrician, cardiologist or therapist and anesthesiologist. The choice of method is strictly individual for each patient, depending on the situation.
Modern medicine has a sufficient arsenal of diagnostic tools to promptly prevent pregnancy complications in women with heart defects. The following methods are usually used:
- Electrocardiography is the recording of electrical phenomena occurring in the heart muscle when it is excited. This study allows you to register changes in the heart muscle by changes in electrical impulses.
- Phonocardiography is a method of recording sounds (tones and noises) resulting from the activity of the heart. It is used to assess heart function and recognize abnormalities, including valve defects.
- Echocardiography (ultrasound of the heart). It is used to study blood circulation and cardiodynamics (heart function), determine the size and volume of the cavities of the heart, and assess the functional state of the heart muscle. The method is harmless to the mother and fetus.
- Load testing is used to assess the functional state of the heart muscle. Load tests on a bicycle ergometer are also used when examining pregnant women - during this test, an ECG is taken for the patient at different intensities of physical activity.
- The study of the function of external respiration and the acid-base state of the blood involves studying lung capacity and blood oxygen saturation at rest and during exercise. The study allows you to determine how adequate the blood oxygen saturation is, i.e. How well the heart can cope with the load at the moment.
- Blood tests are usually a standard test that is performed when examining all pregnant women. However, in this case, the doctor pays special attention to the state of the blood coagulation system.
- Fetal ultrasound, cardiotocography , which are regularly performed after 28 weeks to assess the condition of the placenta and fetus. These studies help determine whether the fetus is suffering from a lack of oxygen and nutrients. In addition, fetal ultrasound allows you to identify possible malformations of the child even before birth and take appropriate measures - from urgent surgery after delivery to termination of pregnancy.
The advantage remains with the natural method of delivery. In case of compensated heart disease, therapy is carried out aimed at preventing heart failure and supporting the heart, preventing pulmonary edema, and, if arrhythmia is possible, ECG monitoring. Adequate labor pain relief is provided, as fear and pain lead to additional stress on the heart.
Advertising
As a rule, the most difficult period of labor - pushing (the period of expulsion of the fetus) - is tried to be shortened with the help of an episiotomy - incision of the perineum. Switching off pushing (application of obstetric forceps) is carried out in case of circulatory problems.
Many doctors believe that full-term delivery by cesarean section reduces the burden on the cardiovascular system and reduces mortality among pregnant women with heart defects.
Caesarean section for heart defects is performed in the following cases:
- with an active rheumatic process (fever, pain in the relevant organs, characteristic changes in tests);
- with severe heart disease with severe left ventricular failure and no effect of drug therapy;
- when a heart defect is combined with obstetric pathology requiring surgical delivery.
Successful delivery of patients suffering from severe congenital and acquired heart defects can be facilitated by delivery under conditions of hyperbaric oxygenation.
After the birth of the fetus and the passage of the placenta, there is a rush of blood to the internal organs (and primarily to the abdominal organs) and a decrease in blood circulation in the vessels of the brain and the vessels feeding the heart muscle. Therefore, to prevent deterioration of the condition, immediately after the birth of the child, the woman is given medications to ensure normal heart function.