Methemoglobinemia is a blood disorder that occurs when there is little oxygen supply to the body's cells. There are two types of methemoglobinemia – congenital and acquired.
Infants can inherit the condition from their parents - this is called congenital methemoglobinemia.
Acquired methemoglobinemia can occur after exposure to certain drugs or chemicals. Acquired methemoglobinemia usually manifests itself mildly and resolves once the cause of the disease is identified and eliminated.
Types and causes of methemoglobinemia
The disease occurs when the blood protein hemoglobin is converted into methemoglobin in the body.
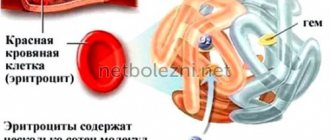
Hemoglobin is present in red blood cells; it carries oxygen throughout the body, distributing it to all tissues and organs. With methemoglobinemia, oxygen is still carried around the body but is not released efficiently.
The causes of methemoglobinemia depend on the type of disease. In the case of a genetic cause, methemoglobinemia is caused by a specific factor.
Inherited (congenital) methemoglobinemia
Methemoglobinemia can be passed on if one or both parents carry a defective gene that causes problems with the cytochrome b5 reductase enzyme.
- Type 1 inherited methemoglobinemia
. This is erythrocyte reductase deficiency which occurs when red blood cells do not have cytochrome b5 reductase. - Type 2, inherited methemoglobinemia
. This is a deficiency of common reductase that occurs when many cells in the body do not have the enzyme. This type can be fatal. - Enzymopenic M methemoglobinemia
. This condition occurs when the hemoglobin protein itself is defective.
Acquired methemoglobinemia
Acquired methemoglobinemia is more common than inherited forms, and methemoglobinemia is caused by exposure to:
- anesthetics such as benzocaine
- nitrobenzene
- some antibiotics, including dapsone and chloroquine
- nitrites, which are used as additives to prevent spoilage of meat
- Some foods, such as spinach, beets or carrots, which contain natural nitrates. They can cause methemoglobinemia if consumed in large quantities
Methemoglobinemia. Clinical and laboratory parallels
Torshin V. A., Ph.D., Associate Professor, Department of Biochemistry, RMAPO, Moscow
Under normal conditions, the blood contains small amounts of hemoglobin derivatives that are unable to carry oxygen, the so-called dyshemoglobins: carboxyhemoglobin, methemoglobin and sulfhemoglobin. With an increase in the content of dyshemoglobins, the oxygen transport function of the blood significantly suffers. The most clinically significant dyshemoglobins are carboxyhemoglobin (COHb) and methemoglobin (MetHb).
Endogenous and exogenous sources of methemoglobin
Methemoglobin is constantly formed as a result of normal metabolism of body cells. There is an endogenous mechanism for regulating the level of methemoglobin in the blood, which allows maintaining the proportion of this fraction not higher than 1.0-1.5% of total Hb. Unlike carboxyhemoglobin, which is formed as a result of the inclusion of carbon monoxide in the hemoglobin molecule, methemoglobin differs from hemoglobin only in the presence of oxidized ferric iron Fe+++ in the heme instead of divalent iron Fe++. There are many compounds in nature that can oxidize Fe++ to Fe+++ in the hemoglobin molecule. In addition to external ones, endogenous influences are also known, as well as congenital disorders of the mechanisms regulating the level of methemoglobin.
Types of exposure and causes of methemoglobinemia
Congenital
- HbM
- Methemoglobin reductase (cytochrome b5 reductase) deficiency
Acquired (drug effects)
- Amyl nitrite
- Novocaine
- Lidocaine/prilocaine
- Dapsone
- Nitroglycerine
- Nitroprusside
- Phenacetin
- Phenazopyridine
- Metoclopramide
- Sulfonamides
- Quinones (chloroquinone, primaquine)
- Nitric oxide
- Dr.
Purchased (chemical agents)
- Aniline dyes
- Butyl nitrite
- Chlorobenzene
- Isobutyl nitrite
- Naphthalene
- Nitrophenol
- Silver nitrate
- Trinitrotoluene
- Foods and drinking water high in nitrates
Endogenous causes (typical for newborns and children of the first year of life)
- Reduced activity of methemoglobin reductase (cytochrome b5 reductase) compared to adults (the norm for adults is 10-20 U/g, in children under 4 months of age it is no more than 60%);
- Diarrhea (intolerance to a number of proteins, viral and bacterial enterocolitis, etc.);
- Conditions causing metabolic acidosis;
- Colonization of the intestine by nitro-forming bacteria.
Oxidizing substances can cause methemoglobinemia either by direct oxidation of iron in hemoglobin or due to the formation of free radicals. In addition to exposure to methemoglobin-forming drugs, infants are predisposed to developing methemoglobinemia when exposed to foods and drinking water high in nitrates. Intestinal flora, which converts nitrates to nitrites, also contributes to an increase in the formation of methemoglobin in childhood. In addition, only by 4 months of a child’s life does cytochrome b5 reductase reach the level of activity of an adult. It should also be noted that fetal hemoglobin, characteristic of newborns, is more easily subject to oxidation compared to the hemoglobin of adults.
Newborns are more likely to experience diarrhea, which can lead to metabolic acidosis. It is known that under conditions of metabolic acidosis, the enzyme system for hemoglobin reduction can lose up to 50% of its activity. Methemoglobinemia associated with diarrhea is caused by a combination of factors, even in the absence of systemic acidosis. In this case, the conversion of nitrates to nitrites by gram-negative bacteria plays a role, as does an idiopathic hypersensitive reaction to certain proteins contained in nutritional mixtures.
Mechanisms of regulation of methemoglobin levels
The main defense system against oxidizing agents, which allows maintaining the methemoglobin fraction in healthy subjects at a level of 1.0-1.5%, includes three components: reduced nicotinamide dinucleotide (NAD-H), the heme-containing hemoprotein cytochrome b5 and the enzyme cytochrome b5- reductase. The electron donor is the glycolysis product NAD-H. The electron is transferred from NAD-H to cytochrome b5 and ultimately to methemoglobin. Electron transport is catalyzed by the enzyme cytochrome b5 reductase. This mechanism is responsible for the recovery of 99% of hemoglobin from methemoglobin. Another pathway for hemoglobin reduction, associated with the activity of NADP-methemoglobin reductase, has little effect under normal conditions. Its role increases in case of cytochrome b5 reductase deficiency. This alternative pathway is also important for the therapeutic effects of the main antidote used for acquired methemoglobinemia, methylene blue. Finally, antioxidants such as reduced glutathione and ascorbic acid have a hemoglobin-reducing effect.
Congenital methemoglobinemia
Hereditary methemoglobinemia is a rare genetic disorder associated with a mutation on chromosome 22q13, on which cytochrome b5 reductase is found. The disease is inherited in an autosomal recessive manner and is manifested by cyanosis and high levels of methemoglobin in the blood shortly after birth. Since the enzyme cytochrome b5 reductase is present in both free and bound forms with the mitochondrial membrane, there are two types of homozygous methemoglobinemia. In type 1, associated with enzyme deficiency only in free form, the disease manifests itself only as cyanosis. In type 2, associated with enzyme deficiency in all cells, the course of the disease is more severe (mental retardation, neurological disorders).
Clinical symptoms of heterozygous methemoglobinemia can only appear under conditions of so-called “oxidative stress.” Hemoglobin-M (HbM) is a group of abnormal hemoglobins with mutations in the globin chain that fix heme iron in an oxidized form. The replacement of histidine by tyrosine in the alpha and beta chains determines the main characteristics of HbM. Congenital pathology associated with the formation of hemoglobin-M is inherited in an autosomal dominant manner.
It is important to note that structural changes in heme in HbM lead to the fact that the absorption spectrum of HbM during absorption spectrophotometry differs from the spectrum of conventional methemoglobin. This creates problems when performing co-oximetry when falsely normal FMetHb values are noted and the analyzer produces falsely elevated FCOHb or FSHb values. In such a situation, it is necessary to conduct a study with potassium cyanide, or using the gas chromatography method.
Clinical manifestations of methemoglobinemia
Clinical manifestations of methemoglobinemia quite clearly correlate with the methemoglobin fraction in the blood, measured using a multi-wavelength co-oximeter. Modern co-oximeters, which are part of blood gas and acid-base balance analyzers, allow analysis by absorption spectrophotometry at 128 wavelengths in steps of 1.5 nm. The relationship between clinical symptoms and the proportion of methemoglobin fraction in the blood is presented in Table 2.
| FMetHb% | Symptoms |
| <3 | No clinical manifestations |
| 3—15 | Grayish tint to the skin |
| 15—30 | Cyanosis, chocolate brown color of blood |
| 30—50 | Dyspnea, headache, weakness, dizziness, fainting, pulse oximeter SpO2 reading about 85% |
| 50—70 | Tachypnea, metabolic acidosis, arrhythmias, convulsions, central nervous system depression up to coma |
| >70 | Severe clinical hypoxia, death |
When assessing the clinical symptoms of methemoglobinemia, the significant vulnerability of young children should be taken into account. Children under 6 months quite often experience diarrhea of various etiologies. You should also take into account the possible effects of oxidizing agents such as surface anesthetics or dental gels, sulfonamides, mothballs, etc. A number of authors have noted the absence of cyanosis in children with severe methemoglobinemia due to diarrhea. Other authors note specific intolerance to the proteins of nutritional mixtures as the main cause of clinically significant methemoglobinemia.
Diagnosis of methemoglobinemia
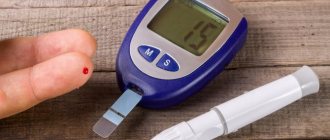
In the diagnosis of methemoglobinemia, undoubtedly, the main test is the measurement of the MetHb fraction on a modern co-oximeter. Interpretation of pulse oximetry and blood gas data can be misleading in the presence of MetHb. Pulse oximetry determines the deoxy- and oxyhemoglobin fractions by measuring the absorption ratio in the red and infrared spectrum using emission spectrometry. In the absence of dishemoglobins, the absorption peak of deoxy- and oxyhemoglobin is observed at waves of 660 and 940 nm with a ratio of 0.43, corresponding to 100% saturation. The peak absorption of methemoglobin can occur at both wavelengths equally, that is, methemoglobinemia creates a ratio of 1.0, corresponding to a saturation of 85%. Thus, with methemoglobinemia over 30%, pulse oximetry data will plateau at 82-85%, regardless of the increase in the level of methemoglobinemia, and, accordingly, the severity of hypoxia. The results of a standard blood gas analysis will also not diagnose methemoglobinemia, since analyzers calculate saturation SaO2%, taking into account paO2, pH, ctHb and assuming a normal position of the oxyhemoglobin dissociation curve. The so-called “pseudohemoglobinemia” occurs quite rarely, when sulfhemoglobin is identified as MetHb by a co-oximeter. The gold standard for diagnosis in such cases is gas chromatography.
Cyanosis is the most common symptom of methemoglobinemia and serves as a reason for differential diagnosis with diseases of the cardiovascular and respiratory systems. Methemoglobinemia is characterized by a discrepancy between the severity of cyanosis and the degree of hypoxemia. Diseases accompanied by severe cyanosis, for example, pulmonary embolism, are manifested by a clear decrease in paO2, which is not observed with methemoglobinia and paO2 can be above 150 mm Hg. Another diagnostic test is inhalation of oxygen, which does not reduce the degree of cyanosis in methemoglobinemia. Arterial blood with methemoglobinemia can be so dark that during puncture of the artery, a specialist may suspect that it has entered a venous vessel. In this case, self-filling syringes for drawing arterial blood may be useful. The pulsating flow of blood into the self-filling syringe confirms that it has entered the arterial vessel, since the pressure of about 10 mm Hg in the veins is not enough for blood to flow by gravity.
Treatment of methemoglobinemia
Treatment of methemoglobinemia is based on the reduction of oxidized ferric iron to ferrous iron. The method of choice is intravenous administration of methylene blue at a dose of 1-2 mg/kg over 3-5 minutes. Methylene blue is recommended for administration to patients with FmetHb 20% in the presence of clinical symptoms, and in the absence of symptoms - at a FmetHb level of 30%. Improvement usually occurs within 1 hour. If there is no improvement, repeated administration of methylene blue at a dose of 1 mg/kg is acceptable. It must be remembered that methylene blue itself is an oxidizing agent and can cause the development of hemolytic anemia, especially when its dose exceeds 4 mg/kg or in patients with glucose-6-phosphate dehydrogenase deficiency. In the presence of concomitant methemoglobinemia and glucose-6-phosphate dehydrogenase deficiency, methylene blue therapy may be ineffective, since these patients have NADP cofactor deficiency. An alternative treatment for these patients is exchange transfusion. The introduction of N-acetylcysteine, as a precursor of glutathione or glucose, as a cofactor for the synthesis of NADP, is also used. Intravenous administration of an antioxidant such as ascorbic acid in a dose of 1-2 g is also useful.
In conclusion, it should be noted that the active introduction of modern co-oximeters into the practice of intensive care units makes it possible to clarify the cause of the development of hypoxic conditions and determine the degree of dyshemoglobinemia, in particular methemoglobinemia, in many urgent situations that previously remained in the realm of “terra incognita”.
Bibliography
- Chelnokov S. B., Yakovleva E. A., Pudina N. A. A case of severe methemoglobinemia in a premature newborn baby // Bulletin of intensive care. 2002. No. 2. P. 18-21.
- Torshin V. A. Clinically significant dysmoglobins. Carboxyhemoglobin // Laboratory. 2007. No. 1. P. 17-18.
- Kyle A. Nelson, Mark A. Hostetler An Infant with Methemoglobinemia // Hospital Physician. 2003. February. pp. 31-38.
- Shannon Haymond, Rohit Cariappa, Charles S. Eby, Mitchell Scott Laboratory Assessment of Oxygenation in Methemoglobinemia // Clinical Chemistry. 2005; 51-2. pp. 434-444.
- Chris Higgins Causes and clinical significance of increased methemoglobin. https://www.bloodgas.org/, 2006, October.
Symptoms
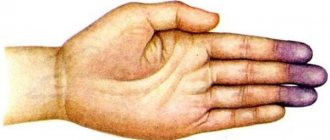
A baby born with this condition may have a bluish tint to the skin, called cyanosis. This color may appear at birth or shortly thereafter.
Child with methemoglobinemia
Signs of methemoglobinemia:
- brown color of blood;
- blueness around the mouth;
- blue hands;
- blueness of legs;
- difficulty breathing, shortness of breath;
- vomit;
- diarrhea.
In severe cases, the following may occur:
- lethargy;
- excessive drooling;
- loss of consciousness.
Symptoms vary depending on the amount of methemoglobin (MetHb) in the blood, which is measured on a scale called methemoglobin concentration.
Methemoglobin (MetHb) concentration levels
- The normal concentration of methemoglobin (MetHb) in human blood is between 0 and 3 percent. If methemoglobin reaches a concentration of 3 to 10 percent, the skin may take on a blue-gray appearance.
- Methemoglobin levels of 15 to 30 percent result in cyanosis, with the blood appearing chocolate brown.
- Concentrations of 30 to 50 percent cause more severe symptoms. These symptoms may include headache, fatigue, dizziness, anxiety and confusion, as well as temporary loss of consciousness, rapid heartbeat and weakness.
- When methemoglobin levels reach 50 to 70 percent, seizures, kidney problems, or abnormal heartbeats may occur.
- MetHb concentrations of 70% or more can be fatal.
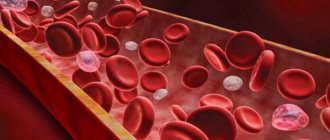
Blood from a patient with methemoglobinemia and normal blood
Typical symptoms of methemoglobinemia in different types of disease
Methemoglobinemia type 1:
- bluish skin color
Methemoglobinemia type 2:
- developmental delay;
- learning problems;
- seizures.
Enzymopenic M methemoglobinemia:
- bluish skin color
Acquired methemoglobinemia:
- bluish skin color;
- headache;
- fatigue;
- shortness of breath, shortness of breath;
- lack of energy.
Symptoms of methemoglobinemia
The symptoms of methemoglobinemia depend on the concentration of methemoglobin in the blood, which is determined using a multi-wavelength co-oximeter. The higher the methemoglobin content, the more pronounced the signs of tissue oxygen starvation will be. Clinical signs of methemoglobinemia are:
- pallor, cyanosis of the skin and mucous membranes;
- breathing problems, shortness of breath;
- dizziness, weakness;
- loss of consciousness;
- heart rhythm disturbance;
- convulsions.
At a methemoglobin concentration of up to 3%, there are no symptoms; from 3 to 15%, the skin acquires a grayish tint. Starting from 15% and up to 30, the patient becomes cyanotic, and his blood becomes chocolate-colored. When the content of methemoglobin in the blood exceeds 30%, breathing becomes impaired, a headache begins, weakness increases, dizziness and episodes of loss of consciousness are possible, while the blood oxygen level drops to 85%.
When methemoglobin exceeds a value of 50%, shortness of breath becomes pronounced, the respiratory rate increases, and signs of acidification of the internal environment of the body (acidosis) appear - heart rhythm disturbances, convulsions, coma. A level of methemoglobin in the blood of 70% or higher is considered fatal, since in this case there is a sharp inhibition of the activity of the cerebral cortex, hypoxia becomes maximum, irreversible changes develop in the tissues, and the patient dies.
In babies in the first months of life, methemoglobinemia can occur due to diarrhea, which quite often occurs in the first six months of a child’s life. Even with severe diarrhea, noticeable cyanosis does not occur in every child, so methemoglobinemia may remain undiagnosed. Symptoms of the pathology in children include cyanosis of the nasolabial triangle, fingers, ears, drowsiness, and possible developmental delays in the chronic course of the pathology.
It is necessary to remember the effect on the child’s body of other oxidizing agents that many young parents use today: dental gels, local anesthetics to relieve teething symptoms. In addition, methemoglobin may increase in food allergies with intolerance to the protein of the nutritional formula.
Methemoglobinemia can occur due to nitrophenol poisoning at work. Cases have been described where this happened not only when chemical vapors were inhaled, but also when it came into contact with the skin from protective clothing. Signs of pathology become noticeable 2-3 hours after contact with phenol and consist of weakness, headache, dizziness, bluish facial skin, respiratory failure, tachycardia, vomiting and nausea.
During the examination, the doctor records not only cyanosis, but also dullness of heart sounds, increased heart rate, decreased blood pressure, significant fever (up to 40ºC and above), enlarged liver and spleen, and possibly wheezing in the lungs. A blood test will show the presence of methemoglobin, a decrease in the level of red blood cells, possibly an increase in bilirubin and slight leukocytosis.
Nitrate methemoglobinemia
Separately, it should be said about the so-called nitrate methemoglobinemia, which occurs due to the ingestion of nitrates contained in drinking water. The upper limit for the concentration of nitrates in drinking water is considered to be 50 mg per liter; above this indicator, water can be dangerous.
The issue of nitrate intoxication is very relevant for infants who grow up on artificial feeding and daily drink formula prepared with poor quality water. In addition, experts are concerned about the quality of water extracted from wells in rural areas, since it does not undergo proper sanitary control.
The extent of the problem of nitrate toxicity and associated methemoglobinemia is not yet clear, but the World Health Organization is collecting information to conduct such a study. Water conditions in rural areas of developing countries and even in some cities still leave much to be desired.
Most often, nitrates enter drinking water from the soil when nitrogenous fertilizers are used in agriculture. Some regions have natural, naturally high levels of nitrogen in the soil, causing both water and grown vegetables to be saturated with nitrates above the acceptable limit.
Nitrates that enter the body with water are metabolized and become nitrites, which form methemoglobin in red blood cells. At high risk are infants prone to diarrhea and intestinal infections, elderly people due to the secretion of hydrochloric acid in the stomach decreasing with age, people with a genetic predisposition to methemoglobinemia, patients with stomach ulcers or chronic gastritis, as well as patients on hemodialysis.
To prevent the development of methemoglobinemia due to nitrates and nitrites, regular sanitary monitoring of the quality of drinking water is necessary, in which nitrates should not be more than 50 mg per liter, and nitrites should not be more than 3 mg/l.
It is easier to control these indicators in the presence of a centralized water supply, so the problem is more relevant for small settlements and villages, as opposed to cities, where, as a rule, everything is fine. Breastfeeding can be considered a preventive measure for babies in the first year of life. It is important to remember that neither boiling nor other manipulations with water remove nitro compounds from it.
Diagnosis of methemoglobinemia
A doctor will diagnose the condition using a blood test that measures the level of functional hemoglobin in the blood.
The doctor may do pulse oximetry, which checks the level of oxygen in the blood, and a blood test, which checks the concentration of gases in the blood.
A complete blood count is examined for the presence of erythrocytosis, reticulocytosis, increased Hb, and decreased ESR.
Photo of a patient with methemoglobinemia
A biochemical blood test is examined for a slight increase in bilirubinemia. Chronic methemoglobinemia is usually accompanied by the presence of Heinz-Ehrlich bodies in red blood cells.
In patients with enzymopenic M methemoglobinemia, with intravenous injection of methylene blue solution, cyanosis disappears, and the skin also acquires a normal color.
Treatment of methemoglobinemia
Some people with congenital methemoglobinemia have no symptoms, meaning they may not need treatment.
Mild cases of acquired methemoglobinemia usually do not require treatment. The doctor will advise the patient to avoid the substance that caused the problem.
Methylene blue is used to treat severe cases of methemoglobinemia, and doctors may prescribe ascorbic acid to lower the level of methemoglobin in the blood.
In severe cases, a person may require a blood transfusion. If cyanosis is severe, oxygen therapy will also be given.
Reasons for the development of methemoglobinemia
All reasons that can lead to an increase in the concentration of methemoglobin in the blood can be divided into several groups:
- Congenital (genetically determined) factors;
- Exogenous - intoxication with chemicals, drugs, industrial poisons, etc.;
- Endogenous - violation of regulatory mechanisms, intestinal microflora, acidosis.
Congenital methemoglobinemia develops due to the circulation of so-called hemoglobin M or due to a lack of the enzyme methemoglobin reductase. In cases where the pathology manifests itself due to a mutation in the gene encoding the enzyme, we speak of a hereditary form of methemoglobinemia. This disease is transmitted in an autosomal recessive manner and gives the first clinical manifestations in the form of cyanosis of the skin and mucous membranes already in the first days of a newborn’s life.
Hemoglobin M is a special form of protein that is formed as a result of a genetic mutation and is capable of combining only with the oxidized, trivalent form of iron. This form of hemoglobin binds oxygen poorly and does not meet the oxygenation needs of tissues. The pathology is transmitted in an autosomal dominant manner.
example of a family with hereditary methemoglobinemia due to a genetic mutation
The influence of exogenous factors contributes to the development of acquired methemoglobinemia . First of all, we are talking about medications - novocaine and some other anesthetics, dapsone, nitroglycerin, sodium nitroprusside, paracetamol, cerucal, nitric oxide, phenacetin (banned for use in most countries of the world due to side effects), amyl nitrite. When taking these medications, it is important not only to monitor blood hemoglobin levels, but also to promptly inform surgeons and anesthesiologists in order to avoid unwanted drug interactions or negative consequences of anesthesia during surgical operations.
In addition to drugs, some chemicals can lead to an increase in the concentration of methemoglobin in the blood: aniline dyes, trinitrotoluene, nitrophenol, chlorobenzene, etc., which can be found in chemical plants and laboratories.
It is known about the negative impact of food and water containing high concentrations of nitrates and nitrites on the content of methemoglobin in erythrocytes. This problem is relevant for rural areas and regions where there is no centralized water supply.
the formation of methemoglobin under the influence of chemicals and the return to hemoglobin under the action of the enzyme methemoglobin reductase. (Its deficiency explains the genetic cause of some cases of exogenous methemoglobinemia)
Endogenous factors in the development of methemoglobinemia especially clearly demonstrate their influence during the neonatal period and during the first year of the baby’s life. These include:
- almost half the activity of the enzyme methemoglobin reductase in the first 4 months of life compared to adults;
- diarrhea - due to food allergies, insufficiency of enzymes of the digestive system, infectious inflammation of the small intestine, etc.;
- conditions accompanied by acidification of the internal environment of the body (acidosis);
- colonization of the intestine by microorganisms that secrete nitrates or are capable of converting nitrates into nitrites.
Oxidizing agents coming from the outside or formed during metabolic reactions in the child’s body can either directly oxidize hemoglobin iron, converting it into a trivalent state, or indirectly through the formation of free radicals. It should also be taken into account that the so-called fetal hemoglobin, circulating in the blood of newborns, is oxidized faster and more easily than “adult” type hemoglobin.
Newborn babies have a fairly high tendency to diarrhea, which can quickly lead to acidosis, which suppresses the activity of enzymes that reduce hemoglobin. Microorganisms and food intolerance to certain proteins only aggravate the situation, leading to the accumulation of nitrates in the intestinal lumen.
Is it possible to prevent the disease?
Congenital methemoglobinemia cannot be prevented. People with a family history of the blood disorder are advised to consult a genetic specialist before having children.
To prevent acquired methemoglobinemia, known causes such as benzocaine, which is one of the most common initiators, should be avoided.
Benzocaine is present in many over-the-counter medications, but topical sprays containing the anesthetic benzocaine cause most severe cases.
Those who suffer from a congenital type of the disease are advised to avoid hypothermia - this can provoke an exacerbation.
Babies under 6 months of age should not eat foods that contain nitrates, such as spinach, beets or carrots.