Relatively infrequently, but not very rarely, when performing a general blood test in a completely healthy patient in the laboratory, it is revealed that there is a pronounced leukocyte shift to the left, and among granulocytes, band cells predominate. They can account for up to 20 and even up to 50% of all granulocytes, that is, eosinophils, basophils and neutrophils.
Typically, such a powerful leukocyte shift to the left occurs with severe inflammation, when not quite mature, young leukocytes, which have a band structure, emerge from the red bone marrow into the blood.
Neutrophils: general information
Not every patient knows about such a pathology as Pelger anomaly of neutrophils. What kind of disease is this? To answer this question, let's try to understand the structure and functions of neutrophils.
Neutrophils are the most numerous type of white blood cells. They are necessary for the body to fight pathogens of infectious diseases. These cells rush to the site of inflammation. and then absorb and digest dangerous bacteria and viruses. This process of neutralizing foreign agents is called phagocytosis.
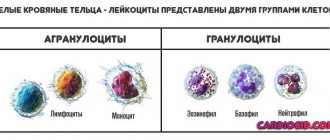
The blood test usually indicates 4 types of neutrophils:
- Myelocytes. These are the youngest cells. Normally, they can only be present in the bone marrow. Their presence in peripheral blood always indicates pathology.
- Young cells. They may be present in a blood test in very small quantities (up to 1% of the total number of neutrophils);
- Band neutrophils. This is a small group of cells. They are 1 - 5% normally.
- Segmented cells. It is this type of neutrophils that predominates in the peripheral blood (40 - 70%).
Mature types of neutrophils include only segmented cells; all other varieties are considered young forms.
With Pelger's anomaly of neutrophils, changes in the structure of segmented cells are noted. They become similar to young forms. This often leads to errors in diagnosing various pathologies.
How does it manifest?
Before studying the manifestations of neutrophilic changes in the blood, you need to understand what function these cells perform. Neutrophils are leukocytes, blood cells that are responsible for ingesting pathogenic bacteria. As soon as pathogenic bacteria or other pathogens enter the blood, neutrophils immediately begin to attack them.
Important! The normal content of segmented neutrophils in the blood is from 45 to 65% in the leukocyte formula. If we talk about the absolute value, the number of neutrophils can vary from 2.0 to 5.5 Giga/liter.
Such an anomaly of leukocytes does not have specific clinical expressions, because their functionality is not impaired. A Pelger anomaly can be detected completely by accident when a person takes a test. Often in people who are carriers of this pathology, ESR and coagulability indicators remain normal. People with this abnormality have the same reactions to blood loss or infection.
Read also: What else you don’t know about the effects of alcohol and cholesterol in the body
Blood picture
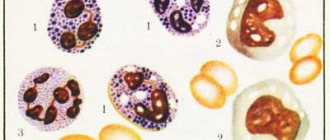
This congenital disorder can only be detected using a clinical blood test. If the nuclei of mature cells have few segments and change shape, this may indicate a Pelger anomaly of neutrophils. In the photo below you can see pathological changes in the nuclei.
The nucleus of Pelger neutrophils can have the following forms:
- oval;
- rod-shaped;
- horseshoe-shaped;
- bean-shaped;
- rounded.
In more rare cases, there are cores with a constriction in the center, similar to a gymnastic weight.
In this case, the core looks too short and consists of only one segment. It has a lumpy structure. Less common are nuclei of altered shape, having two or three segments.
If the nucleus of a segmented neutrophil has a round shape, then it can very easily be mistaken for a myelocyte. Only a detailed blood test can show that this is not a young, but a mature cell. The presence of neutrophils with altered nuclei often leads to errors in the interpretation of analysis results.
Pelger's anomaly: causes and clinical manifestations
Relatively infrequently, but not very rarely, when performing a general blood test in a completely healthy patient in the laboratory, it is revealed that there is a pronounced leukocyte shift to the left, and among granulocytes, band cells predominate. They can account for up to 20 and even up to 50% of all granulocytes, that is, eosinophils, basophils and neutrophils.
Typically, such a powerful leukocyte shift to the left occurs with severe inflammation, when not quite mature, young leukocytes, which have a band structure, emerge from the red bone marrow into the blood.
What is Pelger anomaly?
Normally, a person has few band leukocytes in the leukocyte formula, only a few percent (3-5), but not tens of percent.
Typically, this clinical picture occurs with severe inflammation, for example, with pneumonia with violent chills, fever, aching bones, intense cough and chest pain. However, nothing of the kind is detected in this patient; on the contrary, he is completely healthy.
He continues to undergo tests and is examined in every possible way, but no foci of inflammation appear in the body, and this peculiar band shift in the blood is his lifelong companion. What is it?
In this case we are talking about the so-called Pelger anomaly of neutrophils, or segmented leukocytes. This anomaly got its name in honor of the Dutch specialist Karl Pelger, and he discovered these atypical cells quite a long time ago, in 1928. What kind of anomaly is this, what causes it, what symptoms does it manifest, and does it need to be treated?
Very little time passed after the discovery of this anomaly, only 4 years.
And although modern methods of chromosomal microarray analysis did not yet exist, the human genome had not been studied, and the structure of DNA had not even been discovered, since this was done in 1953, but it was still possible to understand its hereditary nature. Therefore, at present, this anomaly is called Pelger-Huet, after the name of the discoverer, and after the name of the person who proved its innate nature.
The frequency of occurrence of this anomaly (it is no coincidence that it is not called a disease) fluctuates around 0.1%. That is, it occurs in approximately every 1000th person. The number of men and women who carry abnormal white blood cells is approximately the same.
Immediately after the discovery of this anomaly, it was encountered quite rarely in blood tests, since in the twenties and thirties, blood tests were not done for everyone.
Currently, since almost everyone has taken a variety of blood tests, including a general analysis, and more than once, every large laboratory can accumulate even several such abnormal tests from different people within a week.
Causes and laboratory signs
Unfortunately, the causes of this anomaly have not yet been established, but the mechanism of hereditary transmission is known; it is an autosomal dominant type. It can manifest itself to the same extent in homozygous and heterozygous probands. Its development is based on a special change in the leukocyte nuclei.
It is known that ordinary, mature neutrophils have a segmented nucleus, while pelgerization of neutrophil nuclei occurs due to a violation of the segmentation processes. As a result, the kernels retain their youthful shape upon the onset of full maturity.
If in normal neutrophils the nuclei are divided into several lobules, usually four or five, then with Pelger’s anomaly, if you look under a microscope, then mature and fully functional neutrophils have a nucleus that has only two lobules, rarely three.
This lobe has a constriction in the middle, so this core resembles either a peanut, or a gymnastic dumbbell, or pince-nez. In any case, no matter how many segments the nucleus of a Pelger leukocyte contains, it has one more feature.
This core has a lumpy structure, and the bridges between the lobes are quite short.
Such abnormal neutrophils are very similar to young leukocytes - myelocytes of normal structure, and this can be a mistake for laboratory doctors, and even for a hematologist.
At the same time, whatever the variants of the nuclear structure, Pelger neutrophils are absolutely healthy and full-fledged cells.
They can also perform phagocytosis, engulf and digest harmful microorganisms, contain as many enzymes as needed, live as long as normal white blood cells, and respond to all pathological processes in the same way.
Clinical manifestations
Since no dysfunction, as mentioned above, was detected in these neutrophils, there are therefore absolutely no clinical signs of this anomaly. It is not called a disease. Other blood characteristics also do not differ from the norm, such as erythrocyte sedimentation rate and blood clotting.
Their reaction to inflammation, infection, and bleeding is exactly the same as that of normal people, so Pelger’s leukocytes are almost always found as a random finding.
However, some modern researchers still indicate that these cells still have some defects, although not the entire medical community shares this opinion.
Their ability for diapedesis, that is, penetration from the blood into tissues, is slightly reduced, and therefore they may still not sufficiently perform their function of eliminating viruses and bacteria, and the activity of phagocytosis. Therefore, such people may have an increased susceptibility to soft tissue infections, and if an infection is present, it is still more likely to become chronic than in ordinary people.
However, there are also annoying “overlaps”. For example, a patient is admitted with an acute inflammatory disease, and he has a congenital, but still unknown to him, Pelger anomaly. At the same time, a pronounced shift in band neutrophils, as can be seen in the analysis, fits perfectly into the picture of acute bronchitis, or any other inflammation.
The doctor can only be wary that the blood has reacted too violently, and the clinic does not give a reason for such a severe shift of the formula to the left. However, the patient begins to be treated, the symptoms of the disease disappear, and in the control blood test the same supposedly inflammatory picture still remains.
This can mislead the doctor, since according to the rules, the patient cannot be discharged with such a poor blood test.
In the end, the situation is clarified, but this can lead to an unnecessarily long stay of the patient in the hospital for unjustified reasons, and, in the end, the department will be punished with “money” after an examination of the medical history by the insurance fund for “overstaying” the patient.
Analyzes
In order to clarify, Pelger leukocytes are counted according to the usual rules, taking into account the fact that if the nucleus has a thin bridge, then these cells are classified as segmented neutrophils, and this normalizes the situation.
In order to definitively clarify the diagnosis, it is advisable, in addition to the patient, to have the blood of his parents and closest relatives.
This will make it possible to identify family cases of Pelger anomaly, and if relatives do not yet know about it, then it is worth telling their attending physicians about it.
This will allow you to avoid potential mistakes that were described above, but not just for one, but for several people.
Many patients ask what causes Pelger neutrophil anomaly? It only leads to confusion in the laboratory, sometimes to unreasonably long treatment of infectious diseases, but, in the end, everything becomes clear. The patient only needs to warn the doctor about this congenital feature during each appointment for a general blood test, as well as the laboratory assistant.
It is interesting that, in addition to congenital pathology, such hyposegmentation of nuclei can also be acquired. It occurs quite often in chronic tuberculosis, and especially in chronic myeloid leukemia.
If a healthy person who does not have a hereditary Pelger anomaly develops such an acquired change in the nuclear structure, then this is an unfavorable marker of the onset of a myelodysplastic condition, that is, malignant leukemia.
Currently, this phenomenon is being seriously studied, since Pelger cells can appear in the blood long before the first signs of myeloid leukemia and the onset of myelodysplastic manifestations.
Source: //MyAnaliz.ru/blood/obshheklinicheskie/nejtrofily/pelgerovskaya-anomalia/
Causes
The anomaly occurs due to a violation of the formation of segments of neutrophil nuclei. The cause is a failure at the genetic level.
This disease is hereditary. It is transmitted in an autosomal dominant manner. This means that the pathology can be passed on to children if both parents are carriers of the defective gene.
The anomaly occurs in 1 child out of 1000 newborns. It is observed equally often in boys and girls. The pathology is benign. Many doctors consider Pelger's anomaly not a disease, but a constitutional feature.
Why might it appear?
The causes of the leukocyte abnormality have not been precisely established. Until now, scientists have only been able to find out the fact that pelgerization can be transmitted in an autosomal dominant manner. It can appear in both heterozygotes and homozygotes.
Hematologists have found that the main cause of pathogenesis is a violation of the segmentation process of already mature neutrophils. Since this pathology is inherited, it was found that genetic damage occurs due to changes in the structure of the gene responsible for the shape of the nucleus. The presence or absence of this pathology is easy to check if there is a blood test of both parents.
Manifestations
There are no pathological symptoms with Pelger's anomaly of neutrophils. This congenital feature does not affect a person’s well-being in any way and does not pose a health hazard.
Changing the shape of neutrophil nuclei does not affect phagocytosis. Such cells retain the ability to fight foreign microorganisms and produce enzymes. This anomaly does not affect the state of immunity in any way.
A very small proportion of doctors believe that patients with Pelger's neutrophil anomaly are more likely to have short stature and a tendency to have bowed bones. However, these data have not been scientifically confirmed. Most people with this feature are of normal height and do not suffer from bone pathologies.
Consequences
What does Pelger's anomaly of neutrophils lead to? This violation does not cause any complications or negative consequences for human health.
Difficulties arise only when interpreting the results of a blood test. Very often, doctors mistake modified segmented neutrophils for band neutrophils. An increase in con can be a sign of the following diseases:
- oncological diseases of the hematopoietic system;
- bacterial and viral infections;
- injuries and burns;
- myocardial infarction;
- inflammatory processes in various organs;
- allergic reactions;
- sepsis;
- parasitic diseases;
- renal failure.
If the blood picture is altered, the doctor may suspect the above pathologies in the patient. In the history of medicine, there have been cases when patients with Pelger's neutrophil anomaly were mistakenly diagnosed with infectious diseases or leukemia. As a result, people underwent a course of treatment that was absolutely unnecessary for them.
This congenital feature often leads to erroneous interpretation of clinical blood test data. However, a qualified and experienced doctor never makes a diagnosis based on just one test.
Inflammation and neutrophil functions
The inflammatory process has three main components. Firstly, bacterial invasion and tissue perturbation cause a temporary narrowing of the microvasculature or capillary part of the vascular bed. Blood circulation in the affected tissues increases, causing fever and redness of the tissues. Second, the permeability of the microvasculature usually increases as a result of endothelial cell compression, cytokine changes in endothelial cell junctions, endothelial necrosis and cell sloughing, or leukocyte-induced endothelial cell damage. Ultimately, increased vascular permeability allows intervascular, protein-rich fluid to enter the tissue, along with slowing blood flow through the affected tissue.
Clinically, liquid exudate or edema manifests itself as tissue edema. Third, the slowing of blood flow allows white blood cells, especially neutrophils, to accumulate in the inner surface of the capillaries and emigrate from the capillaries into the tissues in order to get rid of bacterial infection.
As blood flow slows during inflammation, white blood cells drop out of the main blood stream and accumulate towards the endothelial surface. After margination (the phenomenon of accumulation of leukocytes at the edge of an area of inflammation), neutrophils first move smoothly and then are attracted or adhere to the endothelial cell surface. The reason for this is the expression of selectin and integrin molecules on the plasma membranes of neutrophils and endothelial cells. The movement of neutrophils is facilitated by the expression of selectin molecules (L-selectin on neutrophils and P- and E-selectin on endothelial cells), while neutrophil adhesion occurs as a result of a closer connection between integrin molecules - CD-11-CD-18 complex and VLA -4 on neutrophils and intracellular adhesion molecules (ICAM-1 ICAM-2) and vascular cell adhesion molecules (VCAM-1) on endothelial cells.
Subsequently, neutrophils expand pseudopodia between endothelial cells, digest a small amount of the base membrane and emigrate from the microvascular part of the bed into the interstitial tissues. Although neutrophils remain viable in healthy tissues for 24-48 hours, their lifespan is significantly reduced during illness. Senescent neutrophils are successively phagocytosed or ingested by monocyte-macrophages or disappear from the surface of the mucosa or wound after migration.
Within tissues, neutrophils follow a chemotastic gradient to one or more sites of infection. The chemotactic factors to which neutrophils respond are varied and include complement constituents, arachidonic acid metabolites, kinin derivatives, and fibrin breakdown products. All these substances are formed after tissue damage, inflammation or infection.
When neutrophils encounter bacteria, they recognize these pathogens if they have been opsonicized by immune serum or have been primed by neutrophils for phagocytosis. Opsonins include both immunoglobulin G (IgG) and fragments of the third component of complement (C3). Specific cellular receptors responsible for neutrophil recognition of opsonized bacteria include the FciR receptor, which detects the Fc portion of IgG, and complement receptors 1, 2, 3 (CR1, 2, 3), which detect both C3b and C3bi (the stable form of C3b). Once recognized, the opsonized bacteria are phagocytosed while the neutrophils simultaneously release an oxygen pulse (Bender and Chickering, 1983). During phagocytosis, bacteria adhere to the plasma membrane of the neutrophil, expansion of the pseudopodia around the bacterium, and fusion of the neutrophil membrane. Following phagocytosis, the internalized bacterium is enclosed in an inverted plasma membrane sac called a phagosome. Subsequently, the phagosome fuses with primary (azurophilic) and secondary (specific) neutrophil granules, forming a phagolysosome.
The destruction of bacteria occurs mainly due to oxygen-dependent mechanisms, including the interaction of atomic oxygen, enzymes and halide ions. The oxygen pulse causes the formation of various toxic oxygen radicals, which are used in the destruction of bacteria. Oxygen and the reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) form superoxide anion in the presence of NADPH oxidase. It is a highly reactive oxygen radical that spontaneously decomposes to form hydrogen peroxide. Hydrogen peroxide in the presence of chloride ions is converted into hypochlorous acid by myeloperoxidase, an enzyme derived from the primary granules of neutrophils. Hypochlorous acid is a highly diluted bleach (such as Clorox) that is very toxic to bacteria. The destruction of bacteria by oxygen-dependent organisms is carried out by covalent bonding of chloride ions or other halides with intracellular proteins or through lipid peroxidation of the membrane and oxidative structuring of membrane proteins by toxic oxygen radicals.
Although most bacteria are killed by the peroxide myeloiroxidase-hydrogen halide system, neutrophils are also capable of killing bacteria by oxygen-dependent mechanisms. These mechanisms include enzymes such as lysozyme or cationic proteins (eg, proteins with increased bactericidal permeability) and defensins. Lysozyme, present in both primary and secondary granules, hydrolyzes the N-acetylglucosamine bonds of muramic acid in the bacterial glucoprotein coat. Defensins present in the primary granule are highly cytotoxic and are cationic proteins saturated with arginine. Defensins form potential-gating ion channels in the bacterial cell membrane, which leads to increased permeability.
After the bacteria are destroyed, acid hydrolases from the primary granules of neutrophils digest these bacteria. The digestion of hydrolase is facilitated by a decrease in pH (to 4-5) inside the phagolysome.
Diagnostics
If the patient’s clinical analysis shows signs of Pelger’s anomaly, the doctor must indicate this in the transcript. To avoid errors in diagnosis, a person needs to additionally take a blood smear for the following indicators:
- kernel maturity;
- chromatin density;
- neutrophil ratio.
With Pelger's anomaly, the patient's chromatin density remains normal, the nucleus has an altered shape, but is old. This study helps distinguish altered mature cells from young forms.
If possible, a clinical test and blood smear should be taken from the patient's parents. Since the pathology is hereditary, the same anomaly is observed in the mother and father of the patient. This is an important diagnostic sign.
Laboratory assessment of neutrophil function
Obtaining documented evidence of neutrophil dysfunction using laboratory tests is expensive, complex and time-consuming and requires special preparation of neutrophils. Unfortunately, many biological assays of neutrophil function are subject to variability or inherited limitations. This can make it difficult to detect subtle abnormalities in neutrophil function. Subsequently, neutrophil function is affected by inflammation, infections, and medications. To reduce the effect of the latter, it is advisable to study the function of neutrophils during the period of remission of the disease and drug withdrawal. If neutrophil dysfunction is suspected, tests should be done to evaluate opsonization activity and the generation of chemotactic factors from serum, as well as neutrophil adhesion, chemotaxis, phagocytosis, and bacterial killing. Opsonization is assessed by the concentration of immunoglobulin (especially IgG) and complement (C3) in the patient's blood serum and by analyzing the ability of the serum to support phagocytosis of bacteria. When the IgG concentration is less than usual, it is desirable to establish the formation of antigens to T-lymphocyte-dependent (tetanus toxoid) and T-lymphocyte-independent (Brucellaabortusantigen, erythrocytes taken from sheep) antigens. In addition, the prudent physician should measure the amount of immune complexes in the circulatory system to assess antibody catabolism. The generation of chemotactic factors can be assessed by scoring the chemotaxis of control neutrophils in the serum of patients who have been “activated” by previous exposure to a yeast infection (zymosan) or purified endotoxin.
Adhesion is measured by the percentage of a patient's neutrophils that adhere to the nylon fiber posts. In the presence of adhesion failure, flow cytometry and fluorescein isothiocyanate (ITS)-conjugated monoclonal antibodies can be used to assess CD-ll-CD-18 integrin expression on the neutrophil plasma membrane. Chemotaxis can be measured in vitro by the migration of neutrophils on agarose or by the migration of a polycarbonate membrane in a blind cell (modified Boyden chamber) in response to a chemotactic moiety. In vivo chemotaxis analysis can be performed by recording the migration of neutrophils into “skin windows” or chambers in areas of minor skin damage. The chemoattractant is usually endotoxin- or zymosan-activated mixed donor serum. Unlike some animal species, including humans, neutrophils in dogs and cats do not respond to N-formyl-methionyl-leucyl-phenylalanine (f-MLP), a synthetic chemotactic tripeptide.
Neutrophil phagocytosis can be quantified using light microscopy or flow cytometry. Neutrophils feed on opsonized fluorescent latex beads, tetrazolium blue latex beads and bacteria. Flow cytometry can count the percentage of phagocytic neutrophils and accurately identify up to five fluorescent latex beads per cell. These parameters can also be calculated using a light microscope, but this is a more labor-intensive method. However, it allows one to distinguish the attachment of bacteria to the cell membrane from their actual engulfment or phagocytosis. The important role of tetrazolium blue color fading will be explained below. The bactericidal activity of neutrophils can be calculated directly or indirectly. Indirect analysis of putative bactericidal capacity is made by measuring the iodination (1251) of the protein by the hydrogen peroxide-myeloperoxidase-halide reaction, the weakening of tetrazolium blue (yellow) to formazan (blue) by oxygen radicals, chemiluminescence with luminol as superoxide releases color during the transition from an excited state to a resting state and a change in cytochemical staining or chemical analysis occurs due to the activity of myeloperoxidase. Direct assay of bactericidal activity is performed by feeding neutrophils with opsonized pathogenic bacteria in log phase. The number of bacteria killed is usually calculated by testing neutrophil lysates for viable bacteria by plating an agar culture.
Is treatment necessary?
Clinical manifestations of Pelger's neutrophil anomaly are completely absent. A person with this feature is considered absolutely healthy and does not need treatment.
It is advisable to identify the anomaly in childhood. This will help avoid diagnostic errors in the future. If a patient with a Pelger neutrophil anomaly is scheduled to undergo a blood test, it is very important to tell the doctor about his congenital abnormality. Otherwise, the test results will be misinterpreted.
Similar anomalies
We examined the congenital type of Pelger anomaly. However, similar changes in neutrophils may also be observed in the following diseases:
- systemic lupus erythematosus;
- tuberculosis;
- malaria;
- flu;
- lymphogranulomatosis;
- decreased thyroid function;
- leukemia
In these cases, the changes are acquired. They are not observed constantly, but only with exacerbation of the underlying pathology.
There is also a very rare congenital disorder that geneticists call SOPH syndrome. It was first described in 2009. This pathology occurs among the peoples of the North, mainly among the Yakuts. Pelger's anomaly is only one of the manifestations of this syndrome. This genetic disease is accompanied by other pathological signs:
- short stature;
- sagging skin;
- atrophy of the optic nerves and retina.
If a child is diagnosed with a Pelger anomaly, then you should take a close look at his well-being and state of health. If no other pathological signs are observed, then there is no cause for concern. This constitutional feature does not affect either the duration or quality of life.