1.General information
The connection between the condition of the kidneys, blood pressure and characteristic swelling has been known for a long time, and not only to doctors. Seeing a pale man with bags under his eyes, rubbing his temples or the back of his head with his fingers, the most discerning among us will immediately ask: is it from the kidneys?
Indeed, in many cases, high blood pressure is caused by kidney dysfunction, and vice versa: lack of blood supply, for example, with atherosclerosis of the renal arteries, leads to damage to the nephron parenchyma (the main tissue of the kidneys, consisting of millions of filtering elements called nephrons) and chronic renal failure.
These processes are complex even for a specialist; research in this area continues, but the widespread prevalence of this type of pathology creates the need to understand at least the basic terms and modern ideas about “renal pressure.”
Arterial hypertension (AH, persistent excess of blood pressure above the conditional threshold of 140/90, hypertension) is classified into two main groups. Essential or primary hypertension is an independent polyetiological disease, against the background of which numerous disorders and complications develop. On the contrary, secondary (symptomatic) hypertension is itself a complication of any pathological process or condition.
Statistical estimates regarding various types of hypertension vary widely, but it is known that the absolute majority of hypertension (up to 95%) are primary.
Regarding secondary hypertension, there are also no clear statistical data or generally accepted classification. The role of renal pathology in this regard is one of the main causes of symptomatic hypertension; According to various estimates, nephrogenic hypertension accounts for from 1-2% to a third of all secondary hypertension. A number of studies highlight an alarming age-related trend: persistent hypertensive blood pressure levels are found in 20-30% of children and adolescents, which is also associated with kidney dysfunction, and in cities this trend is twice as pronounced as in rural areas.
And finally, a few words about terminology. Secondary nephrogenic (renal) hypertension is an indication only of the general cause of high blood pressure; this diagnosis, in fact, is not complete and not specific. The term “renal hypertension” comes from the name of the substance “renin” discovered at the end of the 19th century (in Latin, “kidney” - ren). Renin is a vasopressor produced by the renal glomeruli, i.e. hormone that increases blood pressure. However, the definition of “renal” cannot be considered complete, because An excess of renin in the blood can be caused by both its hypersecretion and disturbances in biochemical processing due to damage to the parenchyma. Therefore, in the literature today one can find clarifying diagnoses of “renoparenchymal”, “renovascular”, “vasorenal” hypertension.
A must read! Help with treatment and hospitalization!
Arterial hypertension in chronic kidney disease: current state of the problem
Summary . Arterial hypertension (AH) is a significant problem in the healthcare system. It is a major modifiable risk factor for cardiovascular disease (CVD), stroke, and kidney failure. Long-term and persistent hypertension accelerates the progression of kidney disease to the terminal stage, and a progressive decrease in kidney function, on the contrary, contributes to an increase in blood pressure (BP) and deterioration of its control. Chronic kidney disease (CKD) is both a common cause of hypertension and a complication of uncontrolled hypertension. Hypertension and CKD are closely related through mixed cause-and-effect relationships. Because hypertension can be a cause and consequence of CKD, its prevalence is higher and more difficult to control. The interaction between hypertension and CKD is complex and increases the risk of adverse cardiovascular and cerebrovascular outcomes. The pathophysiology of hypertension in CKD is complex and is a consequence of multiple factors, including decreased nephron mass, increased sodium retention and extracellular volume expansion, sympathetic hyperactivity, activation of hormones, including the renin-angiotensin-aldosterone system, and endothelial dysfunction. Patients with CKD more often have high-risk hypertension: latent, resistant and nocturnal hypertension. This literature review is devoted to modern ideas about the prevalence, pathogenesis, course, control, and principles of treatment of hypertension in CKD. The relevance of this problem is associated with the growing number of patients with hypertension and CKD around the world and their high morbidity and mortality. The bidirectional nature of the relationship between hypertension and CKD makes it promising to study these two conditions in order to slow down the rate of progression of renal and cardiac dysfunction.
Chronic kidney disease (CKD) is now recognized as a worldwide epidemic. CKD leads to end-stage renal disease (ESRD) and is associated with significant healthcare costs. As kidney function declines, there is an increase in arterial hypertension (AH), which accompanies CKD [1, 2]. Hypertension is a global medical problem. It is the main modifiable risk factor for cardiovascular disease (CVD) and stroke. CKD is both a common cause of hypertension and its complication when uncontrolled. The interaction between hypertension and CKD is complex, increasing the risk of adverse cardiovascular and cerebrovascular outcomes [3]. The incidence of cardiac and renal complications determines hypertension and CKD as significant medical problems [4–6].
The prevalence of hypertension is 25–30%, CKD – 15% among the adult population [1, 5].
The incidence of hypertension in patients with CKD is significantly higher than in the population. According to epidemiological studies, 67–71% of patients in this category have hypertension, and in the elderly it occurs in 82% of cases [1, 7]. In the later stages of CKD, hypertension is found in 90% of patients [8]. Hypertension is widespread in those receiving renal replacement therapy (RRT) [5, 7]. The incidence of hypertension in patients on dialysis varies due to differences in methods of blood pressure (BP) control: before or after dialysis or as an outpatient. According to numerous studies, 50–60% (up to 85%) of patients on hemodialysis and almost 30% of patients on peritoneal dialysis suffer from hypertension [9, 10]. Because hypertension can be a cause and consequence of CKD, its prevalence is higher and more difficult to control. The prevalence, severity and control of hypertension are influenced by: the etiology of CKD, the presence and degree of albuminuria, genetic, socio-economic factors and lifestyle. In addition, there are racial and ethnic disparities in the prevalence, treatment, risks, and outcomes of hypertension in patients with CKD [5].
Pathophysiology of hypertension in CKD
The cause-and-effect nature of hypertension and CKD is bidirectional; these are closely interrelated pathophysiological conditions [11–13]. Patients with CKD are more likely to have high-risk hypertension phenotypes, such as latent, resistant, and nocturnal hypertension. With latent hypertension, target organ damage and other adverse events are more common. Recent studies have shown that latent hypertension in CKD is associated with an increased risk of left ventricular hypertrophy (LVH), proteinuria, and decreased estimated glomerular filtration rate (GFR). Also, latent hypertension is associated with high CVD, end-stage chronic renal failure and all-cause mortality. Further study of latent hypertension and the search for rational methods of diagnosis and treatment are required [12–14]. Resistant hypertension is defined when, when using three antihypertensive drugs of different classes in optimal doses (one of which is a diuretic), blood pressure remains above the target level during “office” and “out-of-office” control [14–17].
The pathophysiology of hypertension in CKD is complex, multifactorial and is a consequence of multiple mechanisms: a decrease in the number of nephrons, increased sodium retention and an increase in extracellular volume, activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system, endothelial dysfunction [3, 12, 18]. In recent years, new information has emerged regarding sodium and water homeostasis and regulation. The multiorgan effects of vasopressin, a high-salt diet, and limited water intake are discussed, as well as the significance of sodium accumulation and the rhythm of its excretion in urine. There is evidence that dysregulation of sodium and water metabolism can have profound effects on the kidneys and blood vessels. Maladjustment to a high-salt diet may be associated with a steady increase in the incidence of hypertension and CKD [19]. The association between erythropoietin (EPA) medications and hypertension in CKD is well known. Research shows that the mechanism of this connection is multifactorial. Thus, some patients with CKD have a limited ability to adapt to a rapid increase in erythrocyte volume due to a decrease in GFR, an increase in vascular resistance and the presence of LVH. In addition, there is a possibility of a direct vasoconstrictor effect and the development of EPA-induced hypertension. It is recommended to exercise caution when using EPA in patients with resistant hypertension and monitor the rate of increase in hemoglobin in uncontrolled blood pressure [20]. The main causes of hypertension in patients on dialysis include: increased fluid volume, sympathetic hyperactivity, activation of the renin-angiotensin-aldosterone system, atherosclerosis, exposure to vasoactive peptides derived from the endothelium, increased intracellular calcium and decreased renalase enzyme secreted by the kidney in response to the release of catecholamines. [21]. The association between uric acid and systemic hypertension, kidney disease and CVD is well known. Recent studies confirm the role of changes in the activity of certain enzymes induced by uric acid in the pathogenesis of hypertension and CKD [22]. There is increasing evidence regarding the involvement of the intestinal microbiota in the regulation of blood pressure and the worsening prognosis of CKD. For example, in hypertension, the amount of short-chain fatty acids decreases, which leads to a deterioration in microbial balance, disruption of the integrity of the epithelial barrier and the development of intestinal inflammation. These changes impair blood pressure regulation and, as a result, contribute to damage to target organs, including the kidneys. On the other hand, in CKD, the accumulation of uremic toxins in the intestine causes changes in the composition of its microbiota and metabolites, promotes the transport of endotoxins into the bloodstream, increases inflammation, kidney damage and worsens the prognosis of CKD [23].
Control and target blood pressure in patients with CKD
Although awareness of hypertension treatment in patients with CKD is improving, BP control remains suboptimal at all stages of CKD [5]. Both random and ambulatory blood pressure measurements can be used to diagnose hypertension. The use of ambulatory monitoring provides additional assessment of diurnal BP variations commonly observed in CKD [3, 7]. A small percentage of patients control their blood pressure, which determines insufficient adherence to medications, suboptimal control of volume overload and, consequently, insufficient treatment [18]. About 30–60% of patients with hypertension do not achieve target blood pressure levels and remain at risk of target organ damage [17]. However, control of blood pressure in the population of patients with CKD can reduce the risk of adverse cardiovascular and cerebral events, mortality and progression of renal failure [7, 12]. Most current recommendations concern intensive blood pressure control and active antihypertensive treatment [12, 24]. Improving the quality of management of patients with hypertension plays an important role in slowing progression, improving prognosis and preventing complications of CKD [2]. The optimal blood pressure target for the treatment of hypertension in general and CKD in particular remains a subject of debate and debate, despite data from numerous clinical studies [3]. The most significant recommendations for the prevention and treatment of hypertension in kidney disease include those coming from the Kidney Disease Improving Global Outcomes Initiative (KDIGO) (2012) and the National Kidney Foundation of the United States - United States NKF KDOQI (2004) [2]. In the KDIGO-2012 clinical guidelines, the upper level of the recommended target blood pressure is < 140/90 mm Hg. Art. with albuminuria < 30 g/day and up to 130/80 mm Hg. Art. with albuminuria more than 30 mg/day [1]. More recently (2017), the American College of Cardiology (ACC) and American Heart Association (AHA) hypertension guidelines established a target BP of <130/day for patients with CKD and those at increased cardiovascular risk. 80 mmHg Art. These recommendations were influenced by the SPRINT (Systolic Blood Pressure Intervention Trial) trial [25]. According to SPRINT, in a group of nondiabetic patients at high cardiovascular risk, many of whom had CKD, those with intensive antihypertensive therapy (target blood pressure < 120 mm Hg) had significantly lower cardiovascular events and mortality from any other cause than in the group with standard therapy (target blood pressure < 140 mm Hg). However, when studying the effect on renal outcomes, a significant decrease in GFR was observed, and the incidence of hypotension and acute kidney injury (AKI) increased in people on intensive care without pre-existing CKD [25, 26]. Thus, blood pressure < 130/80 mmHg. Art. is a scientifically based, target blood pressure in patients with CKD [25]. Comparable results were obtained by D. Ettehad et al. in a MEDLINE meta-analysis. The decrease in systolic blood pressure was accompanied by a decrease in the risk of CVD and all-cause mortality. However, with regard to renal failure, the effect was not significant. Therefore, strict control of blood pressure in relation to cardiovascular outcomes is justified, but as a measure of nephroprotection is questionable. Regardless of the etiology of CKD and the level of proteinuria, it is not recommended to reduce blood pressure to < 120/70 mmHg. Art. When using tight blood pressure control tactics, careful monitoring of renal function is necessary [27].
Principles of treatment of hypertension in CKD
Current issues in the management of hypertension include: control of the circadian rhythm of blood pressure, target blood pressure values, assessment of secondary forms, limiting salt intake, dosing of antihypertensive drugs [11]. Drug therapy for patients with CKD-associated hypertension is a difficult and complex task, since the reasons for the development of hypertension are much greater than in patients with hypertension without CKD. In addition, it may be necessary to exclude pseudoresistance and other secondary causes [3]. There is insufficient information on the treatment tactics of resistant hypertension against the background of CKD in the modern literature. Available data relate to a step-by-step assessment of nutrition, lifestyle, and adherence to antihypertensive therapy. Some authors note the need to study the role of diuretics and mineralocorticoid receptor antagonists, as well as modern methods of treatment, such as renal denervation and baroreceptor stimulation [17]. The key point in the treatment of hypertension in patients with CKD is the use of a combination of antihypertensive drugs with assessment of GFR [3]. The main approaches to the treatment of hypertension in CKD include: restriction of salt intake from food, treatment with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs), diuretic therapy [11, 12]. It is promising to develop clinical guidelines for dosing antihypertensive drugs before bedtime in patients with CKD, which will help restore nocturnal blood pressure fluctuations. [eleven]. Impact on the RAAS is a primary component of nephroprotective therapy. According to recent recommendations, ACE inhibitors should be used as first-line drugs in patients with GFR above 30 ml/min/1.73 m2 [1, 24]. ARBs are also considered first-line drugs used in cases of intolerance to ACE inhibitors [1, 3]. ARBs, both as monotherapy and in combination therapy, have a beneficial effect on proteinuria, and their potential benefit in the treatment of hypertension and CKD is obvious. There is evidence from a study that there are no significant changes in estimated GFR during ARB therapy [28]. ACE inhibitors and ARBs slow down the progression of CKD by reducing intraglomerular pressure and the rate of development of glomerulosclerosis [25]. The simultaneous use of two RAAS blockers is not indicated [14]. To achieve the target blood pressure level, along with ACE inhibitors, combination therapy using calcium channel blockers (CCBs) and diuretics is recommended [24]. Non-dihydropyridine CCBs reduce albuminuria and slow the decline in renal function. Dihydropyridine CCBs are not recommended for use as monotherapy in patients with proteinuric CKD, preferably in combination with RAAS blockers. Diuretics are widely used in the treatment of patients with CKD [1]. When GFR is <30 ml/min/1.73 m2, thiazide diuretics are replaced by loop diuretics [14]. All other groups of drugs should be prescribed when primary treatment is not effective [1]. Reducing blood pressure at the beginning of treatment for hypertension in CKD leads to a decrease in renal perfusion pressure by up to 10–20%. This requires careful monitoring of blood electrolytes and GFR. The decrease in GFR usually occurs during the first two weeks of treatment and then stabilizes. If the decrease in GFR is more severe, it is recommended to stop treatment and exclude renovascular pathology [14]. New promising methods for the treatment of hypertension in CKD include the use of recombinant renalase and its analogues to stimulate the degradation of catecholamines, as well as prebiotics and probiotics to normalize the intestinal microbiota [23, 29].
Additionally, the complex therapy of patients with CKD should include non-drug approaches to the treatment of hypertension (lifestyle modification, dietary salt restriction) [1, 3]. Addressing salt sensitivity is critical for BP management, as the most common cause of hypertension in CKD is a decreased ability of the kidneys to excrete salt [11]. Recently, attention has been paid to the role of physical exercise in CKD. The prevalence of physical inactivity in patients with CKD is 12–50% higher than in the general population. According to a cohort study of this problem in patients on hemodialysis, the advantage of aerobic exercise in improving the quality of life and health has been proven. There is evidence that combined aerobic exercise helps reduce inflammation and insulin resistance in patients with hypertension in the early stages of CKD [4].
Patients with ESRD receiving hemodialysis experience fluctuations in blood pressure during the procedure. Intradialytic hypotension and hypertension are special situations associated with an increased risk of mortality. Patients with intradialytic hypertension have an unexplained increase in vascular resistance during dialysis, and more aggressive management of volume overload is recommended as first-line treatment [7, 10, 30].
Intradialytic hypertension regularly occurs in 10–15% of hemodialysis patients. Patients with intradialytic hypertension usually have a small interdialytic weight gain, but a regular excess of extracellular volume according to bioimpedance spectroscopy. Patients with intradialytic hypertension have lower levels of albumin and predialysis urea, which may contribute to a decrease in plasma osmolarity, which prevents a decrease in blood pressure. Also, endothelin-1 may be a mediator of intradialytic blood pressure surges. Reducing the target “lean” body weight, removing excess sodium, reducing sodium in the dialysate can prevent the increase and help normalize blood pressure in more than 60% of patients [7, 30]. Antihypertensive drugs in this category of patients should be prescribed for persistently elevated ambulatory blood pressure > 140/90 mm Hg. Art. [thirty].
Literature/References
- Kalaitzidis RG, Elisaf MS Treatment of Hypertension in Chronic Kidney Disease // Curr Hypertens Rep. 2018; 20 (8): 64. DOI: 10.1007/s11906-018-0864-0.
- Cai G., Chen X. Hypertension in patients with CKD in China: clinical characteristics and management // Front. Med. 2017; 11 (3): 307–309. DOI: 10.1007/s11684-017-0578-8.
- Hamrahian SM, Falkner B. Hypertension in Chronic Kidney Disease // Adv Exp Med Biol. 2017; 956:307-325. DOI: 10.1007/5584_2016_84.
- Barcellos FC, Del Vecchio FB, Reges A, Mielke G, Santos IS, Umpierre D et al. Exercise in Patients With Hypertension and Chronic Kidney Disease: A Randomized Controlled Trial // J Hum Hypertens. 2018; 32(6):397–407. DOI: 10.1038/s41371-018-0055-0.
- Horowitz B., Miskulin D., Zager P. Epidemiology of Hypertension in CKD // Adv Chronic Kidney Dis. 2015; 22 (2): 88-95. DOI: 10.1053/j.ackd.2014.09.004.
- Zueva T.V., Zhdanova T.V., Urazlina S.E. Comorbidity of renal and cardiac pathologies // Medical Bulletin of the North Caucasus. 2019; 14 (4): 711–717. // Meditsinsky vestnik Severnogo Kavkaza. 2019; 14 (4): 711–717 (In Russ)]. DOI: https://doi.org/10.14300/mnnc.2019.14178.]
- Peco-Antic A., Paripovic D. Renal Hypertension and Cardiovascular Disorder in Children With Chronic Kidney Disease // Srp Arh Celok Lek. 2014; 142 (1–2): 113–117. DOI: 10.2298/sarh1402113p.
- Valika A., Peixoto AJ Hypertension Management in Transition: From CKD to ESRD // Adv Chronic Kidney Dis. 2016; 23 (4): 255–61. DOI: 10.1053/j.ackd.2016.02.002.
- Agarwal R., Flynn J., Pogue V., Rahman M., Reisin E., Weir MR Assessment and management of hypertension in patients on dialysis // J Am Soc Nephrol. 2014; 25: 1630–646. DOI: 10.1681/ASN.2013060601.
- Van Buren PN, Inrig JK Special Situations: Intradialytic Hypertension/Chronic Hypertension and Intradialytic Hypotension // Semin Dial. 2017; 30(6):545–552. DOI: 10.1111/sdi.12631.
- Judd E., Calhoun DA Management of Hypertension in CKD: Beyond the Guidelines // Adv Chronic Kidney Dis. 2015; 22 (2): 116–122. DOI: 10.1053/j.ackd.2014.12.001.
- Ku E., Lee BJ, Wei J., Weir MR Hypertension in CKD: Core Curriculum 2021 // Am J Kidney Dis. 2019; 74 (1): 120–131. DOI: 10.1053/j.ajkd.2018.12.044.
- Babu M., Drawz P. Masked Hypertension in CKD: Increased Prevalence and Risk for Cardiovascular and Renal Events // Curr Cardiol Rep. 2019; 21 (7): 58. DOI: 10.1007/s11886-019-1154-4.
- Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., et al. 2021 ESC/ESH Guidelines for the management of arterial hypertension // Eur Heart J. 2018; 39: 3021-3104. DOI: 10.1093/eurheartj/ehy339.
- Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association // Hypertension. 2018; 72(5):53–90. DOI: 10.1161/HYP.0000000000000084.
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines // Hypertension. 2018; 71 (6): 13–115. DOI: 10.1161/HYP.0000000000000065.
- Braam B., Taler SJ, Rahman M., Fillaus JA, Greco BA, Forman JP, Reisin E. et al. Recognition and Management of Resistant Hypertension // Clin J Am Soc Nephrol. 2017; 12(3):524–535. DOI: 10.2215/CJN.06180616.
- Schmid H., Schiffl H., Lederer SR Erythropoiesis-stimulating Agents, Hypertension and Left Ventricular Hypertrophy in the Chronic Kidney Disease Patient // Curr Opin Nephrol Hypertens. 2011; 20 (5): 465–470. DOI: 10.1097/MNH.0b013e3283497057.
- Qian Q. Salt. Water and Nephron: Mechanisms of Action and Link to Hypertension and Chronic Kidney Disease // Nephrology (Carlton). 2018; 23(Suppl 4): 44–49. DOI: 10.1111/nep.13465.
- Boyle SM, Berns JS Erythropoietin and Resistant Hypertension in CKD // Semin Nephrol. 2014; 34(5):540–549. DOI: 10.1016/j.semnephrol.2014.08.008.
- Nongnuch A., Campbell N., Stern E., El-Kateb S., Fuentes L., Davenport A. et al. Increased postdialysis systolic blood pressure is associated with extracellular overhydration in hemodialysis outpatients // Kidney Int. 2015; 87:452–457. DOI: 10.1038/ki.2014.276.
- Sharaf EI Din UAA, Salem MM, Abdulazim DO Uric Acid in the Pathogenesis of Metabolic, Renal, and Cardiovascular Diseases: A Review // J Adv Res. 2017; 8(5):537–548. DOI: 10.1016/j.jare.2016.11.004.
- Felizardo RJF, Watanabe IKM, Dardi P., Rossoni LV, Camara NOS The Interplay Among Gut Microbiota, Hypertension and Kidney Diseases: The Role of Short-Chain Fatty Acids // Pharmacol Res. 2019; 141:366–377. DOI: 10.1016/j.phrs.2019.01.019.
- Huang QF, Hoshide S, Cheng HM, Park S, Park CG, Chen CH et al. Characteristics On the Management of Hypertension in Asia-Morning Hypertension Discussion Group (COME Asia MHDG) Management of Hypertension in Patients With Chronic Kidney Disease in Asia // Curr Hypertens Rev. 2016; 12 (3): 181–185. DOI: 10.2174/1573402113666161122114854.
- Chang AR, Lóser M, Malhotra R, Appel LJ Blood Pressure Goals in Patients With CKD: A Review of Evidence and Guidelines // Clin J Am Soc Nephrol. 2019; 14 (1): 161–169. DOI: 10.2215/CJN.07440618.
- The SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control // Vnitr Lek. 2016; 62(1):44–47.
- Ettehad D., Emdin CA, Kiran A., Anderson SG, Callender T., Emberson J. et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis // The Lancet. 2016; 387(5):957–967.
- Burnier M., Lin S., Ruilope L., Bader G., Durg S., Brunel P. Effect of Angiotensin Receptor Blockers on Blood Pressure and Renal Function in Patients With Concomitant Hypertension and Chronic Kidney Disease: A Systematic Review and Meta- Analysis // Blood Press. 2019; 28(6):358–374. DOI: 10.1080/08037051.2019.1644155.
- Ficek J., Malyszko J., Chudek J. Renalase and its role in the development of hypertension in patients with chronic renal failure // Przegl Lek. 2015; 72(6):306–308.
- Van Buren PN Pathophysiology and Implications of Intradialytic Hypertension // Curr Opin Nephrol Hypertens. 2017; 26 (4): 303-310.
T. V. Zueva1, Candidate of Medical Sciences T. V. Zhdanova, Doctor of Medical Sciences, Professor
Federal State Budgetary Educational Institution of Higher Education USMU, Ekaterinburg, Russia
1 Contact information
DOI: 10.26295/OS.2020.19.20.002
Arterial hypertension in chronic kidney disease: current state of the problem / T. V. Zueva, T. V. Zhdanova For citation: Attending physician No. 9/2020; Page numbers in issue: 11-14 Tags: cardiovascular diseases, kidneys, sodium metabolism, risk
2. Reasons
The most common congenital cause of renal hypertension is a fibromuscular malformation of the renal artery. Many other anomalies of the structure of the kidneys and renal vessels, bladder, ureters and urethra are also described as congenital causes.
Acquired causes include:
- atherosclerosis in the renal artery basin (statistically the most common cause);
- prolapse of the kidney (nephroptosis);
- an abnormal connection between the renal artery and vein, formed, for example, due to injury;
- arterial inflammation;
- mechanical pressure on the artery (by a growing tumor, cyst, hematoma, etc.).
It should be noted that the two most common causes, one congenital and one acquired (see above), together account for up to 98-99% of renovascular hypertension.
As for renin itself, its secretion and subsequent regulatory role represent a complex endocrine cascade, which involves protein precursor compounds, two forms of angiotensin, angiotensin-converting enzyme (ACE), and aldosterone (a hormone of the adrenal cortex). It is obvious that any failure in this multi-stage process, any damage or injury to secretory and/or receptor cells can cause endocrine imbalance and, accordingly, an excess of vasopressor hormones. Hypersecretion of renin may be a response to a purely mechanical reduction in the vasculature (blood supply) of the kidneys, however, the water-electrolyte balance is also grossly disturbed during inflammatory processes in the kidneys - glomerulo- and pyelonephritis, which leads to an excess volume of blood pumped by the heart and, as a consequence, to hypertension ( for example, bilateral pyelonephritis is accompanied by arterial hypertension in more than 40% of cases).
Visit our Cardiology page
Classification of symptomatic arterial hypertension
According to the primary etiological link, symptomatic arterial hypertension is divided into:
- Neurogenic (caused by diseases and lesions of the central nervous system):
- central (traumas, brain tumors, meningitis, encephalitis, stroke, etc.)
- peripheral (polyneuropathies)
- Nephrogenic (renal):
- interstitial and parenchymal (chronic pyelonephritis, glomerulonephritis, amyloidosis, nephrosclerosis, hydronephrosis, systemic lupus erythematosus, polycystic disease)
- renovascular (atherosclerosis, renal vascular dysplasia, vasculitis, thrombosis, renal artery aneurysms, tumors compressing the renal vessels)
- mixed (nephroptosis, congenital anomalies of the kidneys and blood vessels)
- renoprinic (condition after kidney removal)
- Endocrine:
- adrenal (pheochromocytoma, Conn's syndrome, adrenal hyperplasia)
- thyroid (hypothyroidism, thyrotoxicosis) and parathyroid
- pituitary (acromegaly, Itsenko-Cushing's disease)
- menopausal
- Hemodynamic (caused by damage to the great vessels and heart):
- aortosclerosis
- stenosis of the vertebrobasilar and carotid arteries
- caorctation of the aorta
- aortic valve insufficiency
- Dosage forms when taking mineralo- and glucocorticoids, progesterone- and estrogen-containing contraceptives, levothyroxine, heavy metal salts, indomethacin, licorice powder, etc.
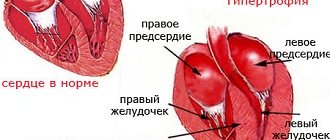
Depending on the magnitude and persistence of blood pressure, the severity of left ventricular hypertrophy, and the nature of changes in the fundus, 4 forms of symptomatic arterial hypertension are distinguished: transient, labile, stable and malignant.
Transient arterial hypertension is characterized by an unstable increase in blood pressure, changes in the fundus vessels are absent, and left ventricular hypertrophy is practically undetectable. With labile arterial hypertension, there is a moderate and unstable increase in blood pressure that does not decrease on its own. There is mild hypertrophy of the left ventricle and narrowing of retinal vessels.
Stable arterial hypertension is characterized by persistent and high blood pressure, myocardial hypertrophy and pronounced vascular changes in the fundus (angioretinopathy I - II degrees). Malignant arterial hypertension is distinguished by sharply increased and stable blood pressure (especially diastolic > 120-130 mm Hg), sudden onset, rapid development, and the risk of severe vascular complications from the heart, brain, and fundus, which determine an unfavorable prognosis.
3. Symptoms and diagnosis
There are some symptoms that are very characteristic of nephrogenic hypertension, but none of them are pathognomonic (characteristic of only one disease and not found in others). Thus, there are sharp and sudden increases in pressure against the background of normal heart rate, therapeutic resistance to conventional antihypertensive treatment (even intensive). However, in general, the symptoms can correspond to any form of arterial hypertension, so diagnosis in this case usually requires the involvement of several related specialists (cardiologist, vascular surgeon, endocrinologist, always an ophthalmologist, etc.). The primary task is to differentiate the renoparenchymal form of hypertension from the renovascular one. The anamnesis is carefully studied, examination, auscultation and percussion are performed, and a detailed and multifaceted laboratory examination is carried out. Of the instrumental diagnostic methods, the most informative are ultrasound, MSCT, various types of contrast urography and angiography (X-ray examination of blood vessels), kidney scintigraphy, radioimmunoassay of renin concentration in the blood, captopril test, etc. It should be noted that if a parenchymal form of renal hypertension is suspected, X-ray examinations should be prescribed with great caution and strictly according to indications, since both radiation exposure and X-ray contrast agents can aggravate damage to renal tissues and structures.
About our clinic Chistye Prudy metro station Medintercom page!
Causes of pathology
Increased pressure is usually associated with occlusion or stenosis of the renal artery. Against the background of pathological processes, the lumen of the vessel narrows, which leads to an increase in blood pressure. The cause of the development of this condition may be pyelonephritis, glomerulonephritis, amyloidosis, hydronephrosis, polycystic kidney disease.
The development of the disease is facilitated by systemic chronic diseases: diabetes mellitus, tuberculosis, fibromuscular dysplasia. The condition is observed with nephropathy in pregnant women.
4.Treatment
As statistical analysis of the data accumulated in the world shows, conservative methods (medication, nutrition, etc.) are much less effective than active therapy and can only be used as auxiliary, preoperative, supportive measures, as well as palliative treatment in the presence of contraindications to surgical intervention. Surgical correction of renal arterial hypertension in most cases brings a lasting and pronounced positive result and significantly increases the life expectancy of such patients. Abdominal, endoscopic or percutaneous surgery is performed (depending on the clinical characteristics of a particular case) to restore the patency of stenotic arteries, eliminate congenital defects, or completely remove the affected and non-functioning kidney, which in some cases is the only effective answer. Approximately one third of patients may no longer take maintenance antihypertensive drugs.
Palliative antihypertensive therapy as an alternative to surgery, however, is also characterized by fairly high efficiency (although we can only talk about constant drug control of blood pressure) and allows one to normalize blood pressure to one degree or another in more than 90% of cases.
Arterial hypertension
Unlike independent essential (primary) hypertension, secondary arterial hypertension serves as symptoms of the diseases that cause them. Arterial hypertension syndrome accompanies the course of over 50 diseases. Among the total number of hypertensive conditions, the proportion of symptomatic arterial hypertension is about 10%. The course of symptomatic arterial hypertension is characterized by signs that allow them to be differentiated from essential hypertension (hypertension):
- The age of patients is up to 20 years and over 60 years;
- Sudden development of arterial hypertension with persistently high blood pressure levels;
- Malignant, rapidly progressing course;
- Development of sympathoadrenal crises;
- A history of etiological diseases;
- Poor response to standard therapy;
- Increased diastolic pressure in renal arterial hypertension.