Hemostasis
Hemostasis is a sequence of tightly regulated processes that maintain blood in a liquid aggregate state and cause the rapid formation of a local thrombus at the site of vessel damage. Thrombosis is a pathological form of hemostasis that leads to intravital blood coagulation in blood vessels after a relatively minor injury [1]. Thromboembolic complications are the third most common cause of death among cardiovascular diseases after ischemic heart disease and stroke. Despite significant progress in diagnosis, the prevalence and mortality from venous thromboembolism have not decreased significantly over the past 30 years [2], which indicates insufficient knowledge of the mechanisms of this disease and the imperfection of its therapy.
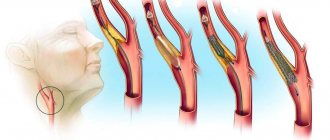
The mechanisms of atherosclerosis leading to arterial thrombosis also remain incompletely understood. A number of operations (balloon angioplasty, stenting, coronary artery bypass grafting) failed to have a significant impact on reducing mortality from cardiovascular diseases. The use of statins, although it improves quality of life and reduces the risk of complications of cardiovascular diseases, only slightly reduces the number of deaths and recurrent myocardial infarctions [3]. When carrying out anticoagulant therapy, laboratory monitoring of blood clotting is required. Treatment may be accompanied by hemorrhagic complications associated with a decrease not only in blood clotting, but also in capillary resistance and an increase in their permeability. These factors force us to look for new causes, as well as methods for preventing and treating vascular thrombosis.
Currently, it is customary to distinguish between two types of hemostasis: vascular-platelet and coagulation .
The first refers to stopping bleeding from small-caliber vessels, the second is used to combat blood loss from arteries and veins. This division is very conditional, since both in case of damage to small and large vessels, blood coagulation always occurs along with the formation of a platelet plug [4].
Vascular-platelet hemostasis leads to the formation of a blood clot and is divided into three stages.
The first of these is vasospasm. The primary spasm begins immediately after injury and is caused by the release of catecholamines into the blood; it lasts about 10 seconds. Then a secondary spasm occurs, which occurs due to the activation of platelets and their release of vasoconstrictors: serotonin, thromboxanes.
The second stage is the formation of a platelet plug. This occurs through platelet adhesion and aggregation. At this stage, adhesion is reversible, but as a result of the reactions of the third stage, which cause the release of prostaglandins and thromboxanes, as well as thrombostenin, the platelet plug contracts and thickens, in other words, retraction [4].
Coagulation hemostasis occurs due to blood coagulation factors and can be divided into 3 phases.
- The first consists of reactions that cause the formation of prothrombinase through the intrinsic or extrinsic pathway.
- The second is the transition of prothrombin to thrombin under the action of prothrombinase.
- The third is the conversion of fibrinogen to fibrin.
At the first stage, the formation of a readily soluble fibrin monomer occurs, which, as a result of polymerization and the action of factor XIII, is converted into a sparingly soluble fibrin polymer [4].
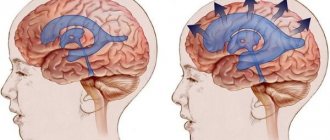
Pathological hemostasis is based on three basic principles formulated by Virchow and entered into history under the name Virchow triad . It consists of pathological changes in the inner layer of blood vessels, changes in blood circulation speed and changes in blood viscosity.
However, from the point of view of modern medicine, these are not all the factors influencing thrombus formation. For example, it is known that the immune system plays a significant role in the development of deep vein thrombosis. Neutrophils are of greater importance. In addition to the usual phagocytosis, they also produce NETs (Neutrophil extracellular traps) , the main function of which is to capture and immobilize microbes in the extracellular space. NETs are composed of intact chromatin fibers and antimicrobial proteins. The multistep process of NET formation is called NETosis. Once activated, some enzymes are transported from granules to the nucleus, causing chromatin decondensation, disrupting nuclear membranes, and causing cytolysis. Activated endothelium, together with neutrophils, causes the formation of NETs, which, in turn, are very large structures and promote platelet adhesion. NET also stimulates fibrin formation and deposition. By cleaving tissue factor inhibitor, stimulating factor Xa, and binding factor XII, NET stimulates the intrinsic and extrinsic coagulation pathways. Histones released during NET formation promote the release of Weibel-Palade bodies upon endothelial activation [5]. Since the immune system is not involved in normal hemostasis, preventing its activation in deep vein thrombosis may be the key to treating this disease. Due to the fact that the endothelial factor is the main factor in the formation of a venous thrombus, suppression of its activation can help in the treatment and prevention of thrombus formation.
Another important aspect affecting hemostasis are inorganic polyphosphates , which consist of linear orthophosphate polymers linked by high-energy phosphoanhydride bonds. There are two types of polyphosphate chains: long (up to several thousand phosphates) and short (60–100 phosphate units), which are stored in dense granules of platelets and released when they are activated. Long-chain polymers activate coagulation factor XII and increase fibrin stability in the thrombus, while short-chain polymers increase factor V activation and inhibit TFPI (Tissue factor pathway inhibitor). In addition, polyphosphates of both types are also cofactors for thrombin activation; weaken fibrinolysis, impairing the binding of plasminogen to fibrin; reduce the activity of anticoagulants such as heparin, direct inhibitors of thrombin and factor Xa [6, 7]. The use of polyphosphate antagonists may be a promising approach to prevent hypercoagulability, with fewer side effects compared with traditional anticoagulant drugs.
In human blood there are microparticles that are released by various cells (platelets, endothelium, leukocytes, erythrocytes) after activation or apoptosis and are small membrane vesicles. They were originally described as "platelet dust" which is released from activated platelets. Regardless of their origin, all microparticles are procoagulants because they provide a membrane surface for the assembly of various components of the coagulation cascade. Also on their surface are anionic phospholipids: phosphatidylserine and procoagulant tissue factor, which are activators of blood clotting. Microparticles play an important role in normal hemostasis [7], but their involvement in deep vein thrombosis and other thrombus-related diseases remains poorly understood.
The problem of spontaneous thrombus formation is one of the main ones today. Modern antiplatelet drugs significantly increase the risk of bleeding, and therefore their use remains limited. Recent studies have made it possible to more deeply understand the mechanisms of thrombosis and hemostasis and the differences between them. New tactics and drugs developed based on these tactics may selectively inhibit pathological thrombosis. All this could be an excellent opportunity for the development of antiplatelet drugs that could provide effective and, most importantly, safe treatment for many patients.
Sources
1. VKMM FRCPath, AKA MBBS, and JCAM PhD, Robbins Basic Pathology, 10 edition. Philadelphia, Pennsylvania: Elsevier, 2021. 2. J. A. Heit, A. Asrani, D. J. Crusan, R. D. McBane, T. M. Petterson, and K. R. Bailey, “Reasons for the persistent incidence of venous thromboembolism,” Thromb. Haemost., vol. 117, no. 2, ss. 390–400, 26 2021. 3. N. E. Nikolaeva, “Relative excess of animal protein in the diet is the initiator of the development of the atherosclerotic process,” Atherosclerosis and Dyslipidemia, vol. 2 (27), 2021. 4. Lecture 16. Physiology of platelets. Concept of hemostasis (vascular platelet and coagulation hemostasis) 5. T. A. Fuchs, A. Brill, and D. D. Wagner, “Neutrophil Extracellular Trap (NET) Impact on Deep Vein Thrombosis,” Arterioscler. Thromb. Vasc. Biol., vol. 32, no. 8, pp. 1777–1783, Aug. 2012. 6. J. H. Morrissey, S. H. Choi, and S. A. Smith, “Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation,” Blood, vol. 119, no. 25, ss. 5972–5979, Jun. 2012. 7. J. Geddings and N. Mackman, “New players in haemostasis and thrombosis,” Thromb. Haemost., vol. 111, no. 04, ss. 570–574, 2014.
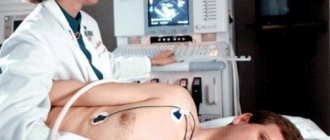
Methods for diagnosing disorders of the hemostatic system
The following methods can be used to identify pathology:
- determination of bleeding time (according to Duke, according to Ivey, according to Lee-White);
- general blood test with formula;
- determination of platelet aggregation;
- prothrombin time (PTT) and prothrombin index (PTI);
- activated partial thromboplastin time (aPTT), etc.
Depending on the specific disorders of the hemostatic system, the doctor may prescribe both individual indicators and a comprehensive study - a coagulogram. It includes indicators such as PTT, PTI, APTT, determination of fibrinogen and D-dimer levels, etc.
In order to confirm or refute the hereditary nature of disorders of the hemostatic system, genetic testing is prescribed. You can take it in medical genetics.
Preparation for a hemostasiogram
Blood is drawn from a vein on an empty stomach - that is, the patient’s last meal should be 8-12 hours before the procedure. In the morning before the analysis, you can drink some pure still water. On the eve of the test, you should avoid stress, physical and mental fatigue, and do not drink alcohol. Do not smoke for at least 2 hours before taking blood.
Biomaterial should not be submitted for analysis earlier than 3-5 days after ultrasound, x-rays or physical procedures.
If it is necessary to monitor indicators over time, subsequent studies should be carried out under identical conditions: in the same laboratory, at the same time of day, etc.
Important! A few days before the test, you need to stop taking medications, especially those that affect clotting (for example, aspirin). If the patient takes such drugs on a regular basis, it is necessary to discuss this issue with the attending physician!
It is not advisable for women to undergo screening during menstruation - real data on hemostasis during this period may be distorted.
How to study coagulation?
To study coagulation, various models are created - experimental and mathematical. What exactly do they allow you to get?
On the one hand, it seems that the best approximation for studying an object is the object itself. In this case, a person or an animal. This allows you to take into account all factors, including blood flow through the vessels, interactions with the walls of blood vessels and much more. However, in this case the complexity of the problem exceeds reasonable limits. Convolution models make it possible to simplify the object of study without losing its essential features.
Let's try to get an idea of what requirements these models must meet in order to correctly reflect the coagulation process in vivo.
The experimental model must contain the same biochemical reactions as in the body. Not only proteins of the coagulation system must be present, but also other participants in the coagulation process - blood cells, endothelial and subendothelial cells. The system must take into account the spatial heterogeneity of coagulation in vivo: activation from a damaged area of the endothelium, the spread of active factors, the presence of blood flow.
It is natural to begin the consideration of coagulation models with methods for studying coagulation in vivo. The basis of almost all of these approaches used is to inflict controlled injury on the experimental animal in order to induce a hemostatic or thrombotic response. This reaction is studied by various methods:
- monitoring bleeding time;
- analysis of plasma taken from an animal;
- autopsy of a euthanized animal and histological examination;
- Real-time thrombus monitoring using microscopy or nuclear magnetic resonance (Figure 4).
Figure 4. In vivo thrombus formation in a laser-induced thrombosis model. This picture is reproduced from a historical work where scientists were able to observe the development of a blood clot “live” for the first time. To do this, a concentrate of fluorescently labeled antibodies to coagulation proteins and platelets was injected into the mouse’s blood, and, placing the animal under the lens of a confocal microscope (allowing for three-dimensional scanning), they selected an arteriole under the skin accessible for optical observation and damaged the endothelium with a laser. Antibodies began to attach to the growing clot, making it possible to observe it.
[7]
The classic setup of an in vitro coagulation experiment is that blood plasma (or whole blood) is mixed in a container with an activator, after which the coagulation process is observed. According to the observation method, experimental techniques can be divided into the following types:
- monitoring the coagulation process itself;
- monitoring changes in concentrations of coagulation factors over time.
The second approach provides incomparably more information. Theoretically, knowing the concentrations of all factors at an arbitrary point in time, one can obtain complete information about the system. In practice, studying even two proteins simultaneously is expensive and involves great technical difficulties.
Finally, coagulation in the body is heterogeneous. The formation of a clot starts on the damaged wall, spreads with the participation of activated platelets in the plasma volume, and is stopped with the help of the vascular endothelium. It is impossible to adequately study these processes using classical methods. The second important factor is the presence of blood flow in the vessels.
Awareness of these problems has led to the development of a variety of in vitro flow experimental systems since the 1970s. It took a little more time to understand the spatial aspects of the problem. Only in the 1990s did methods begin to appear that take into account spatial heterogeneity and diffusion of coagulation factors, and only in the last decade have they begun to be actively used in scientific laboratories (Fig. 5).
Figure 5. Spatial growth of a fibrin clot in normal and pathological conditions. Coagulation in a thin layer of blood plasma was activated by tissue factor immobilized on the wall. In the photographs the activator is located on the left. The gray expanding stripe is a growing fibrin clot.
Along with experimental approaches, mathematical models are also used to study hemostasis and thrombosis (this research method is often called in silico [8]). Mathematical modeling in biology makes it possible to establish deep and complex relationships between biological theory and experience. Conducting an experiment has certain boundaries and is associated with a number of difficulties. In addition, some theoretically possible experiments are infeasible or prohibitively expensive due to limitations in experimental technology. Modeling simplifies experiments, since it is possible to select in advance the necessary conditions for in vitro and in vivo experiments under which the effect of interest will be observed.
Blood test for hemostasis: main indications:
- age category over 50 years;
- infertility;
- taking oral contraceptives/indirect anticoagulants, antiplatelet agents, heparins;
- miscarriage;
- excessively heavy menstruation;
- history of failed IVF attempts;
- at least two missed pregnancies in history or severe gestosis;
- cardiovascular diseases;
- signs of excessive bleeding;
- preoperative examination;
- examination before IVF, pregnancy;
- the presence of thrombophilia, hemophilia, etc.;
- assessment of liver function, etc.