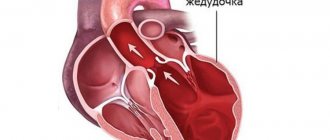
The term cardiomyopathy (CM) is a collective name for a variety of myocardial diseases associated with disruption of its structure and function, not associated with arterial hypertension, circulatory disorders and valve diseases.
Most often, cardiomyopathy causes abnormal thickening of the heart muscle (hypertrophic cardiomyopathy) or enlargement of the heart chambers (dilated cardiomyopathy) due to genetic mutations or other causes.
1
Ultrasound of the heart for cardiomyopathy
2 Holter for cardiomyopathy
3 Diagnosis of cardiomyopathy
Classifications of cardiomyopathies
Classification of cardiomyopathies by origin
Based on their origin, they distinguish between primary (myocardial diseases of unknown origin) and secondary cardiomyopathy (the cause of myocardial damage is known or is associated with diseases of other organs).
Causes of primary cardiomyopathy:
- Genetic disorders;
- viral infections (Coxsackie, etc.);
- replacement of the heart muscle with connective or fatty tissue.
Causes of secondary cardiomyopathy:
- endocrine disorders (thyrotoxicosis, diabetes mellitus);
- intoxication (toxic, alcoholic, etc.);
- stress (Takotsubo cardiomyopathy).
Classification of cardiomyopathy according to clinical signs (main forms):
Dilated cardiomyopathy is the most severe and common. With this disease, cardiomegaly occurs - a significant increase in myocardial cavities. As a result, the heart loses its ability to fully pump blood. Dilated cardiomyopathy inevitably leads to the development of heart failure.
Peripartum (postpartum) cardiomyopathy is one of the types of dilated cardiomyopathy. Paradoxically, the development of heart failure in a woman and its characteristic symptoms is practically the main sign of the peripartum form. Other common options include alcohol.
Hypertrophic cardiomyopathy (HCM) is one of the main causes of sudden death in young, physically active people (athletes, military personnel). This cardiomyopathy is associated with thickening of the myocardium (cardiac muscle hypertrophy). The increase in wall thickness outpaces the development of blood vessels and, as a result, the blood supply to the heart muscle is disrupted, and the filling of the ventricles with blood also suffers.
HCM is more common in men.
Obstructive cardiomyopathy (subaortic subvalvular stenosis) is a type of hypertrophic cardiomyopathy in which blood flow into the aorta from the left ventricle is limited by a locally thickened interventricular septum.
Restrictive cardiomyopathy is a rare pathology in which the elastic properties of the heart walls deteriorate. The myocardium loses its ability to stretch and relax, which impairs its ability to fill with blood. The result is stagnation of blood in the veins and a lack of it in the arteries. The probable cause of this is abnormal proliferation of connective tissue.
Arrhythmogenic right ventricular cardiomyopathy (Fontan disease) - accompanied by life-threatening arrhythmias.
Arrhythmogenic CMP can cause ventricular arrhythmia in children and young adults with apparently normal hearts, as well as in older patients. The outcome of this disease is often sudden death, especially at a young age.
Takotsubo cardiomyopathy (broken heart syndrome) is a very rare disease in which a sudden, transient decrease in myocardial contractility develops. The shape of the left ventricle, due to pathological expansion, is externally similar to takotsubo - an octopus trap in Japan (it was in this country that this syndrome was discovered and described).
Takotsubo CMP is a cause of acute heart failure. It is more often detected in postmenopausal women and develops against the background of severe psycho-emotional shock. On the ECG it can masquerade as myocardial infarction.
1 Tests for cardiomyopathies
2 Diagnosis of cardiomyopathy
3 ECG for cardiomyopathies
Peripartum cardiomyopathy (Meadows Cardiomyopathy, Peripartum disease)
Conservative therapy
After confirmation of the diagnosis, patients are necessarily hospitalized in a cardiology hospital.
In case of severe circulatory failure, transfer to the intensive care unit for intra-aortic balloon counterpulsation is indicated. Despite the rapidly progressive course, almost half of the patients experience spontaneous recovery. Non-drug treatment includes limiting fluid intake to 2 liters per day and table salt to 3-4 grams per day. When prescribing drug therapy to pregnant women, it is necessary to take into account the potential undesirable effect of certain medications on the fetus. Recommended:
- Dopamine receptor agonists.
The drugs act on the main pathogenetic link of PPCM. By suppressing the production of prolactin, they reduce the formation of its cardiotoxic fragment (16 kDa). Bromocriptine is used in pregnant women; after childbirth, it is possible to switch to cabergoline or quinagolide. - ACE inhibitors.
Essential medications that slow the progression of heart failure. - Beta blockers.
Prescribed as an alternative to ACE inhibitors for pregnant women, as well as in the presence of tachyarrhythmias. - Vasodilators.
Vasodilator medications are used to relieve vasospasm when systolic pressure is more than 110 mmHg. Art. - Diuretics.
To reduce congestion in the lungs and remove fluid, thiazide and loop diuretics are used. - Cardiac glycosides.
To maintain adequate cardiac output during arterial hypotension, digoxin and levosimendan are indicated. - Anticoagulants.
Since dilatation of the cardiac cavities is accompanied by a high risk of thrombus formation, patients are recommended to take anticoagulants (warfarin, rivaroxaban). Only heparin is allowed for pregnant women.
Surgery
In case of severe CHF (left ventricular ejection fraction reaches less than 40%), which develops in pregnant women, termination of pregnancy by cesarean section is required. Extremely severe peripartum cardiomyopathy, resistant to conservative therapy, is an indication for heart transplantation. For persistent tachyarrhythmias accompanied by severe hemodynamic disturbances (critical drop in blood pressure), implantation of a cardioverter-defibrillator is used.
Symptoms of cardiomyopathy
Unfortunately, cardiomyopathy can occur for a long time without any symptoms, and then manifest itself with symptoms common to many cardiac diseases:
- shortness of breath with minor physical effort, walking;
- arrhythmias;
- pain in the heart area;
- constant loss of strength;
- dizziness;
- fainting states.
Often, cardiomyopathy can be suspected only when the disease is already in the stage of decompensation and all the symptoms of heart failure are present:
- shortness of breath even at rest;
- presence of swelling in the legs;
- ascites (accumulation of fluid in the abdominal cavity), enlarged liver, etc.;
- atrial fibrillation and other types of arrhythmias;
- cough that gets worse when lying down;
- attacks of suffocation;
- pulmonary edema.
1 Ultrasound of the heart for cardiopathy
2 ECGs for cardiomyopathies
3 Blood tests for cardiomyopathy
Peripartum (pregnancy and childbirth associated) cardiomyopathy (PPCM) is a rare disease that develops in women in the last month of pregnancy or 5 months after childbirth. Cases of its development earlier than 1 month before birth have been described [26].
A number of authors [2, 3, 17] consider PPCM to be one of the forms of dilated cardiomyopathy (DCM), occurring with left ventricular heart failure (HF).
The diagnostic criteria for this pathology are:
1) development of HF in the last month of pregnancy or 5 months after birth;
2) absence or uncertainty of the causes of heart failure;
3) absence of diagnosed heart disease more than 1 month before birth;
4) signs of impaired left ventricular function according to echocardiography (EchoCG), expressed in a decrease in ejection fraction (EF) [22].
An extremely rare type of myocardial damage is the formation of the so-called non-compact myocardium, considered by a number of authors as an independent nosological form - non-compact cardiomyopathy (NCCM). In modern literature, only 7 cases of this pathology in pregnant women are described [18, 19].
The purpose of this publication is to familiarize practicing physicians with the issues of diagnosis and differential diagnosis of severe myocardial diseases in pregnant women.
Patient P.,
26 years old, primigravida. Previously, I was involved in sports (volleyball, swimming). My father died at the age of 36 from DCM. From the 34th week of pregnancy, she began to notice increasing shortness of breath on exertion, then suffocation at night, a sharp decrease in appetite, and swelling of the legs. I didn’t go to the doctors. She entered the Moscow Regional Research Institute of Obstetrics and Gynecology at 36 weeks of pregnancy. Objectively: the condition is serious. Cardiomegaly is determined by percussion, the systolic murmur of mitral insufficiency, gallop rhythm (tachycardia up to 120 beats per minute, additional third sound in diastole), accent of the second tone on the pulmonary artery are heard. Blood pressure 90/60 mm Hg. The liver is not clearly palpable. Swelling of the legs. Clinical, biochemical blood tests, urine tests without pathology.
On the ECG (Fig. 1):
Figure 1. ECG of patient P., 26 years old. sinus rhythm, low-voltage ECG from the extremities, normal direction of the electrical axis of the heart. Signs of hypertrophy and dilatation of the left atrium and both ventricles. Daily Holter ECG monitoring revealed 2 “runs” of paroxysmal ventricular tachycardia from 3 to 5 QRS complexes.
With echocardiography (Fig. 2 and 3):
Figure 2. EchoCG of patient P., 26 years old (four-chamber position). Dilatation of the left ventricle is determined. Layer designations: K - compact myocardium; NC - non-compact myocardium (indicated by an arrow).
Figure 3. Doppler echocardiography of patient P., 26 years old (four-chamber position). Areas of blood flow from the ventricular cavity into the intertrabecular recesses in the area of the apex and lateral wall of the left ventricle are determined (indicated by arrows). dilatation of all chambers of the heart. The end systolic size (ESD) of the left ventricle (LV) is 7.1 cm (normal is up to 4.3 cm), the end diastolic size (EDD) of the LV is 7.8 cm (normal is up to 5.5 cm); the size of the left atrium is 4.8 × 8 cm (normal 1.85 × 3.3 cm). The myocardium is hypertrophied, penetrated by multiple lacunae, pronounced trabecularity and hypoplasia of the papillary muscles are determined. Global myocardial contractility is reduced (LV RF during dynamic observation ranged from 18 to 22%). Restrictive type of diastolic blood flow. Mitral insufficiency of II-III degree. The estimated pressure in the right ventricle is 55-60 mmHg. (normal is 25-30 mm Hg).
Results of computed tomography of the chest (Fig. 4):
Figure 4. X-ray computed tomogram (with the introduction of omnimac) of the heart of patient P., 26 years old. The total thickness of the myocardium is 25.3 mm, the thickness of the compact layer is 6.1 mm, RA is the right atrium. the right sections are expanded. Their condition is unremarkable. The LV cavity is expanded, the walls are sharply thickened, represented by compact and non-compact layers, the latter occupies more than 2/3 of the posterolateral wall.
Diagnosis: dilated cardiomyopathy. Non-compact myocardium. Relative mitral insufficiency of II-III degree. Pulmonary hypertension degree II. CH, IV functional class.
Delivery was permitted in the operating room of the cardiac surgery hospital of the Moscow Regional Research Clinical Institute named after. M.F. Vladimirsky using cesarean section under regional anesthesia at 38 weeks of pregnancy. A live, full-term girl was extracted, weighing 3000 g, height 49 cm, with an Apgar score of 7-8 points. Lactation was terminated using bromocriptine. After delivery, the somatic condition of the postpartum woman stabilized, and the disappearance of edema and shortness of breath was noted. Transferred for further treatment to the cardiology department. She received therapy with cardiac glycosides, riboxin, amiodarone, angiotensin-converting enzyme (ACE) inhibitors, and saluretics. After 1 year, she suddenly died from the underlying disease.
Discussion
The development of severe HF in pregnant women in the absence of other causes is most often associated with PPCM. Its actual frequency is unknown and depends on the geographic region. Thus, it is 1:3000-1:4000 births in the USA [16], 1:1000 in South Africa, 1:6000 in Japan, 1:400 in Haiti, and in Nigeria - 1 in 100 births [12 ].
The pathogenesis of PPCM is not fully understood. In its development, the complex effects of infection, hemodynamic load during pregnancy and genetic factors are important. In a number of studies, the importance of previous myocarditis is attached to the genesis of the development of PPCM. It is believed that a viral infection suffered in childhood leads to the persistence of a virus that has no pathogenetic significance. However, during pregnancy, which is accompanied by a decrease in immunity, reactivation of the virus with myocardial damage is possible [8]. Another theory considers the cause of PPCM to be the entry of fetal antigens into the maternal bloodstream (fetal microchimerism) against the background of reduced immunity of the pregnant woman. In this pathology, high titers of autoantibodies against the main myocardial proteins are often detected [24]. A. Ansari et al. [4] found high titers of autoantibodies in the blood serum of patients with PPCM, which were not detected in patients with idiopathic DCM [4].
DCM is always considered as a differential diagnosis, which is one of the most common and irreversible forms of cardiomyopathies, occurs with a frequency of 1:2500 healthy people, can be hereditary and in 1/3 of cases is the reason for heart transplantation [28]. However, in the presented clinical observation, the development of HF was clearly associated with pregnancy, which allowed us to settle on the diagnosis of PPCM.
An extremely rare observation is the so-called non-compact myocardium, and a number of authors attribute this condition of the heart muscle to the development of NCCM, which is a rare congenital form of LV cardiomyopathy, described in 1984 by R. Engberding and F. Bender and is characterized by an autosomal dominant type of inheritance (in 44%), and also occurs both as an independent form and in other heart diseases. For example, non-compact myocardium can be detected in myocarditis in 12%, in DCM in 52%, as well as in other heart diseases [14]. The mechanism of NCCM is considered to be a violation of the formation of normal myocardium from embryonic spongy, which, in the absence of coronary blood flow, is supplied with blood from the cavities of the heart [1]. Although the process most often affects the apex and inferior wall of the LV, it can be detected in any wall of the left and right ventricles [10].
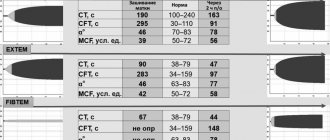
The differential differences between NCMP and DCM relate to a greater extent to the quantitative characteristics determined by echocardiography, as well as to a more favorable clinical picture and the possibility of recovery after childbirth with NCMP (see table).
The criterion for a poor prognosis of the disease is the ratio of the thickness of the non-compact and compact layers of the myocardium exceeding 3:1, the involvement of more than 3 myocardial segments in the process, and pronounced signs of heart failure.
Due to the absence of typical clinical manifestations of NCMP, the main method of diagnosis is echocardiography. Diagnosis is based on the following main criteria: the presence of pronounced trabecularity and deep intertrabecular grooves in at least 4 places, the presence of blood flow from the ventricular cavity into the intertrabecular grooves and a typical two-layer structure in the affected part of the LV. Nuclear magnetic X-ray tomography can be used in the diagnosis of NCCM [15].
Treatment.
Observation and treatment of pregnant women with PPCM is carried out by a cardiologist and an obstetrician-gynecologist at a specialized maternity hospital. The treatment uses diuretics (hydrochlorothiazide, furosemide), which reduce the severity of edema syndrome, congestion in the lungs, as well as cardiac glycosides (digoxin). It must be taken into account that long-term use of diuretics can reduce uteroplacental blood flow and contribute to the development of acidosis in the mother, which requires monitoring of the acid-base status [5]. As a vasodilator, sodium nitroprusside can be used.
Intravenously administered drugs with a positive inotropic effect (increasing myocardial contractility) occupy an important place in the short-term treatment of acute HF, including in pregnant women. The most commonly used drugs are β-adrenergic agonists (dopamine, dobutamine). In addition to the use of these drugs, the issue of using in pregnant women a representative of a new class of non-glycoside cardiotonic drugs called calcium sensitizers - levosimendan, the main mechanism of action of which is to increase the sensitivity of the contractile proteins of cardiomyocytes to calcium, which leads to stabilization of the conformation of troponin C, which triggers myofibril contractions. A number of authors [5, 6] believe that the use of levosimendan can reduce the degree of pulmonary hypertension and increase cardiac output in PPCM. However, in a randomized study by M. Biteker et al. [7] did not reveal a positive effect of the drug after 24-hour use in this group of patients. In addition, experimental studies have shown its toxic effect on the fetus, and therefore levosimendan should be prescribed to pregnant women only if the benefit to the mother outweighs the potential risk to the fetus.
The use of other drugs with a positive inotropic effect (dopamine, dobutamine) is allowed in cases of a significant decrease in cardiac output, and their withdrawal is carried out when the patient’s condition has stabilized [25]. The use of cardioselective β-blockers (carvedilol) can reduce myocardial oxygen consumption, reduce preload, and is safe in pregnant women with PPCM [22].
Treatment of arrhythmias complicating the course of PPCM is carried out according to general indications. Implantation of a cardioverter-defibrillator is possible (class of recommendations IIb, level C) [20].
In women with LVEF less than 20% or with evidence of intracavitary thrombosis, the use of unfractionated heparin (UF), low molecular weight heparin (LMWH) or warfarin is recommended (C. Nelson-Piercy et al., 1997).
Immunosuppressive therapy for PPCM is not indicated. There are isolated studies of the successful use of immunoglobulin for this disease (A. Keogh et al., 1994).
Cases of severe PPCM require balloon pumping or the use of an artificial LV [13]. The positive result of using the latter within 3-6 months after birth in women with PPCM can significantly reduce the frequency of heart transplants from 33 to 4-7% [9].
Preparation for childbirth and delivery
Preparing for childbirth in pregnant women with PPCM includes the use of cardiotonic drugs and positive inotropic drugs (as indicated). Delivery is carried out in a cardiac surgery hospital under the supervision of an experienced cardiologist and anesthesiologist-resuscitator with constant monitoring of ECG and blood pressure measurements with the possibility of performing balloon counterpulsation or “switching on” an artificial LV.
Delivery is most often carried out by caesarean section using epidural anesthesia with a slow titration of the anesthetic dose or general anesthesia, which is used in cases of emergency delivery [21, 23]. It is also possible to use spinal-epidural anesthesia [27].
Postpartum period.
Restoration of LV contractile function occurs in 23-54% of patients with PPCM and depends on the initial value of LVEF [9, 14]. The normalization process usually takes from 3 to 24 months. The mortality rate for PPCM in the United States ranges from 6 to 10%, and the 6-month and 2-year mortality rates in South Africa are 10 and 28%, respectively [11, 25]. Women who undergo PPCM with further improvement in LVEF remain at risk of developing severe HF and sudden death in the postpartum period [17].
Monitoring and treatment of women who have undergone PPCM are carried out by a cardiologist and a cardiac surgeon. Along with conventional drugs used in the treatment of heart failure, ACE inhibitors can be used in the postpartum period. EchoCG examination is carried out 6 weeks and 6 months after birth, and more often if indicated [25].
In the postpartum period, in women with LVEF less than 30%, the use of NG or LMWH is recommended (C. Nelson-Piercy et al., 1997).
Thus, in the presented clinical observation, a rare combination of PPCM and non-compact myocardium was identified in a pregnant woman, possibly representing a separate nosological form, and the hereditary nature of the pathology, severe ventricular arrhythmias and the absence of positive EchoCG dynamics after delivery caused, despite active drug therapy, a fatal outcome of the disease.
Diagnosis of cardiomyopathy
Diagnosis of CMP should be trusted only to an experienced specialist, since the clinical picture for various pathologies has similar signs.
First, the doctor collects anamnesis and examines the patient (pays attention to skin color, clarifies the presence of edema, measures heart rate and blood pressure, listens to the heart and lungs with a stethoscope).
Next, laboratory and instrumental studies are prescribed:
- blood tests (clinical, biochemical, hormonal status, lipid profile, etc.);
- electrocardiography (ECG);
- Holter monitoring ECG;
- echocardiography (ultrasound of the heart);
- radiography;
- coronary angiography;
- MRI, CT scan of the heart.
Pregnancy in patients with cardiomyopathy/HF: HFA, ESC consensus document
Pregnancy is a condition accompanied by increased stress on a woman’s body. In this connection, it is obvious that its onset can contribute to the decompensation of any chronic diseases, including heart disease. In addition, pregnancy itself can be a factor in one of the forms of dilated cardiomyopathy - periportal cardiomyopathy. In this regard, experts from the Heart Failure Association of the European Society of Cardiology decided on the need to create a consensus document on risk stratification and management of women with cardiomyopathies/heart disease who are planning pregnancy or are pregnant. First of all, the document highlights general approaches to the management of pregnant and lactating patients with heart failure. It is emphasized that, if possible, pregnancy should be prolonged to 32 weeks. The preferred method of delivery, as in healthy pregnant women, is vaginal delivery, accompanied by adequate pain relief. At the same time, it is noted that in most cases, in the presence of heart failure, delivery is possible only by cesarean section. Next, we discuss general issues of managing pregnant patients with both congenital forms of cardiomyopathies (hypertrophic, arrhythmogenic dysplasia of the right ventricle) and acquired ones (periportal, dilated, Takotsubo syndrome). Particularly relevant is the section that discusses some aspects of the management of pregnant women who have previously undergone treatment for cancer and who have developed heart failure during pregnancy. Risk factors for the development of heart failure in such patients have been identified. They are: a decrease in left ventricular ejection fraction <50%, chemotherapy and radiation therapy for cancer, diagnosis of cancer at a very young age <10 years, time from cancer to pregnancy >15 years. In general, it is obvious that any research in this area is almost completely absent. In this regard, the decision on the optimal treatment method should be made by a team of specialists, including obstetricians-gynecologists and cardiologists. In addition, detailed counseling of such patients when planning pregnancy is important.
Source:
Sliwa K, et al. Eur J Heart Fail. 2021. doi: 10.1002/ejhf.2133.