© Author: A. Olesya Valerievna, candidate of medical sciences, practicing physician, teacher at a medical university, especially for SosudInfo.ru (about the authors)
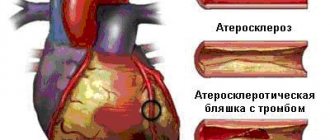
Ebstein's anomaly (EA) is a very rare cardiac defect that develops during fetal development. With pathology, the normal arrangement of the tricuspid valve leaflets is disrupted, resulting in its insufficiency and circulatory disorders. According to statistics, Ebstein's anomaly accounts for no more than 1% of cases of all congenital heart anomalies.
In general, congenital malformations of organs are becoming more common. Constantly deteriorating environmental conditions, unfavorable factors affecting the expectant mother, and the pathology of pregnancy are considered to be to blame. Heart anomalies constitute a large group of defects, which, even if compatible with life, very often lead to serious hemodynamic disorders requiring surgical correction.
When signs of a heart defect are detected in the fetus during an ultrasound examination of a pregnant woman, the expectant mother, of course, begins to worry and look for information about the disease in order to be ready to help her baby after birth. Let's try to figure out what Ebstein's anomaly is and what possible ways to correct this rare defect.
Ebstein's anomaly
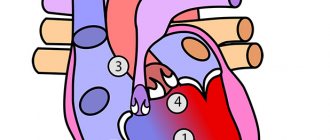
Normally, the septal leaflets of the mitral and tricuspid valves are attached at the same level; with Ebstein’s anomaly, this distance is increased to 1.4-3.2 cm, the tricuspid foramen (fibrous annulus) remains in its normal position. The displaced cusps of the tricuspid valve into the right ventricle divide it into two parts: the atrialized part (the portion of the right ventricle between the fibrous ring and the displaced cusps) and the cavity of the right ventricle itself. The defect is combined with a secondary ASD or an open oval window (Fig. 112).
Fig. 112.
Ebstein's anomaly (diagram).
Fig. 113.
Ebstein's anomaly. Open oval window.
EchoCG criteria
One-dimensional echocardiography:
- Delayed closure of the tricuspid valve (over 0.03 s).
- Increased excursion of the anterior leaflet of the tricuspid valve.
- Simultaneous visualization of two atrioventricular valves.
- Gentle EF tilt of the anterior tricuspid valve leaflet.
- Delayed tricuspid valve closure.
Two-dimensional echocardiography:
- Displacement of the septal leaflet into the cavity of the right ventricle in the projection of the 4 chambers from the apex (more than 20 mm in adults and 15 mm in children) (Fig. 113).
- The presence of an atrialized portion of the right ventricle (the distance between the displaced valve and the tricuspid ring).
- Dilation of the right atrioventricular orifice.
- Visualization of a patent foramen ovale or ASD (observed in 85% of cases).
Doppler EchoCG:
- Detects tricuspid valve insufficiency.
- Assessment of the magnitude of pulmonary hypertension.
- Determination of associated anomalies (ASD, patent foramen ovale).
Ebstein's anomaly (echographic, clinical and pathomorphological comparisons)
Ultrasound machine RS85
Revolutionary changes in expert diagnostics.
Impeccable image quality, lightning-fast operating speed, a new generation of visualization technologies and quantitative analysis of ultrasound scanning data.
Introduction
An unusual heart defect was first described by the German scientist Wilhelm Ebstein in 1866 in a 19-year-old boy with cyanosis, shortness of breath, and dilated jugular veins. In 1927 it was named Ebstein's disease (anomaly) [1]. Ebstein's anomaly is a rare congenital heart defect characterized by displacement of the atrioventricular ring of the tricuspid valve towards the apex of the heart. Part of the right ventricular cavity is atrialized, so that the ventricular cavity itself becomes smaller. Heart failure inevitably develops, the degree of which and the time of manifestation are completely determined by the magnitude of the displacement of the right atrioventricular ring. The rarity of the defect affects its diagnosis. But remembering the disease means diagnosing it by 50%.
results
The prevalence of Ebstein's anomaly is low. For more than 40 years of work of the pathology department of the regional hospital, this defect was diagnosed in only 2 cases (in more than 6000 sectional studies): one adult and one child [2, 3]. Over a 40-year period (1979–2019) of work in ultrasound diagnostic rooms of multidisciplinary children's hospitals, we identified Ebstein's anomaly in 2 cases.
The manifestation of heart disease in all the children we observed developed early, in the first 3 months of life. The adult patient was engaged in heavy physical labor (mason). He developed manifest heart failure at the age of 62 years. Clinical symptoms in all cases were reduced to tachycardia, expansion of the borders of the heart, shortness of breath, congestion in the lungs and an increase in liver size. Auscultation revealed a rough systolic murmur at the base of the heart and on the vessels.
Radiologically, cardiomegaly, pulmonary oligemia, enlargement of the right atrium and enlargement of the right heart silhouette were noteworthy. In the absence of cyanosis, the vascular pattern of the lungs did not change.
Ultrasound examination is decisive for the diagnosis. For Ebstein's anomaly, the typical finding was apical displacement of the septal and/or posterior leaflets of the tricuspid valve. Normally, the rings of the atrioventricular valves are located at the same level. In Ebstein's anomaly, the degree of displacement of the right atrioventricular ring directly correlates with the severity of the defect. The atypically large anterior leaflet prolapsed, causing a stream of regurgitation (Fig. 1).
Rice. 1.
Echogram - Ebstein's anomaly.
A sharply enlarged right atrium and a reduced right ventricle are clearly visible. Displacement of the right atrioventricular ring relative to the left.
RV - right ventricle, TV - tricuspid valve, RA - right atrium, LV - left ventricle, PM - papillary muscle, MV - mitral valve.
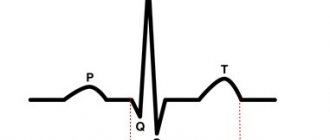
The ECG showed a high sharp P wave (dilatation of the right atrium), prolongation of the PR interval, deviation of the electrical axis of the heart to the right, and right bundle branch block.
During anatomical and histological studies (2 observations), the heart was sharply increased in size. The fibrous right atrioventricular ring is sharply displaced towards the apex of the heart. The right atrium was a large thin-walled cavity. The inner surface of the right atrium is devoid of trabecular muscles and is smooth. The papillary muscles are shifted towards the apex and are thin. The oval window is open. In an adult, the displacement of the right attroventricular ring relative to the left was less. Due to the long-term existence of the defect, the walls of the right ventricle were thickened up to 1.5 cm, in the apex area - up to 3.5 cm. Hypertrophy of the interventricular septum. The endocardium of the right ventricle is thickened and white. Fibrous cords from the endocardium penetrate the entire thickness of the wall of the right ventricle. Between the hypertrophied muscle bundles of the wall of the right ventricle there are wide layers of mature connective tissue. Fibroblasts are located among the collagen fibers.
Discussion
Ebstein's anomaly is a rare congenital heart defect occurring in approximately 1 in 200,000 live births, accounting for significantly less than 1% of all congenital heart defects. The causes of Ebstein's anomaly are heterogeneous. Based on the results of family and twin studies, genetic, reproductive and environmental factors (benzodiazepenes and lithium drugs in mothers) are indicated as possible causative factors. The vast majority of cases of this anomaly are sporadic; familial cases are rare. In rare cases, mutations of the cardiac transcription factor NKX2.5, deletions 10p13-p14 and 1p34.3-p36.11 are found in relatives [4, 5].
The morphogenesis of Ebstein's anomaly consists of incomplete detachment of the septal and posterior leaflets of the tricuspid valve during development from the myocardium, which leads to annular displacement and atrialization of the right ventricle. This entails overload of the remaining part of the right ventricle and its dysfunction. The anterior leaflet of the tricuspid valve is often redundant and is usually described as "sail-like". An unbalanced deformity results in rotational displacement of the tricuspid valve into the right ventricular outflow tract, causing varying degrees of obstruction. The severity of the disease varies and depends on the degree of valve displacement, the degree of atrialization of the right ventricle, outflow tract obstruction, and ventricular dysfunction.
The clinical picture is nonspecific. The degree of heart failure and its severity vary depending on the severity of atrialization of the right ventricle. In some patients, the defect, as in one of our observations, can manifest itself in adulthood. According to foreign data, the age of patients ranges from 1 day to the elderly. The earlier the symptoms develop, the more severe the defect. Newborns with Ebstein's anomaly in the presence of cardiomegaly, heart failure, increased pulmonary artery pressure, cyanosis and acidosis should be immediately referred to a specialized cardiac surgery department [5, 6].
Depending on the anatomical disorders of the valve apparatus, the following options are distinguished (as the prognosis worsens):
- The volume of the preserved part of the right ventricle is sufficient for full hemodynamics.
- The atrialized portion of the right ventricle is large, but the anterior leaflet of the tricuspid valve moves freely.
- The anterior leaflet is significantly limited in movement and creates obstruction of the outflow tract.
- Almost complete atrialization of the right ventricle [4, 7].
Conclusion
Echocardiography is the non-invasive method of choice in diagnosing Ebstein's anomaly. An ultrasound examination can reveal the degree of displacement of the valves, the function of the ventricles of the heart, and the presence of additional defects. Identifying the morphological details of Ebstein's anomaly is the main condition for determining patient management tactics. Echocardiography makes it possible to diagnose heart defects in utero.
Literature
- Balaguru D. Pediatric Ebstein Anomaly. Updated: Aug 14, 2018.
- Delyagin V.M. Congenital heart defects in children of the Aktobe region according to sectional data for 22 years // Pediatrics. 1977; 9:87.
- Delyagin V.M. Congenital heart defects in twins // Medical practice. 1978; 12:42–44.
- Attenhofer Ch., Connolly H., Dearani J., Edwards W. et al. Ebstein's Anomaly. Circulation. 2007; 115:277–285.
- Cherry C., DeBord S., Moustapha-Nadler N. Ebstein's Anomaly: A Complex Congenital Heart Defect. AORN J. The official voice of perioperative nursing. 2009; 89(6):1098–1114.
- Nihoyannopoulos P., McKenna W., Smith G., Foale R. Echocardiographic Assessment of the Right Ventricle in Ebstein's Anomaly: Relation to Clinical Outcome. JACC 1986; 8(3):627–635.
- Munoz-Castellanos L., Espinola-Zavaleta N., Kuri-Nivon M., Candace Keirns C. Ebstein's Anomaly: Anatomoechocardiographic correlation. Bio Med Central. Cardiovascular Ultrasound; 2007; 5: 43. doi: 10.1186/1476-7120-5-43.
Ultrasound machine RS85
Revolutionary changes in expert diagnostics.
Impeccable image quality, lightning-fast operating speed, a new generation of visualization technologies and quantitative analysis of ultrasound scanning data.
Tricuspid valve atresia
With this defect, the structures of the tricuspid valve are not visualized. Since there is no communication between the right atrium and the right ventricle, there is always an atrial septal defect and usually a ventricular septal defect. Other anomalies often associated with tricuspid atresia include transposition of the great vessels and pulmonary stenosis.
There are three types of defect depending on the position of the great vessels, the size of the VSD and the presence of pulmonary artery stenosis (J.Keith, M.Paul).
Type I
- with normal position of the great vessels:
- A. with pulmonary artery atresia (combined with PDA),
- B. with stenosis (hypoplasia) of the pulmonary artery and a small VSD,
- V. with stenosis (hypoplasia) of the pulmonary artery and a large VSD.
Type II
— with D-transposition of the great vessels:
- A. with pulmonary artery atresia (combined with PDA),
- B. with pulmonary artery stenosis,
- V. with a wide pulmonary artery (usually combined with stenosis or coarctation of the aorta),
III type
- with L-transposition of the great vessels (with subpulmonary or subaortic stenosis).
The most typical combination: moderate pulmonary artery stenosis, normally located great vessels, small ventricular septal defect.
EchoCG criteria
One-dimensional echocardiography:
- Small right ventricle.
- Enlargement of the left ventricle and left atrium.
- Volume overload of the left ventricle.
- Inability to visualize tricuspid valve structures.
Two-dimensional echocardiography:
- Dense echo signals at the level of the tricuspid ring without visualization of the leaflets.
- Large atrial septal defect.
- The small right ventricle is connected to the left through a ventricular septal defect.
- Hypoplasia of the right ventricular outflow tract.
- Identifies concomitant anomalies of the great vessels.
Doppler EchoCG:
- Lack of communication between the right atrium and the right ventricle.
- Turbulent flow through a ventricular septal defect.
How does AE manifest itself?
The time of onset of AE symptoms depends on the depth of valve damage, the severity of its insufficiency, and combination with other congenital heart defects. In children who have severe changes in valve structures, signs of the defect are diagnosed immediately after birth. Characteristic:
- Blueness of the skin;
- Weak sucking reflex;
- The child gets tired quickly when feeding and slowly gains weight.
The presence of an open foramen ovale in a newborn baby allows, to some extent, to compensate for the load on the right atrium, because part of the blood goes to the left half of the heart. If this hole is absent or very small, then the child’s condition can quickly become critical, and he can die from severe heart failure within the first weeks of life. Thus, the combination of AE with a defect in the septum in this case can even play a positive role, providing at least some unloading of the “right heart”.
With a moderate or small degree of valve displacement into the right ventricle for a long time, the only sign may be cyanosis. Such patients live with it for up to 10-15 years, and sometimes the defect is even diagnosed in adults. Another, no less dangerous manifestation is arrhythmia, which may also require surgical treatment.
Symptoms of AE include:
- Cyanosis of the skin and mucous membranes;
- Shortness of breath;
- Fatigue, weakness;
- Various arrhythmias;
- When heart failure occurs, edema occurs.
The lack of oxygen in the blood delivered to the organs causes not only an external change in the form of cyanosis, but also a metabolic disorder in the tissues caused by hypoxia. The consequence of this is a change in nails in the form of “watch glasses” and fingers in the form of “drumsticks”. These signs accompany many congenital heart diseases and indicate an insufficient concentration of oxygen in the blood or a discharge of venous blood into the arterial bed.
The enlarged right atrium, the volume of which can reach a liter or more, puts pressure on the anterior surface of the chest, which is especially pronounced in a growing child, whose bones are very pliable. This phenomenon leads to the appearance of such an external sign of pathology as a “heart hump” - a bulging of the front part of the chest in the area of the heart.
Complications of AE include fatal arrhythmias, cardiac arrest, and thromboembolism. The cause of death can be a stroke, sudden cardiac death, or an increase in congestive heart failure with an uncompensated defect.
Tetralogy of Fallot
The vice includes 4 components:
- pulmonary stenosis
- ventricular septal defect
- dextraposition of the aorta
- right ventricular hypertrophy.
The most important 2 components of this defect are pulmonary stenosis and ventricular septal defect. Pulmonary artery stenosis can be infundibular, at the level of the valve, pulmonary trunk, or in the subinfundibular zone. The most typical combination is valvular and infundibular stenosis. Dextraposition of the aorta can be of varying degrees of severity. Conotruncal ventricular septal defect. Right ventricular hypertrophy is secondary and results from outflow tract obstruction. The right-sided aortic arch is observed in 20-30% (Fig. 114).
Fig. 114.
Tetralogy of Fallot: ventricular septal defect. Valvular and infundibular pulmonary artery stenosis. Right ventricular hypertrophy. Atrial septal defect (diagram).
Fig. 115.
Tetralogy of Fallot: the aorta “riding” over the interventricular septum. Large septal defect.
Fig. 116.
Tetralogy of Fallot.
EchoCG criteria
One-dimensional echocardiography:
- Lack of anterior continuation.
- Dilatation of the aorta.
- Dextraposition of the aorta (location of the anterior wall of the aorta and the interventricular septum at different depths).
- Hypertrophy of the anterior wall of the right ventricle.
- Hypertrophy of the interventricular septum.
- Reduction in the diameter of the pulmonary artery.
- Reduction of the left atrium.
Two-dimensional echocardiography:
- Direct visualization of the ventricular septal defect, aortic displacement and dilatation in the parasternal long-axis projection (Fig. 115).
- Direct visualization of pulmonary artery stenosis, its location and severity.
Doppler EchoCG:
- Turbulent flow through a ventricular septal defect.
- Turbulent flow in the pulmonary artery trunk is more than 1.1 m/s.
Differential diagnosis:
- Common arterial trunk.
- Pulmonary atresia with ventricular septal defect.
Pulmonary atresia with ventricular septal defect
The defect includes 5 components (Fig. 117):
- pulmonary atresia;
- occlusion of the right ventricular outflow tract;
- large VSD;
- dextraposition of the aortic root;
- any source of collateral blood supply to the lungs (PDA, aortopulmonary collateral arteries).
Fig. 117.
Pulmonary atresia. Ventricular septal defect. Patent ductus arteriosus. This variant of the defect is often defined as an extreme form of tetralogy of Fallot.
EchoCG criteria
One-dimensional echocardiography:
- Lack of anterior continuation.
- A break in the echo signal from the interventricular septum during M-scanning from the apex to the base of the heart.
- Dilatation of the aorta.
- Dextraposition of the aorta.
- Inability to visualize the pulmonary valve.
- Hypertrophy of the interventricular septum.
- Hypertrophy of the anterior wall of the right ventricle.
- Normal or reduced (40%) size of the left ventricle.
Two-dimensional echocardiography:
- Direct visualization of a large VSD is usually in the subaortic area.
- Inability to visualize the pulmonary artery trunk or its severe hypoplasia.
- Dextraposition of the aorta.
- Detection of concomitant congenital anomalies (PDA, ASD, abnormal drainage of the pulmonary veins).
Doppler EchoCG:
- Turbulent flow through a ventricular septal defect with determination of the pressure gradient in the right ventricle.
- Identification of concomitant congenital anomalies.
Differential diagnosis:
- Common arterial trunk.
- Tetralogy of Fallot.
Complete overview of Ebstein's anomaly: etiology, clinical picture, treatment
Ebstein's anomaly is a rare congenital heart defect. With this congenital anomaly, the chordae tendineae of one or two leaflets of the tricuspid valve are attached not to the papillary muscles, but to the walls of the right ventricle. The posterior leaflet of the tricuspid valve is almost always displaced into the cavity of the right ventricle; however, the septal leaflet is also often displaced. Displaced valves are usually deformed, dystrophic, and their chords are shortened. Only the anterior leaflet of the tricuspid valve remains attached to the atrioventricular fibrous ring. However, it is significantly increased in size and has a trapezoidal shape, and in most cases is the only normally functioning leaflet of the tricuspid valve. The presence of such a defect leads to a reduction in the cavity of the right ventricle and its division by the plane of the downwardly displaced valve opening into two parts. Thus, the part that is below this plane is the ventricle itself, and the part that is located above the plane (supravalvular) is united with the right atrium. All this leads to deformation and expansion of the fibrous ring, and subsequently to tricuspid valve insufficiency.
According to the literature, Ebstein's anomaly is a rather rare pathology, the frequency of which among all congenital heart defects does not exceed 1%. This pathology occurs in 1 case per 20,000 newborns. Currently, the causes of Ebstein's anomaly remain poorly understood. Genetic predisposition and environmental factors play a large role in the occurrence of this pathology. The occurrence of this pathology is most often associated with the intake of lithium into the mother’s body in the early stages of pregnancy. Various infectious diseases (measles, rubella, etc.) and severe concomitant diseases (diabetes mellitus, anemia of various origins, etc.) in a pregnant woman can lead to not only this anomaly, but also other congenital heart defects.
According to the new classification (1988) of this defect, there are 4 types of Ebstein's anomaly, differing in the level of attachment and degree of deformation of the anterior leaflet of the tricuspid valve.
Type A: the anterior valve leaflet is enlarged and moves freely. The septal and posterior leaflets are moderately displaced towards the right ventricle. The lower part of the right ventricle is small, its wall is thinned, and contractility is normal. The trabecular part of the right ventricle is developed normally. The cavity of the right ventricle is of normal size.
Type B: the anterior valve leaflet is thickened and significantly increased in size, its movement is free. Interchordal spaces are reduced in size. The posterior and septal leaflets of the valve are lowered deep into the right ventricle and attached to the myocardium. The lower (atrialized) part is larger than the ventricle itself, and therefore its contractility is significantly impaired.
Type C: the anterior valve leaflet is fixed to the anterior wall of the right ventricle, which leads to restriction of its movement. The interchordal spaces are significantly reduced in size. With this type of anomaly, a pronounced displacement of the posterior and septal valves is observed. The atrialized part is significantly enlarged, its wall is thin, and contractility is impaired. Right ventricle
Type D. The right ventricle is almost completely atrialized, surrounded by fibrous tissue of the anterior valve leaflet that has adhered to it. The interchordal spaces are completely obliterated. The posterior and septal valves are absent. The inflow part of the right ventricle is a non-contractile cavity where there is a small outflow tract.
Clinical manifestations of Ebstein's anomaly depend on the severity of anatomical defects and hemodynamic disorders. Most children born with this defect survive infancy. Patients with minor anatomical changes may not have clinical manifestations. The main clinical symptoms of this pathology are: shortness of breath, rapid fatigue with minor physical exertion, dizziness, diffuse cyanosis, swelling of the lower extremities, various rhythm disturbances (the most common are attacks of paroxysmal tachycardia, WPW syndrome).
Various diagnostic methods are used to identify Ebstein's anomaly. The ECG reveals signs of hypertrophy of the right atrium, deviation of the EOS to the right, various heart rhythm disturbances: extrasystoles, paroxysmal tachycardia, atrial fibrillation. When performing phonocardiography, a weakening of the first tone is determined at the left edge of the sternum in the 3rd and 4th intercostal spaces. In the second intercostal space at the left edge of the sternum, a weakening of the second tone is noted. In the fourth to fifth intercostal spaces on the left, a systolic murmur is recorded, caused by tricuspid valve insufficiency. Echocardiography reveals the main anatomical defects corresponding to Ebstein's anomaly. Doppler echocardiography reveals signs of tricuspid valve insufficiency. An X-ray of the OGK reveals signs of hypertrophy of the right atrium and upper parts of the right ventricle. When conducting angiocardiography, intense contrast is detected in the dilated right atrium and the atrialized part of the right ventricle. The distal (terminal) part of the right ventricle is contrasted weaker and slower, and a decrease in its diameter is determined.
Both conservative and surgical treatment methods are used to treat Ebstein's anomaly. Drug therapy is usually used to treat arrhythmias and to reduce symptoms of heart failure. Anticoagulant therapy is carried out for patients with atrial fibrillation and attacks of paroxysmal tachycardia. For patients with severe heart failure, therapy with cardiac glycosides and diuretics is carried out. In case of severe rhythm disturbances, a pacemaker or cardioverter is implanted. Surgical treatment: patients under 15 years old undergo valve plastic surgery, patients over 15 years old undergo tricuspid valve replacement. The operation is performed on an open heart, under the conditions of a heart-lung machine. The mortality rate for these surgical interventions ranges from 10 to 50% and depends on the severity of the defect and its complications. Restoration of patients' ability to work is possible within a year.
Pulmonary atresia with intact interventricular septum
With this defect, there is no communication between the trunk of the pulmonary artery and the right ventricle; survival is possible in the presence of a PDA or ASD.
EchoCG criteria
One-dimensional echocardiography:
- Dilatation of the aorta.
- Dilation of the left ventricle and left atrium.
- Decreased right ventricular outflow tract.
- Increased echo signal from the endocardium of the right ventricle.
- Inability to visualize the pulmonary valve.
- Dilatation of the right atrium (especially with concomitant tricuspid valve insufficiency).
- Hypertrophy of the interventricular septum.
- Decreased excursion of the tricuspid valve.
Two-dimensional echocardiography:
- Hypoplasia of the right ventricle.
- Hypoplasia of the right atrioventricular orifice.
- Hypoplasia or atresia of the pulmonary artery; pulmonary valve leaflets are not visualized.
- Atrial septal defect.
- Patent ductus arteriosus.
Doppler EchoCG:
- Determination of the gradient between the right and left atrium.
- Detection of patent ductus arteriosus.
Differential diagnosis:
- Pulmonary atresia with VSD.
- Right ventricular hypoplasia syndrome.
Ebstein's anomaly: cone reconstruction surgery - the first experience of anatomical correction
The most complete first coverage of the features of Ebstein’s anomaly in Russian literature was given by R.P. Zubarev in the book “Ebstein’s Anomaly” [1].
Currently, a large amount of material has been accumulated on the embryogenesis of this congenital heart defect (CHD). The mechanisms causing disruption of valve separation have not been established. However, there is evidence of the frequent occurrence of this defect with mutations of the NKX2.5, 10p13-p14, 1p34.3-p36.11 gene and in women who took benzodiazepines or drugs containing lithium in early pregnancy [2]. There are known familial cases of this defect [3]. We have two such patients under observation. In one case, these are identical twins, since the mother implanted one egg through in vitro fertilization, and in the other case, these are monochorionic twins. In one child, Ebstein's anomaly was combined with a ventricular and atrial septal defect and pulmonary artery atresia, and in another, with an atrial septal defect. The second children do not have congenital heart defects. The genetic material in these cases is identical, as is the nature of the influence of environmental factors.
The anatomical features of this pathology are described in detail by N.A. Belokon and V.P. Podzolkov (1990) [4]. It should be noted that this congenital defect has pronounced individual variability. The main anatomical feature of the defect is the displacement of the tricuspid valve into the cavity of the right ventricle towards the apex of the heart, usually to the junction of its inflow and trabecular parts [5, 6]. The degree of dysplasia, deformation of the valves and their structures varies widely:
- the process of delamination (separation) of the tissue of the tricuspid valve leaflets from the endocardium of the right ventricle is disrupted (Fig. 2);
- the posterior and septal leaflets are spread across the endocardium of the right ventricle, and the free parts of the leaflets are displaced apically from the valve ring;
- the chordal apparatus of the posterior and septal valves is shortened or absent;
- there is a shift in the anteropical direction of the level of cooptation of the tricuspid valve leaflets;
- the anterior leaflet of the tricuspid valve compensatory enlarges, thickens, and fenestrations often appear in it;
- the zone of the true tricuspid ring expands;
- dilatation of the atrialized part of the right ventricle occurs with thinning of the myocardium in it and replacement of the latter by fibrosis;
- the cavity of the right atrium increases significantly.
In 90% of patients with Ebstein's anomaly under observation, an atrial septal defect was detected. One patient had pulmonary atresia, and two patients had mitral valve regurgitation. Additional pathways for conducting electrical impulses from the atria to the ventricles were recorded in 28.5% of patients.
The natural history of patients with Ebstein's anomaly is unfavorable. According to a study by A. Yetman et al. (1997), who analyzed the survival of 46 newborn patients with Ebstein's anomaly and cyanosis, 70% of patients died. According to the cardiac surgery department of Children's Hospital No. 1 in St. Petersburg, one woman was diagnosed with frozen pregnancy of a fetus that was observed with a diagnosis of Ebstein's anomaly, and two children with Ebstein's anomaly died in the first month of life. A child with a severe form of Ebstein's anomaly was observed at the Samara Cardiac Dispensary. The child died suddenly at home at the age of 7 months.
In connection with the emergence of the possibility of anatomical correction of this heart defect using one’s own tissues at an early age, performing a cone reconstruction operation, it can be argued that a radical change has appeared in solving this problem. An important problem remains the ability to identify patients whose risk of sudden death exceeds the risk of undergoing surgical treatment at an early age.
Materials and research methods
This study was conducted based on the analysis of clinical material from 2014 to the present. The results of surgery for Ebstein's anomaly in three cardiac surgery centers were taken into account. The clinical characteristics of the patients are shown in Table.
results
In patients operated on before the age of 5 years, various complications were observed with a high frequency, including: reciprocal atrioventricular (28.5%) and non-reciprocal atrial rhythm disturbances (33.3%), the presence of concomitant heart defects and hemodynamic decompensation .
Among them was a patient aged 11 months. The child was admitted to the hospital for emergency reasons due to severe thrombocytopenic purpura. Chronic hypoxemia led to decompensation of DIC syndrome. During surgery, a flat, organized thrombus was identified and removed in this patient's right ventricular cavity.
Another patient was operated on for Ebstein's anomaly on the second day of life. The child's body weight was 2 kg 300 g. He was the second of twins. The first child is healthy. The operation was required for emergency reasons due to the presence of pulmonary atresia in the child and hemodynamics, depending on the functioning of the ductus arteriosus. The child underwent anatomical correction of Ebstein's anomaly under artificial circulation, the atrial septal defect and ventricular septal defect were closed, and the pulmonary artery was reconstructed with a venous valve-containing homograft. According to the literature, such a successful correction was performed for the first time in the world.
The third child had progressive pulmonary hypertension secondary to a large ventricular septal defect.
The fourth patient was 5 years old. His surgery was performed for urgent reasons due to acutely developed bradyarrhythmia (heart rate 36–48 beats per minute) 2 months after repeated radiofrequency ablation. Radiofrequency ablation was performed for accessory pathways leading to persistently recurrent atrioventricular reciprocal hemodynamically significant tachycardia. He developed complete atrioventricular block. During the operation, he was implanted with a permanent dual-chamber pacemaker, and after 2 months, according to Holter monitoring, he had correct sinus rhythm 86% of the time. The results are summarized in Fig. 1.
When analyzing the indications for surgical treatment of Ebstein's anomaly at an early age, the following main links of hemodynamic disturbances identified in all operated patients were identified:
- reduction of the right ventricular cavity due to a shift in the level of cooptation of the valves to the apex of the heart, leading to impaired diastolic function of the right ventricle;
- regurgitation on the tricuspid valve caused volume overload of the right heart and led to progressive dilatation of the atrialized part of the right ventricle and right atrium when observed over time;
- the atrialized part of the right ventricle ejects blood in a retrograde direction, increasing the volume overload of the right parts of the heart;
- tachyarrhythmias disrupted compensation mechanisms, causing acute cardiovascular failure.
These hemodynamic disturbances are interrelated, progress over time, and when rhythm disturbances occur, they are life-threatening. In this regard, the risk of delaying surgical treatment exceeds the risk of performing surgery in young children.
The progressive replacement of the muscle tissue of the right ventricle in the atrialized part with fibrosis plays a significant role in the pathogenesis of the disease. Based on the surgical material, morphological preparations presented in Fig. 1 were obtained. 2, 3 and 4.
This specimen shows pronounced thickening of the endocardium. Pronounced signs of fibrosis in the endomysium and sclerotic changes in the thinned myocardium.
All preparations show pronounced replacement of the myocardial connective tissue matrix.
A convincing example of morphological changes in the atrialized part of the right ventricle is an operational photograph that characterizes the native appearance of the atrialized wall of the right ventricle after separation of the posterior leaflet of the tricuspid valve. This is what the myocardium of the inflow tract of the right ventricle looked like in all patients (Fig. 5).
The wall of the atrialized part of the right ventricle after separation of the leaflets is a layer of epicardium and myocardium 2–3 mm thick. Its functionality is questionable. The risk of developing an aneurysm and the risk of circular electric currents occurring in it is quite high.
Summarizing the anatomical changes that cause hemodynamic disturbances and morphofunctional changes in the cavity of the right ventricle, we can conclude that patients with a severe form of Ebstein’s anomaly should be operated on in early childhood, can be operated on in adolescence, and not all adult patients with Ebstein’s anomaly will be able to undergo surgery to radically correct a heart defect.
Nineteen patients with Ebstein's anomaly underwent cone reconstruction.
In all patients, the operation was performed on a stopped heart. The average aortic cross-clamp time was 127 ± 34 minutes. Cardioplegia was performed with Custodiol solution. Cardioplegia was repeated after 90–110 minutes. Hypothermia during correction was 28–32 °C.
The operation diagram is shown in Fig. 6.
The purpose of this operation is to create a cone from the tissues of the tricuspid valve without pulling the leaflets to the fibrous ring. It is important that the mobility and windage of the valves be maintained, and that the papillary muscles of the notochordal apparatus be as close to each other as possible. In 7 patients, the marginal chords of the posterior and septal leaflets, and the two and anterior leaflets of the tricuspid valve were absent. The chordae were created after separating the leaflets by making cuts 1/4 of the length of the leaflets, from the edge of their attachment to the myocardium. The essential point of this operation was the movement of the posterior leaflet 180 degrees clockwise to the septal leaflet. Its mobility was ensured by cutting off the apical edge of the valve from the myocardium and re-fixing it to areas of the septal valve displaced to the apex of the heart or directly to the endocardium of the right ventricle. The need for excision of the atrialized part of the right ventricle or its plication remains a controversial issue. In this study, in 7 out of 19 patients, including a newborn child, the atrialized part of the myocardium in the intervascular zone was excised. It was in these patients that the best restoration of the shape of the inflow portion of the right ventricle was achieved. It should be noted that in 8 patients this was impossible due to the scattered type of coronary arteries. Plication of the atrialized part of the right ventricle was ensured by U-shaped sutures with large velor pads to prevent cutting of the sutures. This risk is due to thinning and impaired myocardial strength in the atrialized part of the right ventricle. All patients underwent narrowing of the annulus fibrosus. In two patients, narrowing of the annulus fibrosus was performed at the “3” and “10” o’clock levels. All patients required suturing of the holes in the valve leaflets to create a cone. Of great importance is the creation of support for the posterior valve thanks to additional fixing sutures between the posterior and septal valves, which provide the role of opening the “sail”.
In 16 patients, the tricuspid valve was reimplanted into the annulus fibrosus with a continuous enveloping suture. According to the presented material, a continuous suture in the area of the atrioventricular node was carried out with an “exit” to the right atrium. In one patient, a dual-chamber pacemaker with two epicardial electrodes was immediately implanted, since he was operated on urgently due to the development of complete atrioventricular block two months after repeated radiofrequency ablation. This patient had continuously recurrent atrioventricular reentrant tachycardia for 4 years.
In the early postoperative period, 16 patients required dopamine and 4 patients required a short course of 1–5 days of adrenaline.
One patient required surgery to create a bidirectional cavopulmonary anastomosis on the fourth day.
The follow-up time for patients after surgery ranged from 2 months to 3.5 years. On average 1.8 ± 0.74 years. All patients underwent ultrasound examination every 3–6 months. An important prognostic sign is effective reconstruction of the inflow tract of the right ventricle: suturing or resection of the atrialized part of the right ventricle. Bringing papillary muscles or chordae attachment sites closer together.
Three months after discharge, only 6 patients were receiving angiotensin-converting enzyme inhibitors. All patients maintained high exercise tolerance. According to dynamic monitoring of the electrocardiogram, no relapses of paroxysmal changes were detected in the group of operated patients.
It should be noted that the anatomy of Ebstein's anomaly is always individual. In connection with this, the decision on the nature of treatment is always made individually. The cone reconstruction operation successfully corrects anatomical disorders and is also always of a strictly individual nature. As experience and clinical material accumulate, this operation should become part of the main arsenal of pediatric and adult cardiac surgery departments.
conclusions
- Cone reconstruction is an operation that allows you to perform anatomical correction of congenital heart disease - Ebstein's anomaly at any age.
- If life-threatening complications occur, this operation can be performed at an early age, including newborns.
- Based on the results obtained, this operation can also be performed in older people, with mandatory consideration of the contractile function of the right and left ventricles.
Literature
- Zubarev R.P. Ebstein's anomaly. M.: Medicine, 1975. 112 p.
- Park JM Ebsten's anomaly of the tricuspid valve associated with prenatal exposure to lihium carbonate // Amer. J. Dis. Child. 1980. Vol. 134. No. 7. P. 703–704.
- Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK Ebstein's anomaly. Circulation. 2007. 115 (2): 277–285. doi:10.1161/ CIRCULATIONAHA.106.619338. PMID 17228014.
- Belokon N. A., Podzolkov V. P. Congenital heart defects. M.: Medicine, 1990. 352 p.
- Dearani JA, Bacha E., da Silva JP Cone Reconstruction of the Tricuspid Valve for Ebstein's Anomaly: Anatomic Repair.
- Reddin G, Poterucha JT, Dearani JA, Warnes CA et al. Cone Reconstruction of Atypical Ebstein Anomaly Associated with Right Ventricular Apical Hypoplasia // Tex Heart Inst J. 2021 Feb; 43(1):78–80. DOI: 10.14503/THIJ-15–5011.
V. A. Bolsunovsky*, 1, Candidate of Medical Sciences G. G. Khubulava**, Doctor of Medical Sciences, Professor, Academician of the Russian Academy of Sciences G. A. Novik*, Doctor of Medical Sciences, Professor M. V. Zhdanova*, Candidate of Medical Sciences R. R. Movsesyan***, Doctor of Medical Sciences, Professor, Corresponding Member of the Russian Academy of Sciences S. E. Shorokhov****, Doctor of Medical Sciences M. S. Khokhlunov**** A. V. Bolsunovsky*
* Federal State Budgetary Educational Institution of Higher Education St. Petersburg State Pediatric Medical University of the Ministry of Health of the Russian Federation, St. Petersburg ** Federal State Budgetary Educational Institution of Higher Education Military Medical Academy named after. S. M. Kirova Ministry of Defense of the Russian Federation, St. Petersburg *** St. Petersburg State Budgetary Healthcare Institution Children's City Hospital No. 1, St. Petersburg **** State Budgetary Healthcare Institution SOKKD, Samara
1 Contact information
Common truncus arteriosus
With this defect, one vessel departs from the base of the heart, which provides systemic, coronary and pulmonary blood supply.
There are 4 types of defect (classification by K. Collett, J Edwards):
1 type
- the pulmonary arteries arise from the posterior or lateral wall of the truncus with a short common trunk (Fig. 118),
Type 2
- pulmonary arteries arise separately from the posterior wall of the truncus,
Type 3
- pulmonary arteries arise from the lateral walls of the truncus separately, are hypoplastic,
4 type
- there are no pulmonary arteries, pulmonary circulation is carried out through the bronchial arteries.
Fig. 118.
Common truncus arteriosus type 1. Ventricular septal defect. Atrial septal defect. Left-sided aortic arch.
EchoCG criteria
One-dimensional echocardiography:
- A wide great vessel, the diameter of which is usually more than 4 cm (a distinctive feature from the tetralogy of Fallot).
- Lack of anterior continuation.
- Absence of posterior continuation (when the truncus predominantly originates from the right ventricle, when it originates from the left ventricle, the posterior continuation is preserved).
- Interruption of the signal from the interventricular septum (IVS defect).
- Truncus valve insufficiency (see aortic valve insufficiency).
- Dilatation of the left atrium.
- Inability to visualize the second (pulmonary) semilunar valve.
- Diastolic flutter of the anterior leaflet of the mitral valve (with truncus valve insufficiency).
Two-dimensional echocardiography:
- In cross section at the level of the great vessels, one large great vessel is visible.
- Visualization of a ventricular septal defect in a longitudinal section.
- Visualization of the origin of the pulmonary arteries from the truncus from the suprasternal approach.
Doppler EchoCG:
- Determination of the magnitude of regurgitant flow in case of truncus valve insufficiency.
- Turbulent blood flow through a ventricular septal defect with determination of the gradient and right ventricular pressure.
Differential diagnosis:
- Tetralogy of Fallot.
- Transposition of the great vessels.
Transposition of the great vessels
In transposition, the aorta is located to the right and in front of the pulmonary artery (D-transposition) or in front and to the left (L-transposition and communicates with the right ventricle; the pulmonary artery is located to the left and behind and communicates with the left ventricle. The connection between the pulmonary and systemic circulation is through the VSD , ASD, PDA or large bronchial arteries (Fig. 119).
Fig. 119.
Transposition of the great vessels (diagram).
Fig. 120.
Transposition of the great vessels in two-dimensional mode.
Fig. 121.
Transposition of the great vessels in one-dimensional mode.
EchoCG criteria
One-dimensional echocardiography:
- Simultaneous recording of two semilunar valves, with the pulmonary valve having a shorter ejection period.
- Dilatation of the right ventricle.
- Right ventricular hypertrophy.
- Increased excursion of the anterior leaflet of the tricuspid valve.
Two-dimensional echocardiography:
- Identification of the anterior and right (D-transposition) or anterior and left (L-transposition) aorta and posterior pulmonary artery. These vessels lie one below the other. The pulmonary artery lies posteriorly and its bifurcation can be seen.
- In the longitudinal section, a parallel orientation of the outflow tracts of both ventricles and both great vessels is visible, while the pulmonary artery does not bend around the aorta.
- The pulmonary artery arises from the left ventricle and forms the mitral-lunate continuation.
- The aorta arises from the right ventricle.
- Detection of associated intracardiac anomalies: large ventricular septal defect, patent ductus arteriosus, common atrioventricular canal, pulmonary stenosis or atresia, hypoplasia (atresia) of the atrioventricular valve.
Doppler EchoCG:
- Turbulent blood flow through a ventricular septal defect.
- Assessment of the degree of pulmonary blood flow (TMS with increased pulmonary blood flow, TMS with weakened pulmonary blood flow).
- Identification of concomitant congenital anomalies.
Corrected transposition of the great vessels
The defect is characterized by atrioventricular and ventricular-arterial discordance, while the blood flow has a physiological direction (Fig. 122).
Fig. 122.
Corrected transposition of the great vessels: Inversion of the ventricles, Transposition of the great vessels, Intact interventricular septum, Left-sided aortic arch (diagram).
EchoCG criteria
One-dimensional echocardiography:
- The posterior great vessel (pulmonary artery) passes into the anterior leaflet of the right-sided (mitral) valve.
- The anterior leaflet of the posterior (tricuspid) valve continues into the anterior great vessel (aorta) and has a large excursion.
- Simultaneous visualization of two atrioventricular valves.
- Paradoxical movement of the interventricular septum (in 60% of cases).
Fig. 123.
Corrected transposition of the great vessels: Left-sided valve - tricuspid.
Two-dimensional echocardiography:
- The anterior great vessel (aorta) arises from the anterior wall of the left ventricle and does not continue into the atrioventricular valve.
- Both main vessels run parallel and do not intersect.
- The right ventricle is located on the left and contains the tricuspid valve (Fig. 123).
- The left ventricle is on the right and contains the mitral valve.
- Detection of associated anomalies: VSD (70%), pulmonary stenosis, arterial (tricuspid) valve insufficiency.
Doppler EchoCG:
- Assessment of arterial (tricuspid) valve function.
- Identification of concomitant congenital anomalies.
Double origin of the great vessels from the right ventricle
With this defect, the pulmonary artery and aorta communicate with the right ventricle, and the VSD provides exit from the left ventricle. The defect is classified depending on the position of the interventricular defect and the presence or absence of pulmonary stenosis (Fig. 124).
I type DOS
with subaortic VSD
- A. without pulmonary stenosis
- B. with pulmonary artery stenosis
II type DOS
with subpulmonary VSD
- A. without pulmonary artery stenosis (Taussig-Bing anomaly)
- B. with pulmonary artery stenosis.
Fig. 124.
Double origin of the great vessels from the right ventricle, ventricular septal defect.
EchoCG criteria
One-dimensional echocardiography:
- Lack of anterior continuation.
- Lack of posterior continuation.
- Interruption of the echo signal from the interventricular septum.
- Right ventricular hypertrophy.
- Reduction of the left ventricular cavity.
Two-dimensional echocardiography:
- Visualization of two parallel great vessels arising from the right ventricle (the posterior vessel is the pulmonary artery).
- Determination of VSD localization: subaortic, subpulmonary, under both great vessels, distant from the great vessels.
- Confirmation of the presence or absence of pulmonary stenosis.
Doppler EchoCG:
- Determination of the systolic gradient between the left and right ventricles.
- Determination of the systolic gradient between the aorta and the right ventricle.
- Determination of the systolic gradient between the pulmonary artery and the right ventricle.
Treatment methods for Ebstein's anomaly in Germany
Ebstein's anomaly is one of the rarest congenital heart defects, in which there is an abnormal position of the tricuspid valve and its leaflets. They are located below normal. As a result, the leaflets do not close completely and tricuspid valve insufficiency occurs. The downward displacement of the valve leaflets leads to the fact that the volume of the right ventricle decreases, and the volume of the right atrium increases. Ebstein's anomaly is also usually combined with another congenital defect, a patent foramen ovale. An enlarged right atrium with a defect in the septum between the atria leads to oxygen-poor blood entering the left side of the heart. In addition, tricuspid valve insufficiency leads to overfilling of the right ventricle, which leads to its dilation. Over time, all this leads to the appearance of symptoms of heart failure.
Manifestations of Ebstein's anomaly occur in cases of moderate and severe defects. With a mild degree of Ebstein's anomaly, no symptoms may be noted. Symptoms of this heart defect include shortness of breath, especially with exertion, fatigue, heart flutters or arrhythmia, cyanosis - blueness of the lips and skin.
Ebstein's anomaly can also be combined with various types of heart rhythm disturbances - tachycardia, syncope, Wolff-Parkinson-White syndrome.
Diagnosis of Ebstein's anomaly in Germany is based on echocardiography, ECG, chest X-ray data, CT or MRI of the heart, electrophysiological studies and some other methods.
Treatment of Ebstein's anomaly depends on the degree of manifestation of this defect and the presence of concomitant cardiac arrhythmias. For arrhythmias, antiarrhythmic drugs may be prescribed. Drugs are also used to treat heart failure and prevent blood clots in the cavities of the heart. In some cases, infants may undergo an intervention to preserve the ductus arteriosus between the aorta and the pulmonary trunk to improve blood flow to the lungs.
The main treatment method for Ebstein's anomaly in German clinics is surgical. Surgical treatment is recommended in patients with moderate to severe symptoms of heart disease that affect the quality of life. Also, surgery can be prescribed for patients with mild manifestations of Ebstein's anomaly, when dilation of the cavities of the heart and disruption of its function are detected.
Currently, cardiac surgeons have several surgical methods for treating this rare heart defect.
Tricuspid valve reconstruction in Germany
During this operation, the size of the tricuspid valve opening is reduced and its insufficiency is eliminated. A synthetic band may be implanted around the valve to stabilize it. This intervention is usually performed when there is enough tissue to reconstruct the tricuspid valve.
Some surgeons are using a new method of surgical reconstruction of the tricuspid valve - cone reconstruction. In this process, the leaflets of the tricuspid valve are separated from the walls of the heart, rotated and sutured into place, resulting in a cone of leaflets.
Sometimes it may be necessary to reconstruct the tricuspid valve again or replace it.
Tricuspid valve replacement in German clinics
If reconstructive intervention is not possible, tricuspid valve replacement is performed in patients with Ebstein's anomaly. Currently, biosynthetic valve prostheses are most often used in cardiac surgery.
Closing a patent foramen ovale in Germany
As stated, a patent foramen ovale is the most common associated atrial septal defect in patients with Ebstein's anomaly. Closing of such a defect is usually carried out simultaneously with intervention on the tricuspid valve. In this case, the defect is closed with a synthetic “patch”.
Operation Maze in Germany
Such an intervention is carried out if Ebstein's anomaly is combined with cardiac arrhythmias, namely tachycardia. The essence of the operation is that the surgeon uses a scalpel to make small, shallow incisions on the wall of the atrium. As these cuts heal, they turn into scar tissue, which is not known to conduct electrical impulses. As a result of this procedure, it is possible to prevent severe forms of tachycardia, for example, ventricular tachycardia. Today, cryodestruction or radiofrequency ablation are also used for this procedure. In addition to the Maze procedure, electrophysiological studies with radiofrequency ablation of the area of the heart responsible for pathological impulses can also be used to treat rhythm disturbances in patients with Ebstein's anomaly.
Modern cardiac surgery centers in Germany annually attract thousands of patients, including from Russia, for the treatment of many heart diseases. Foreign specialists have extensive experience in the surgical correction of such a rare heart defect as Ebstein's anomaly, and the availability of first-class equipment and the active introduction of innovative methods of intervention allows such treatment to be carried out in accordance with international standards. Our online consultants can help you choose the most suitable clinic for you, or you can fill out the form on our website.
Double origin of the great vessels from the left ventricle
With this defect, the aorta and pulmonary artery are located side by side, completely departing from the left ventricle, the VSD is an outlet of the right ventricle.
EchoCG criteria
One-dimensional echocardiography:
- Lack of anterior continuation.
- Lack of posterior (mitral-aortic) continuation.
- Interruption of the echo signal from the interventricular septum.
Two-dimensional echocardiography:
- Parallel course of the great vessels.
- Communication of both great vessels with the cavity of the left ventricle.
- Large ventricular septal defect (usually in the subaortic region).
- Detection of associated abnormalities (usually pulmonary stenosis).
Doppler EchoCG:
- Determination of the systolic gradient between the left and right ventricles.
- Determination of the systolic gradient between the left ventricle and the pulmonary artery.
- Assessment of pulmonary hemodynamics.
Differential diagnosis:
Complete transposition of the great vessels with VSD and pulmonary stenosis.