© Author: Sazykina Oksana Yuryevna, cardiologist, especially for SosudInfo.ru (about the authors)
Analyzing a cardiogram is not always an easy task even for experienced doctors. What can we say about novice doctors, because they need to decipher ECGs with such disorders that were sometimes mentioned in textbooks only in a few words.
However, the ECG signs of some diseases, and even more so their clinical manifestations, must be known to a doctor of any specialty, since if left untreated they can provoke the sudden death of the patient. Exactly such a disease is long QT syndrome.
What is the QT interval responsible for?
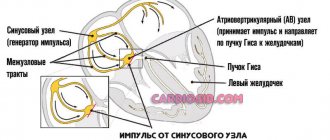
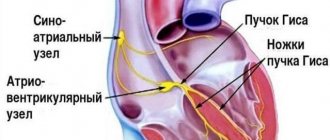
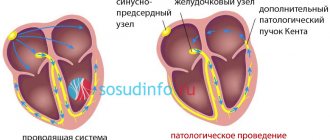
Each contraction of the atria and ventricles of the heart, providing a cardiac cycle, is reflected on the electrocardiogram. Thus, the P wave on the cardiogram reflects the contraction of the atria, and the QRST complex – the contraction of the ventricles. At the same time, the QT interval characterizes atrioventricular conduction, that is, the conduction of an electrical impulse through the connection between the atria and ventricles (through the AV node).
Thus, the QT interval on the ECG characterizes the conduction of an impulse along the Purkinje fibers in the wall of the ventricles, more precisely, the time during which electrical excitation of the myocardium ensures systole (contraction) of the ventricles.
Normally, the QT interval is no less than 0.36 seconds and no more than 0.44 seconds. Typically, students and doctors use this cheat sheet - on a regular ECG with a tape speed of 50 mm/sec, each small cell (1 mm of graph paper) corresponds to a time period of 0.02 seconds, and each large cell (including five small ones) corresponds to 0.1 second. In other words, the QT interval should normally be at least three and a half large cells and no more than four and a half large cells.
Due to the fact that the time of the QT interval depends on the heart rate, the determination of the corrected QT interval is used for a more accurate calculation. For patients with normal heart rate (from 60 to 100 per minute), the Bazett formula is used:
QTс = QT/ √RR,
For patients with bradycardia or tachycardia (heart rate less than 60 or more than 100 per minute, respectively), use the Frederick formula:
QTс = QT/ 3√RR, where RR is the distance between the R waves of two adjacent complexes.
Groups of drugs that can affect the length of the QT interval
Due to the fact that LQT syndrome can be caused by the direct effects of drugs, and their withdrawal often leads to normalization of all indicators, we will take a closer look at which drugs can change the length of the QT interval:
- antiarrhythmics (amiodarone, procainamide, sotalol, propafenone, disopyramide);
- antibiotics (erythromycin, spiramycin, clarithromycin, isoniazid);
- antihistamines (ebastine, astemizole);
- anesthetics;
- antimycotics (fluconazole, ketoconazole);
- antitumor drugs;
- psychotropic drugs (droperidol, amitriptyline);
- diuretics (indapamide), etc.
They should not be prescribed to persons who already have a prolongation of this interval. And with a late onset of the disease, their role as a provoking factor is necessarily excluded.
What are the differences between short and long QT and PQ intervals?
Terminology can sometimes be confusing for medical students and patients. To prevent this, it is necessary to clearly understand what the PQ interval is responsible for and what the QT interval is responsible for, and what the difference is between shortening and lengthening the interval. As already mentioned, analysis of the PQ interval is necessary to assess conduction between the atria and ventricles, and the QT interval is necessary to assess intraventricular conduction.
So, PQ prolongation can be differently regarded as atrioventricular block, that is, the longer the interval, the longer the impulse is conducted through the atrioventricular connection. With complete block, hemodynamics can be significantly impaired, accompanied by an extremely low heart rate (less than 20-30 per minute), as well as low cardiac output, insufficient to ensure blood flow to the brain.
Shortening the PQ interval (more details at the link) means reducing the time it takes for an impulse to pass through the atrioventricular junction - the shorter the interval, the faster the impulse passes, and in the normal rhythm of heart contractions there is a constant “reset” of impulses from the atria to the ventricles. More often, this phenomenon is characteristic of Clerk-Levy-Christesco syndrome (CLC syndrome) and Wolff-Parkinson-White syndrome (SVC syndrome). The latter syndromes are also fraught with the risk of developing paroxysmal ventricular tachycardia with a heart rate of more than 200 per minute.
The lengthening of the QT interval reflects an increase in the time of excitation through the ventricles, but such a delay in the impulse leads to the emergence of prerequisites for the formation of a re-entry mechanism (the mechanism of re-entry of the excitation wave), that is, for repeated circulation of the impulse in the same pathological focus. Such a focus of impulse circulation (hyper-impulse) can provoke a paroxysm of ventricular tachycardia.
QT shortening is characteristic of rapid conduction of the impulse through the ventricles, again with the occurrence of paroxysmal atrial fibrillation and ventricular tachycardia.
This syndrome (Short QTS) was first described in 2000, and its prevalence among the population is currently poorly understood.
Causes of Long QT Interval
The causes of this disease have now been studied quite well. There are two forms of long QT syndrome - caused by congenital and acquired factors.
The congenital form is a rare pathology (about 1 case per 10 thousand newborns) and, as a rule, is combined with congenital deafness. It is caused by genetic changes in the structure of genes encoding the corresponding proteins on the membranes of cardiomyocytes. In this regard, the permeability of the membrane changes, contributing to changes in cell contractility. As a result, electrical excitation is carried out more slowly than normal - repeated circulation of the impulse occurs in the source.
The genetically determined form of long QT syndrome, combined with congenital deaf-muteness, is called Jervell-Lange-Nielsen syndrome, and the form not accompanied by deaf-muteness is called Roman-Ward syndrome.
The acquired form of a prolonged QT interval may be due to the side effects of antiarrhythmic drugs used for the basic treatment of other rhythm disorders - atrial fibrillation, atrial flutter, etc. Typically, quinidine and sotalol (Sotalex, Sotahexal and other trade names) have an arrhythmogenic side effect. In addition to taking antiarrhythmics, a prolonged QT interval can occur with coronary heart disease, intracranial hemorrhage, alcohol poisoning, and also with myocarditis.
Acquired form
Previously, it was believed that the occurrence of acquired LQT syndrome is associated with a disruption in the functioning of ion channels, which is caused not by a mutation, but by the influence of some external or internal factors. This statement is true, but it has been proven that a genetic defect contributes to the development of the pathological process. At the same time, it is difficult to distinguish the acquired syndrome from congenital pathology, since they have much in common. Typically, this pathology goes undetected for a long time and manifests itself under unfavorable conditions, for example under stress or physical exertion. Factors that contribute to prolongation of the QT interval include:
- taking medications (we’ll look at which ones below);
- electrolyte disturbances (lack of potassium, sodium, magnesium);
- heart rhythm disturbances;
- cardiac ischemia;
- myocarditis;
- hypertrophic cardiomyopathy;
- diseases of the nervous system (trauma, infection, tumor);
- changes in hormonal status (diabetes mellitus, pathology of the thyroid gland or adrenal glands);
- alcoholism;
- fasting, etc.
Of particular danger is the exposure of a susceptible organism to several risk factors.
How does long QT syndrome manifest clinically?
Symptoms of the congenital form of the syndrome begin to appear in childhood. If a child was born deaf and mute, the doctor already has the right to suspect Jervell-Lange-Nielsen syndrome. If a child hears well and is able to make sounds (humming, speaking), but experiences episodes of loss of consciousness, you need to think about Roman-Ward syndrome. Loss of consciousness can occur during screaming, crying, stress or physical activity. Usually, fainting is accompanied by a rapid pulse (more than 150-200 per minute) and a feeling of rapid heartbeat - the heart flutters in the chest. Episodes of fainting can occur infrequently or up to several times a day.
As people get older, these symptoms persist if left untreated and can lead to sudden cardiac death.
Clinical manifestations of the acquired form are also characterized by fainting with tachycardia, and in the interictal period there is dizziness, general weakness and fatigue caused by sinus bradycardia (pulse less than 50 per minute).
Prolongation of the QT interval when using medications. Cardiotoxicity of drugs
The fact that drug antiarrhythmic therapy does not reduce overall mortality, but partially even leads to an increase in mortality, is due to the risk of a paradoxical increase in arrhythmias - that is, the proarrhythmic effect of Vaughan-Williams class I and III substances. Indicative results of the CAST study (Cardiac Arrhytmia Suppression Trial), in which, in a comparative assessment, it was strikingly discovered that more post-infarction patients died under the influence of the IC antiarrhythmics Flecainid and Encainid than with placebo, which confirmed the proarrhythmic potential of sodium channel blocking substances. But also antiarrhythmics acting through blockade of repolarizing potassium channels (class III) carry a risk of ventricular proarrhythmia. With these groups of substances, the prolongation of repolarization caused by early afterdepolarizations and Torsade-de-Pointes tachycardia (TdP) come to the fore. The SWORD (Survival With Oral d-Sotalol) study was stopped because more new arrhythmias and deaths occurred with d-Sotalol (a pure class III antiarrhythmic without additional beta-blocking activity) in patients with cardiac infarction than with placebo. Even antiarrhythmic therapy with amiodarone in post-infarction patients does not provide benefit compared with placebo in terms of all-cause and cardiac mortality. For some time, undesirable cardiovascular effects have also been described under certain circumstances of non-antiarrhythmic substances, which partially led to the withdrawal from the market by the manufacturer independently or by order of the government. We will discuss these adverse side effects of non-cardiac substances in more detail later.
QT interval
The time required for ventricular repolarization can be measured on the ECG as the QT interval. Prolonged repolarization is recognized by prolongation of the QT interval. Prolongation of the QT interval, on the one hand, can have an antiarrhythmic effect, and on the other hand, favor the onset of early post-repolarizations and is associated with the occurrence of TdP tachycardias, which either stop spontaneously or can lead to sudden cardiac death. Clearly prolongation of the QT time (or frequency corrected QT time (QRc)) is one of the main signs of TdP tachycardias. QT intervals from 350 to 440 ms (men <430 ms, women <450 ms) are normal, potentially causing values from 450 to 500 ms are considered concern, an increased risk of arrhythmias occurs from values of 500 ms. Along with congenital forms of QT prolongation (with or without deafness), acquired forms play an important clinical role. Along with QT prolongation, an additional increase in QT dispersion is described, a measure heterogeneity of repolarization.
QT prolongation by antiarrhythmics
QT prolongation and TdP tachycardia are typical side effects of various antiarrhythmics (Table 1). They occur partly in a dose-dependent manner and in the early phase of therapy. Predominantly, TdP tachycardias are observed only after conversion of sinus rhythm (during relative bradycardia), and not during atrial flutter. The frequency of such rhythm disturbances ranges from 1% to 8%. Coplen conducted a meta-analysis of a number of randomized trials of quinidine to achieve sinus rhythm after cardioversion of atrial flutter. Quinidine therapy was associated with higher mortality (2.9% vs 0.8% of controls). Some substances, such as amiodarone and Bepridil, even cause QT prolongation, but rarely TdP. Amiodarone is even used in patients who have developed TdP as a result of other drugs. This is due to the fact that amiodarone blocks not only K+ channels, but also Na+ and Ca++ channels, as well as beta-adrenergic receptors, and reduces the risk of early post-repolarizations and triggered arrhythmias.
| Table 1. QT prolongation after antiarrhythmics ( mod . Nach Thomas et al .) | |
| A drug | Mechanism of action |
| Class I.A. Chinidin, Disopyramid (Norpace, Rythmodul), Procainamid* | Na+ channel blockade Prolongation of repolarization |
| Class III N-Acetylprocainamid*, Amiodaron (Amiobeta, Amiodarex, Amiohexal, Cordarex, Tachydarin, etc.), Bretylium*, Sotalol (Darob, Sotabeta, Sotagamma, Sotalex, etc.) | K+ channel blockade Prolongation of repolarization |
| Class IV Bepridil*, Lidoflazin*, Prenylamin* | Calcium channel blockade |
| *No longer sold in Germany | |
Using the example of amiodarne, we can also draw attention to another problem. We are talking about the pharmacokinetic aspect. The half-elimination time for amiodarone is 15-100 days (average 30 days); for the active metabolites of desethylamiodarone, an average of 60 days. Since the Kumulations-steady-state is established after almost 5 half-life values, it is easy to imagine that such substances are very difficult to control. In 27 patients (55.4 + 2.4 years) receiving amiodarone for 1 year, initial QTc values were 453 + 7 ms. Between 9 and 12 months they quickly reached values of 479 + 9 ms. Patient monitoring should appropriately include blood levels and ECG analysis. The Drug Commission of the German Society of Physicians already pointed out quite early on the danger of QT prolongation with class I and III antiarrhythmics. Also, with regard to the fixed combination of Cordichin (160 mg Chinidin plus 80 mg Verapamil), the risk of developing TdP tachyarrhythmias and ventricular flutter was indicated.
QT prolongation with non-cardiac drugs
Along with class IA and class III antiarrhythmics, some other pharmacological drugs that are not considered antiarrhythmics can also lead to the development of QT prolongation and TdP tachycardias.
Withdrawals from the market In recent years, several drugs have been withdrawn from both the German and American markets due to severe adverse cardiovascular effects. Already in early 1998, the antihistamine Terfenadin (Teldane) was recalled in the United States. Astemizol followed in Germany and the USA in 1999, after the first indications of severe arrhythmias and cardiac arrest appeared - mainly in patients with severe liver dysfunction and/or while taking enzyme inhibitors. In a "Rote-Hand" letter (October 27, 1999), Glaxo Wellcome in Germany and the United States called attention to the withdrawal of Grepafloxacin after it - although very rarely - was associated with QT prolongation with a risk of severe arrhythmias (TdP). Also, the antipsychotic Sertindol was withdrawn from the German market due to the risk of severe adverse cardiovascular events (dose-dependent QT prolongation, sudden cardiac death). Sertindol has never been used in the United States. In April 2000, Janssen withdrew the prokinetic drug Cisaprid from the market after the FDA documented more than 340 reports of cardiac arrhythmias, including 80 deaths. After which the German authorities revoked the approval of cisapride-containing drugs due to severe side effects. Janssen-Cilag protested about this. In addition, other QT prolonging drugs have been described (Table 2), which have a wide variety of clinical implications. This often involved individual observations, sometimes probands or patients in clinical trials.
| Table 2. QT prolongation after “non-cardiac” drugs | |
| A drug | Notes |
| Antipsychotics/neuroleptics | |
| Chlorpromazin (Propaphenin)* | Case description (100 mg/d) |
| Haloperidol (Haldol, etc.)* | 4 mg orally to >100 mg iv (case report) |
| Primozid (Orap)* | Healthy probands (6 mg orally), TdP and fatal arrhythmias in patients |
| Quetiapin (Seroquel)* | Case description (comedication with the CYP3A4 inhibitor Lovastatin |
| Thioridazin (Melleril)* | Healthy probands (59 mg orally), overdose (500 mg) |
| Antidepressive drugs | |
| Desipramin (Pertofran, Petylyl)* | Case description (2.5 mg/kg/d) |
| Doxepin (Aponal, Doneurin, etc.)* | Clinical study patients (169 mg/d) |
| Nortriptylin (Nortrilen)* | Case description (0.51 mg/kg/d) |
| Amitriptylin (Amineurin, Saroten, etc.) | Clinical trial patients. (150-200 mg/d) |
| Fluoxetin (Fluctin, Fluxet, etc.) | Patients wedge. Research (37 mg/d) |
| Maprotilin (Deprilept, Ludiomil, etc.) | Case description (patient 69 years old, severe heart failure) |
| Antihistamines (2nd generation) | |
| Terfenadin (Histedin etc.)* | Healthy probands, patients with cardiovascular diseases (120-360 mg), Case description (combination with enzyme inhibitors), healthy probands (slow metabilizers) |
| Cetirizin (Alerid, Zyrtec) | Healthy probands (up to 60 mg/d) |
| Fexofenadin (Telfast) | Healthy probands, patients with allergic rhinitis (180-240 mg/d), description of a case with an attempt at reexposition |
| Loratadin )Lisino) | Healthy probands (10 mg/d in combination with erythromycin), case report of attempted suicide (300 mg) |
| Mizolastin (Mizollen, zolium) | Healthy probands (40 mg/d) |
| Antihistamines (1st generation) | |
| Chlorphenamine (Codicaps, Contac, etc.) | |
| Diphenhydramine (Emesan, etc.) | |
| Hydroxyzin (AN 3 N, Atarax, etc.) | |
| Promethazin (Atosil, Prothazin, etc.) | |
| Macrolide antibiotics | |
| Clarithromycin (Cylinid, Klacid, etc.)* | Case description (1000 mg/d orally) |
| Erythromycin* | Patients (500-1000 mg iv) Case Description (2000-4000 mg iv) |
| Spiramycin (Rovamycine, Selectomycin)* | Newborns (350,000 IE/kg/d orally |
| Gyrase inhibitors | |
| Levoflaxin (Tavanic)* | Case description (500 mg/d) |
| Moxiflocxacin (Avalox)* | Patients in a clinical study (400 mg/d) |
| Beta-2 adrenergic agonists | |
| Fenoterol (Berotec, Partsisten)* | Patients with mild asthma in a clinical study |
| Salbutamol (Apsomol, Sultanol, etc.) | Patients with mild asthma in a clinical study |
| Terbutalin (Bricanyl, Contimit, Terbul, etc.) | Patients with mild asthma in a clinical study |
| Antimalarial | |
| Chinin* | Patients (1800 mg/d iv), healthy probands, patients with hepatitis (10 mg/kg/iv) |
| Halofantrin (Halfan)* | Case description (1000 mg/d orally). Especially in women, high doses should be avoided. |
| A drug | Notes |
| Others | |
| Arsentrioxide | Patients in clinical trial (phase II), 0.15 mg/kg iv/d max 60 days |
| Cyclophosphamide (Endoxan, etc.)* | 5 out of 19 patients on high dose therapy |
| Ketoconazol (Nizoral, Terzolin)* | Healthy probands (400 mg/d orally) |
| Pentamidin (Pentacarinat)* | HIV-infected patients (4 mg/kg/d) Women in a clinical study of gynecological surgery |
| Tacrolimus (Prograf)* | Case description (5 mg iv daily, 0.25 mg/hour iv) |
| Tiaprid (Tiapridex) | Case description (300 mg/), 76 years old, additionally mild heart failure. |
| * We found the data to be particularly clinically significant | |
Antipsychotics In one very carefully conducted comparative study, it was found that patients with schizophrenia who received antipsychotic medications (Chlorpromazin, Thioridazin, Levomepromazin and Haloperidol) at a conventional dosage (n = 59) compared with patients not receiving antipsychotics (n = 5) and with healthy people (n=45), both QTc values and QTc dispersion increased. Ventricular tachycardias, however, were not observed in this study, possibly because other risk factors were absent. In a recent review, abnormal QTc prolongation (>456 ms) was particularly common in patients over 65 years of age receiving Droperidol or Thioridazine. Thioridazin and Mesoridazin (not commercially available in Germany) have been classified by the FDA and WHO as having a particularly increased risk. Droperidol intravenously has been primarily used for neuroleptanalgesia. Janssen-Cilag began producing it in 2001. Psychiatric emergency patients who received their psychotics parenterally and often experienced hypokalemia were particularly susceptible. Conversely, QTc prolongations caused by the atypical antipsychotics Risperidon, Quetiapine or Olanzapine were not significant. Even comedication with enzyme inhibitors, such as Ketoconarazol, Fluvoxamine or Paroxetin, did not have a negative effect.
Antidepressants Undesirable cardiovascular events have been described with various tricyclic antidepressants (Clomidin, Imipramin, Desipramin, Doxepin, Nortriptylin) not only with their overdoses, but in some cases also with the use of usual therapeutic doses. Reports of sudden cardiac death have been noted following Desipramin, Clomipramin, and Imipramin. A 69-year-old female patient with severe heart failure developed TdP tachycardia (QTc=700 ms) while taking Maprotilin (50 mg/d for several years). In this case, comorbidity definitely played a decisive role. There should be clear indications of the meaning of comorbidity of “cardiovascular disease.” In contrast, it appears that QT prolongation does not occur after Fluoxetin or after Amitriptylin at recommended dosages. Also, QT prolongation has not yet been described with the use of Citalopram.
Antihistamines One of the case-controlled studies determined the incidence rates (95% confidentiality interval) of ventricular arrhythmias per 10,000 person/years, for example, for Astemizol 8.5 (2.8-26.5), for Cetrizin 3.6 (0.9-14.2), for Loratadin 1.5 (0.2-10.3) and for Terfenadin 1.0 (0.3-3.0). Women appeared to be slightly more susceptible than men, and patients >50 years of age were clearly more affected than younger patients. This risk assessment of the predominantly non-sedating 2nd generation H1 antihistamines has also been shared by other authors. It is especially important to note the dose-dependence of these conditions, since it is with self-medication with antihistamines that the danger is especially great, since patients are “titrated” until the symptoms completely disappear. The cardiotoxicity of Astemizol appears to be played by its two main metabolites Desmethylastemozol and Norastemizol. The maternal substance is primarily responsible for cardiac incidents associated with Terfenadine. This is also supported by the fact that cardiotoxicity is enhanced by enzyme inhibitors, for example, macrolide antibiotics or antimycotics. In healthy men and women, it can be demonstrated that QTc values can positively correlate with blood levels of Terfenadine and Loratadine. Blood levels increase with additional administration of the antidepressant drug Nefazodon. The latter is an inhibitor of cytochrome P-450-3A (CYP3A). Currently, however, the lack of cardiotoxicity of Fexofenadine, a metabolite of Tefenadine, is questioned. In a 67-year-old man, the post-exposure and re-exposure QTc values to Fexofenadine (180 mg/d) were 532 ms. — 512 ms. The baseline values were however slightly prolonged (482-494 ms). In addition, data from animal experiments and individual clinical observations deserve attention that even classical sedating antihistamines, and, above all, Diphenhydramine and even Hydrozysin in high dosages can induce QT prolongation and abnormal ventricular repolarization. Arrhythmogenic features have also been described for Promethazin, Pheniramin and Chlorphenamine. It is possible that with increased attention, such incidents could be identified and classified more often.
Macrolide Antibiotics Between 1970 and 1996, 346 cases of cardiac arrhythmias associated with erythromycin were reported to the FDA (58% women, 32% men, 10% missing data). In 49 patients, life-threatening arrhythmias (ventricular tachycardias, TdP, ventricular flutter) and death were reported (33). Risk factors were primarily high dosages and intravenous administration. Erythromycin dose-dependently prolonged the duration of the action potential and decreased the maximum rise of the action potential in Purkinje fibers. These electrophysiological effects are very similar to those of Chinididn. For Claritromycin, there were two incidents of QT prolongation and TdP as early as 1998. In healthy probands, QT prolongation was significant only in combination with the prokineticum Cisaprid. In an animal experiment on rats, it was shown that Roxithromycin and Azithromycin were clearly less likely to provoke arrhythmias than erythromycin or clarithromycin. For this reason, Roxithromycin should be preferred in therapy.
Gyrase inhibitors Among the new fluoroquinolones, Glaxo Wellcome's Grepafloxacin was withdrawn from the market due to the development of TdP. There have also been reports regarding Sparfloxacin and Moxifloxacin. Zagam was no longer listed in the Roten Liste 2002. Also with regard to Moxifloxacin (Avalox), the manufacturer clearly indicates limitations of use and contraindications; Doses of 400 mg/d should not be exceeded. Comedication with other proarrhythmic drugs should not occur. Use is not recommended in patients with electrolyte disturbances and/or bradycardia. There are separate descriptions of cardiac arrhythmias with the use of Ofloxacin, Levofloxacin and Enoxacin. The approval for the use of Clinafloxicin was withdrawn by the manufacturers Gödecke (or Parke-Davis) due to significant side effects, including QT prolongation.
Beta-2 adrenergic agonists An epidemic of asthma deaths in Japan was reported in the 1960s in association with Isoprenalin forte. 10 years later the same phenomenon was noted in connection with Fenoterol (200 mg per aerosol burst) in New Zealand, in Sasktchewan (Canada) and in Japan. The mechanisms of this association are not well known. However, cardiovascular effects cannot be excluded. In a double-blind cross-over study, the effects of Fenoterol, Salbutamol and Terbutalin were compared with placebo on 8 patients with asthma. A pronounced dose-dependent prolongation of QT values was detected with the use of Fenoterol. There was a slightly smaller, but obvious, prolongation of QTc when using the highest doses of Salbutamol and Terbutalin. There was a decrease in plasma potassium levels in almost the same proportions. With restrained use of inhaled beta-agonists, such problems could be resolved in the future. The attitude of health officials towards this phenomenon varies from country to country. Fenoterol is not approved in the US.
Halofantin 21 healthy probands received 500 mg Halofantin daily for 42 days and were monitored for a further 138 days. The average half-life was 7 + 5 days. It was possible to demonstrate a clear concentration-dependent prolongation of QTc intervals.
Cyclophosphamide, Ketoconazol High doses (1400 mg/m2 for 4 days) Cyclophosphamide caused prolongation of QT-dispersion values (43.2-83.2 ms) in some patients; in this case, acute failure of the left heart then occurred. It is possible that these incidents mainly occur when additional anthracycline-related cardiac damage is at play. Also, Ketoconazol (200 mg 12 hours for 5 days), an antimycotic, caused small but significant prolongations of QTc values in healthy probands.
Vasodilatoren Also formerly used as vasodilators, substances now excluded from sale in Germany such as Lidoflazin, Prenylamin, Bepridil have a dose-dependent class-1A effect, which was of particular clinical significance in elderly patients and could cause TdP tachycardias.
Serotonin antagonists: Apparent QT prolongation and TdP tachycardia have also been described during treatment with the serotonin antagonists Ketanserin and Zimedin; and almost always in the presence of additional favorable factors (hypokalemia, bradycardia). Both substances are not sold in Germany. Zimedin was abandoned worldwide in 1983.
Risk factors for QT prolongation and TdP
Gender In general, women are at higher risk for QT prolongation and TdP than men (Table 3).
| Table 3 Congenital and acquired forms of altered QT | |
| Gender dependent Women have a greater risk of QT changes and the occurrence of Torsades-de-Pointes, clearly dependent on the menstrual cycle | |
| Congenital forms* Romano-Ward-Syndrome Jervell-Lange-Nielsen-Syndrome (with inner ear deafness) | |
| Acquired forms | |
| Electrolyte disturbances | Hypokalemia, hypomagnesemia, hypocalcemia |
| Metabolic disorders | Hypothyroidism, hyperparathyroidism, hyperaldosteronism, pheochromocytoma, diabetes (autonomic neuropathy) |
| Central nervous system disorders | Intracranial, subarachnoid hemorrhages, acute sinus thrombosis, encephalitis, head injuries |
| Cardiac disorders | Myocarditis, cardiac tumor, high degree AV block, sinus node dysfunction, clinically significant bradycardia (<50 el|vby/) |
| Eating disorders | Fasting, liquid protein diet |
| * Ion channel diseases with cardiac arrhythmias | |
Of the 346 erythromycin-related arrhythmias, 58% occurred in women and 32% in men (10% had missing data). This effect was confirmed in isolated rabbit hearts perfused with erythromycin. This effect has now been described again in relation to Chinidin. Among the participating probands, in any case, women already had higher baseline QTc values (407 = 7 ms) than men (395 + 9 ms), Chinidin-induced prolongations ranged from 42 + 3 ms to 29 + 3 ms. Using experimentally induced (antiarrhythmic Ibutilid 0.003 mg/kg iv 10 min.) QT prolongations in women, it was possible to show that the greatest changes were determined during the first half of the menstrual cycle (follicle maturation/proliferation phase).
Sudden death in childhood There are indications that prolongation of the QT interval in newborns at 1 week of life is clearly associated with sudden infant death syndrome. Routine ECG screening of newborns, however, is not yet recommended.
Electrolyte changes Electrolyte disturbances, whether induced by drugs (eg, diuretics), or in the form of concomitant diseases such as metabolic disorders, diseases of the central nervous system, heart and nutritional disorders, can favor the occurrence of TdP tachycardias. QTc prolongation secondary to pseudohypoparathyroidism-induced hypocalcemia was recently described in a 12-year-old girl. It should be recalled that hypokalemia can be caused by diuretics (Thiazid, Furosemid), Amphotericin B iv, corticosteroids and Laxanzien abuse. Hypomagnesiumemia known as “soft-water-factor”. Causes can be varied, such as geographical areas with “soft water”, phosphate-poor plant foods, modern cooking methods, phosphate-containing drinks such as cola, excessive sweating (sports, sauna), diseases and many medications.
Bradycardias Bradycardias favoring the onset of early afterdepolarizations can, among other things, be caused by cardiac glycosides or beta-receptor blockers. Also, in bradycardias enhanced by antiarrhythmics (sinus bradycardia or AV block) and after His bundle ablation in patients with pre-intervention tachycardial superconducting atrial flutter, TdP tachycardias are described.
Overdose of medications Since toxic side effects occur depending on the dose, overdose of medications is always associated with a special risk. The reasons for this are manifold: completely careless erroneous overdose by a doctor or patient, overdose of drugs as a result of underestimation when setting the dose of limited function of the kidneys, liver and/or thyroid gland. In old age, the often reduced volume of distribution plays a special role. It may also be important that for many substances there are slow and fast metabolizers. Poor metabolizers are most at risk. In relation to the Cytochrome P-450 isoenzyme, among people of the Caucasian race there are 5-8% of slow excretors. Drug interactions In the early 90s, it became apparent that terfenadine-containing drugs were contraindicated not only in patients with severe liver dysfunction, but also the concomitant use of other drugs, for example, Ketoconazol or the macrolide antibiotics erythromycin, Josamycin, Troleandomycin, which may be associated with a high risk of life-threatening ventricular arrhythmias. Subsequently, relevant findings were again described, for example, QTc prolongation in healthy probands when Cisaprid was combined with Clarithromycin was significantly more intense than when using either substance separately. Enzyme inhibitors include various macrolide antibiotics, primarily Erythromycin, Clarithromicin and Troleandomycin (and vice versa, not Rqxithromycin, Rulid), Chloramphenicol, Ciprofloxacin, Azol-Antmycotica, for example Fluvoxamin, Fluoxetin, HIV protease inhibitors, for example, Indinavir, Nelfinavir, Ritonavir , Saquinavir, an H2 receptor antagonist (but not Famotidine), and the HMG-CoA reductase inhibitor Lovastatin, which inhibits the CYP3A4 isoenzyme; here Pravastatin could be an alternative. There is increasing interest in the fact that grapefruit juice inhibits the metabolism of many substances metabolized by CYP3A4, such as Dihydropyridine calcium antagonists, Cyclosporin, Midazolam, Triazolam, Terfenadin and Amiodaron. Complications may also develop.
Conclusion If patients develop TdP during treatment, all suspected medications should be discontinued and all electrolyte abnormalities corrected. If there are no alternative medications, it is necessary to carry out a very careful individual dose selection, taking into account the comorbidity and comedication of patients. The relevant incident must be reported to the pharmacological commission of the German Society of Physicians or to the pharmaceutical industry.
Diagnosis of long QT
To clarify the diagnosis, a standard ECG is sufficient. Even in the absence of paroxysm of ventricular tachycardia, signs characteristic of the syndrome can be seen on the cardiogram. These include:
- Increase in the duration of the QT interval from the beginning of the Q wave to the end of the T wave.
- Very high heart rate (150-200 or more) with wide, deformed QRST complexes during paroxysm of ventricular tachycardia.
- Sinus bradycardia during the interictal period.
- Negative or flattened T waves, as well as ST segment depression.
Treatment of long QT syndrome
The treatment tactics for congenital forms of the disease involve the prescription of drug therapy, and if treatment fails, implantation of an artificial pacemaker (PAC).
Drug therapy consists of taking beta-blockers (metoprolol, bisoprolol, nebivalol, etc.) according to the age-specific dosage, which can prevent paroxysms of ventricular tachycardia. If there is resistance to the therapy, the patient is advised to install a stimulator with cardioversion and defibrillation functions. That is, the pacemaker detects the onset of ventricular tachycardia and, by electrically “rebooting” the heart, helps maintain a normal heart rhythm and adequate cardiac output.
The cardioverter-defibrillator requires annual examination by an arrhythmologist and cardiac surgeon, but in general it can remain operational for several years, perfectly preventing paroxysms of ventricular tachycardia. Thanks to a pacemaker, the risk of sudden cardiac death is minimized, and the patient, whether a child or an adult, can perform normal household activities without fear of losing consciousness or dying.
In the acquired form, it is quite sufficient to discontinue the antiarrhythmic drug taken with the correction of antiarrhythmic therapy with other drugs.
Complications and prognosis
Of the complications of this syndrome, of course, it should be noted sudden cardiac death caused by ventricular tachycardia, which turned into ventricular fibrillation followed by asystole (cardiac arrest).
According to studies, the prognosis of this syndrome without treatment is unfavorable, since long QT interval syndrome causes the development of sudden cardiac death in 30% of all cases. That is why this syndrome requires the close attention of cardiologists and arrhythmologists, since in the absence of effect from drug therapy, the only method that can prolong the life of a child with a congenital form of the syndrome is pacemaker implantation. When it is installed, the prognosis for life and health becomes favorable, since life expectancy reliably increases and its quality also improves.
Clinical manifestations
This disease is characterized by attacks of sudden loss of consciousness.
The clinical picture of the syndrome is characterized by polymorphism of symptoms. Their severity can vary from mild dizziness to loss of consciousness and sudden death. Sometimes the latter can act as the first sign of illness. The most typical manifestations of this pathology are:
- attacks of loss of consciousness;
- congenital deafness;
- cases of sudden death in the family;
- changes in the electrocardiogram (QT more than 450 ms, T wave alternans, ventricular tachycardia of the “pirouette” type, slow heart rate).
With congenital variants of the syndrome, other symptoms characteristic only of it may appear.
It should be noted that syncope with this pathology has its own characteristics:
- occur against a background of stress, under the influence of strong sound stimuli (alarm clock, phone call), physical activity, sports (swimming, diving), during a sharp awakening from a night's sleep, in women - after childbirth;
- the presence of symptoms preceding loss of consciousness (dizziness, severe weakness, ringing in the ears, darkening of the eyes, a feeling of palpitations, heaviness in the chest);
- rapid restoration of consciousness with a favorable outcome;
- absence of amnesia and personality changes (as with epilepsy).
Sometimes loss of consciousness may be accompanied by convulsions and involuntary urination. In such cases, differential diagnosis with epileptic seizures is carried out.
The course of the pathological process in each patient may have certain differences. It depends both on the genotype and on living conditions. The following options are considered the most common:
- syncope occurring against the background of prolongation of the QT interval;
- isolated prolongation of this interval;
- syncope in the absence of changes on the ECG;
- complete absence of symptoms (high risk of sudden death without phenotypic manifestations of the disease).
The most unfavorable course is complicated by the development of ventricular fibrillation and cardiac arrest.
With congenital variants of the disease, fainting appears in childhood (5-15 years). Moreover, their occurrence in preschool children is a prognostically unfavorable sign. And paroxysm of ventricular tachycardia, which requires emergency care, increases the likelihood of repeated cardiac arrest in the near future by 10 times.
Patients with asymptomatic long QT syndrome may be unaware of their diagnosis and have a normal life expectancy, but pass the mutation on to their children. This trend is observed very often.