© Author: Maria Petrovna Ivanova, operating obstetrician-gynecologist, especially for SosudInfo.ru (about the authors)
About 100 years ago, the diagnosis of “tetralogy of Fallot” sounded like a death sentence. The complexity of this defect, of course, allowed for the possibility of surgical treatment, but the operation was carried out for a long time only to alleviate the patient’s suffering, since it could not eliminate the cause of the disease. Medical science moved forward, the best minds, developing new methods, never ceased to hope that the disease could be dealt with. And they were not mistaken - thanks to the efforts of people who dedicated their lives to the fight against heart defects, it became possible to treat, prolong life and improve its quality even with such ailments as tetralogy of Fallot. Now new technologies in cardiac surgery make it possible to successfully correct the course of this pathology.
with the only condition that the operation will be performed in infancy or early childhood.
The very name of the disease says that its appearance is due not to one, but to four defects that determine a person’s condition: tetralogy of Fallot is a congenital heart defect that combines 4 anomalies:
- A defect in the septum between the ventricles of the heart, usually the membranous part of the septum is missing. The length of this defect is quite large.
- Increased volume of the right ventricle.
- Narrowing of the lumen of the pulmonary trunk.
- Displacement of the aorta to the right (dextroposition), to the point where it partially or even completely moves away from the right ventricle.
Basically, tetralogy of Fallot is associated with childhood, this is understandable: the disease is congenital, and life expectancy depends on the degree of heart failure, which is formed as a result of pathological changes. It is not a fact that a person can expect to live happily ever after - such “blue” people do not live to an old age, and, moreover, they often die during the infancy period if surgical intervention is postponed for some reason. In addition, the tetralogy of Fallot may be accompanied by a fifth anomaly of cardiac development, which turns it into the pentade of Fallot - atrial septal defect.
Circulatory disorders with tetralogy of Fallot
Tetralogy of Fallot refers to the so-called “blue” or cyanotic defects. A defect in the septum between the ventricles of the heart leads to a change in blood flow, resulting in blood entering the systemic circulation that does not bring enough oxygen to the tissues and they, in turn, begin to experience starvation.
Due to increasing hypoxia, the patient’s skin acquires a cyanotic (bluish) tint, which is why this defect is called “blue”. The situation with tetralogy of Fallot is aggravated by the presence of narrowing in the area of the pulmonary trunk. This leads to the fact that a sufficient volume of venous blood cannot escape through the narrowed opening of the pulmonary artery into the lungs, so a significant amount of it remains in the right ventricle and in the venous part of the systemic circulation (this is why patients turn blue). This mechanism of venous stagnation, in addition to reducing blood oxygenation in the lungs, contributes to the fairly rapid progression of CHF (chronic heart failure), which manifests itself:
cyanosis in tetralogy of Fallot
- Worsening cyanosis;
- Violation of metabolism in tissues;
- Accumulation of fluid in cavities;
- Presence of edema.
In order to prevent such a development of events, the patient is indicated for cardiac surgery (radical or palliative surgery).
Video: Tetralogy of Fallot – medical animation
Diagnostics
The diagnosis of tetralogy of Fallot is confirmed by clinical examination and physical examination. A variety of specialized tests may be performed to assist in diagnosis and treatment, including an electrocardiogram, echocardiogram, and cardiac catheterization. When tetralogy of Fallot is present, x-rays usually reveal a normal-sized heart that has a characteristic "wooden shoe" shape. Periodic measurement of blood oxygen saturation and hemoglobin is also recommended. Infants with this condition usually have a relatively loud murmur in the upper left side of the sternum.
Symptoms of the disease
Due to the fact that the disease manifests itself quite early, in the article we will focus on childhood, starting from birth. The main manifestations of tetralogy of Fallot are caused by an increase in CHF, although in such babies the development of acute heart failure (arrhythmia, shortness of breath, anxiety, breast refusal) cannot be excluded. The appearance of the child largely depends on the severity of the narrowing of the pulmonary trunk, as well as on the extent of the defect in the septum. The greater these disturbances, the faster the clinical picture develops. The appearance of the child largely depends on the severity of the narrowing of the pulmonary trunk, as well as on the extent of the defect in the septum. The greater these disturbances, the faster the clinical picture develops.
On average, the first manifestations begin at 4 weeks of a child’s life. Main symptoms:
- A bluish coloration of the skin of a child first appears when crying or sucking, then cyanosis can persist even at rest. At first, only the nasolabial triangle, fingertips, and ears appear blue (acrocyanosis), then, as hypoxia progresses, total cyanosis may develop.
- The child lags behind in physical development (later begins to hold his head, sit up, crawl).
- Thickening of the terminal phalanges of the fingers in the form of “drum sticks”.
- Nails become flattened and round.
- The chest is flattened, and in rare cases, a “heart hump” forms.
- Decreased muscle mass.
- Irregular growth of teeth (wide gaps between teeth), caries develops quickly.
- Spinal deformity (scoliosis).
- Flat feet develops.
- A characteristic feature is the appearance of cyanotic attacks, during which the child experiences:
- breathing becomes more frequent (up to 80 breaths per minute) and deeper;
- the skin becomes bluish-purple;
- pupils dilate sharply;
- shortness of breath appears;
- characterized by weakness, up to loss of consciousness as a result of the development of hypoxic coma;
- Muscle cramps may occur.
typical areas of cyanosis
Older children tend to squat during attacks, as this position makes their condition a little easier. On average, such an attack lasts from 20 seconds to 5 minutes. However, after it, children complain of severe weakness. In severe cases, such an attack can lead to a stroke or even death.
Choosing a clinic and cost of treatment
Treatment of tetralogy of Fallot requires the collaboration of cardiologists, cardiac surgeons, therapists and rehabilitation specialists. Large university clinics are leaders in the treatment of this heart defect:
- University Hospital Oldenburg, Department of Cardiac Surgery
- University Hospital Essen, Department of Cardiothoracic Surgery
- University Hospital Ulm, Department of Cardiothoracic Surgery
- University Clinic named after. Goethe Frankfurt am Main, Department of Cardiothoracic Surgery
- University Hospital Tübingen, Department of Adult and Pediatric Cardiothoracic Surgery
The estimated cost of examination and treatment is:
- Examination for suspected tetralogy of Fallot – from €485
- Radical surgical treatment of tetralogy of Fallot – from €14,104
- Cardiac rehabilitation after surgery – from €566 per day
The full cost of the medical program is determined based on the results of the examination, taking into account the duration of hospitalization, the presence of concomitant diseases and other factors.
Algorithm of actions when an attack occurs
- You need to help the child squat down, or take a “knee-elbow” position. This position helps reduce venous blood flow from the lower body to the heart, and therefore reduces the load on the heart muscle.
- Oxygen supply through an oxygen mask at a rate of 6-7 l/min.
- Intravenous administration of beta blockers (for example, Propranolol at 0.01 mg/kg body weight) eliminates tachycardia.
- The administration of opioid analgesics (“Morphine”) helps to reduce the sensitivity of the respiratory center to hypoxia and reduce the frequency of respiratory movements.
- If the attack does not stop within 30 minutes, emergency surgery may be required.
Important! During an attack, drugs that increase heart contractions (cardiotonics, cardiac glycosides) should not be used! The action of these drugs leads to an increase in the contractility of the right ventricle, which entails additional blood discharge through the defect in the septum. This means that venous blood, which contains practically no oxygen, enters the systemic circulation, which leads to an increase in hypoxia. This is how a “vicious circle” arises.
Associated disorders
Symptoms of the following conditions may be similar to those of tetralogy of Fallot. Comparisons can be useful for differential diagnosis:
- Atrial septal defect (ASD) is a common congenital heart defect characterized by a small hole between the two atria of the heart. These defects result in increased workload on the right side of the heart, as well as excessive blood flow to the lungs. Symptoms vary widely among sufferers and may appear in infancy, childhood, or adulthood, depending on the severity of the defect. At first, symptoms are usually mild and may include difficulty breathing (shortness of breath), increased susceptibility to respiratory infections, and an abnormal bluish discoloration of the skin and/or mucous membranes (cyanosis). Some people with an ASD may be at increased risk of developing blood clots that can travel to major arteries (embolism), blocking circulation.
- Ventricular septal defect (VSD) is a group of common congenital heart defects characterized by the absence of one ventricle. Infants with these defects may have 2 atria and 1 large ventricle. Symptoms of these conditions are similar to those of other congenital heart defects and may include abnormally fast breathing (tachypnea), blue skin color (cyanosis), wheezing, fast heartbeat (tachycardia), and/or an abnormally enlarged liver (hepatomegaly). A VSD can also cause excessive fluid to accumulate around the heart, leading to congestive heart failure.
- Atrioventricular canal defect (AVTD) is a rare heart defect that is present at birth (congenital) and is characterized by abnormal development of the septa and valves of the heart. Infants with the full form of the defect usually develop congestive heart failure. Excess fluid accumulates in other parts of the body, especially the lungs. Congestion in the lungs can lead to difficulty breathing (dyspnea). Other symptoms may include blue skin (cyanosis), poor feeding, rapid breathing (tachycardia) and heart rate (tachycardia), and/or excessive sweating (hyperhidrosis). Adults with DAVID may experience abnormally low blood pressure, irregular heartbeat, and/or rapid heartbeat.
- Triatrial heart is an extremely rare congenital heart defect characterized by the presence of an additional chamber above the left atrium of the heart. The pulmonary veins, returning blood from the lungs, drain into this additional “third atrium”. Symptoms of the disease vary greatly and depend on the size of the opening between the chambers. Symptoms may include abnormally rapid breathing (tachypnea), blue skin (cyanosis), wheezing, coughing, and/or abnormal accumulation of fluid in the lungs.
- Double origin of the great vessels from the right ventricle is an extremely rare congenital heart defect characterized by the absence of one ventricle. Infants with this defect have 2 atria and 1 large ventricle. Symptoms are similar to those of other congenital heart defects and may include difficulty breathing (shortness of breath), excessive fluid build-up in the lungs and around the heart (pulmonary edema), and/or blue discoloration of the skin and mucous membranes (cyanosis). Other symptoms may include poor feeding, abnormally rapid breathing (tachypnea), and/or rapid heartbeat (tachycardia).
- Mitral valve stenosis is a rare heart defect that can be present at birth (congenital) or acquired. It is characterized by abnormal narrowing of the mitral valve opening. In the congenital form, symptoms vary greatly and may include cough, difficulty breathing, rapid heartbeat and/or frequent respiratory infections. With acquired mitral valve stenosis, symptoms may also include weakness, abdominal discomfort, chest pain (angina) and/or intermittent loss of consciousness.
Based on what studies is the diagnosis of “tetralogy of Fallot” made?
- When listening to the heart, a weakening of the second sound is revealed; a rough, “scraping” noise is detected in the second intercostal space on the left.
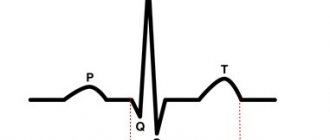
- Electrocardiography data can reveal ECG signs of enlargement of the right chambers of the heart, as well as a shift of the heart axis to the right.
- The most informative is an ultrasound of the heart, which can reveal a defect in the interventricular septum and displacement of the aorta.
Thanks to Doppler ultrasound, it is possible to study in detail the blood flow in the heart: the discharge of blood from the right ventricle to the left, as well as the difficulty of blood flow into the pulmonary trunk. ECG fragment with tetralogy of Fallot - X-rays reveal a “Dutch boot”-shaped outline of the heart, in which the apex of the heart is slightly elevated.
- In a blood test, red blood cells can be almost twice the permissible limit. This reaction of the body to hypoxia is compensatory. However, this may cause increased thrombosis.
Affected Populations
Tetralogy of Fallot is a rare congenital heart defect that occurs more often in men than women. Approximately 1 percent of newborns have congenital heart defects. About 10 percent of these babies are diagnosed with tetralogy of Fallot. This heart defect is usually detected several weeks or months after birth. The prevalence of the disorder is estimated at 1 in 3000 live births.
Children with chromosomal disorders such as Down syndrome often have tetralogy of Fallot and other congenital heart defects.
Treatment
If a patient has tetralogy of Fallot, it is important to remember one simple rule: surgery is indicated for all (without exception!) patients with this heart defect.
The main treatment method for this heart defect is surgery. The most optimal age for surgery is considered to be 3-5 months. It is best to perform surgery as planned.
There may be situations where emergency surgery may be required at an earlier age:
- Frequent attacks.
- The appearance of bluish skin, shortness of breath, increased heart rate at rest.
- Marked retardation of physical development.
Usually, a so-called palliative operation is performed as an emergency. During this time, an artificial shunt (connection) is not created between the aorta and the pulmonary trunk. This intervention allows the patient to temporarily gain strength before undergoing a complex, multicomponent and lengthy operation aimed at eliminating all defects in tetralogy of Fallot.
How is the operation performed?
Considering the combination of four anomalies in this heart defect, surgical intervention for this pathology is particularly difficult in cardiac surgery.
Progress of the operation:
- Under general anesthesia, a dissection of the chest along the anterior line is performed.
- After providing access to the heart, a heart-lung machine is connected.
- An incision is made into the heart muscle from the right ventricle so as not to touch the coronary arteries.
- From the cavity of the right ventricle, access to the pulmonary trunk is made, the narrowed opening is dissected, and the valves are repaired.
- The next step is to close the ventricular septal defect using synthetic hypoallergenic (Dacron) or biological (from the tissue of the heart sac - pericardium) material. This part of the operation is quite complex, since the anatomical defect of the septum is located close to the heart pacemaker.
- After successful completion of the previous stages, the wall of the right ventricle is sutured and blood circulation is restored.
This operation is performed exclusively in highly specialized cardiac surgery centers, where relevant experience has been accumulated in the management of such patients.
Advantages of heart surgery in German clinics
To objectively assess the success rates of cardiac surgery, the German Society of Thoracic and Cardiovascular Surgery has established a corresponding registry. It includes data on all heart operations performed in 78 German specialized centers and cardiac surgery departments. The aggregated data for 2021 shows a survival rate of 97.1%, which is quite impressive. Cardiac surgery in Germany is based on the following principles:
- High-quality preoperative examination to identify concomitant diseases. This is important for children with genetic disorders, since tetralogy of Fallot may be accompanied by DiGeorge syndrome or pulmonary pathology.
- Safe anesthesia . In children, general anesthesia should provide adequate sedation and analgesia with no withdrawal symptoms or hemodynamic changes. The optimal drug for the perioperative period is dexmedetomidine, other options include intravenous acetaminophen and propofol.
- Endovascular and minimally invasive interventions . Without a doubt, complex cardiac reconstruction is performed through an open surgical approach, with a clear view of the surgical field. Often two or three surgeons are involved in the operation. However, preliminary surgeries (such as Blalock-Taussig bypass) can be performed in a minimally invasive manner.
- Use of reliable vascular prostheses . In most cases, the patient's own tissue is sufficient to correct the structures of the heart. Otherwise, the surgeon uses certified synthetic prostheses.
- Aesthetic aspect . Regardless of the patient’s age, German surgeons strive to avoid the formation of large hypertrophic scars on the chest.
- Careful postoperative monitoring . In the early postoperative period, patients spend up to 24 hours in the intensive care unit. Thanks to this, medical personnel constantly monitor vital signs and promptly respond to their changes.
- Detailed recommendations regarding further observation, rehabilitation . After surgical treatment in Germany, postoperative rehabilitation and supportive drug treatment are always carried out. Cardiac rehabilitation with the participation of qualified medical professionals promotes rapid postoperative recovery and normal further development of the cardiovascular system in children. Regular medical examinations include laboratory (blood test, coagulogram, etc.) and instrumental (ECG, EchoCG, etc.) examinations, providing the doctor with objective information about the condition of the heart.
The updated annual registry of the German Society of Thoracic and Cardiovascular Surgery reflects current treatment success rates and serves as an independent benchmark for quality control of cardiac surgery. All medical institutions strive to achieve better results and improve their ratings.
Send a request for treatment
Possible complications and prognosis
The most common complications after surgery are:
- Preservation of narrowing of the pulmonary trunk (with insufficient dissection of the valve).
- When the fibers that conduct excitation in the heart muscle are injured, various arrhythmias may develop.
On average, postoperative mortality is up to 8-10%. But without surgical treatment, the life expectancy of children does not exceed 12-13 years. In 30% of cases, the death of a child occurs in infancy from heart failure, stroke, or increasing hypoxia.
However, with surgical treatment performed on children under 5 years of age, the vast majority of children (90%) upon re-examination at the age of 14 years do not reveal any signs of developmental lag from their peers.
Moreover, 80% of operated children lead a normal lifestyle, practically no different from their peers, except for restrictions on excessive physical activity. It has been proven that the earlier a radical operation is performed to eliminate this defect, the faster the child recovers and catches up with his peers in development.
Is registration of a disability group indicated for illness?
All patients before undergoing radical heart surgery, as well as 2 years after the operation, are required to register for disability, after which re-examination is carried out.
When determining the disability group, the following indicators are of great importance:
- Are there any circulatory problems after surgery?
- Does pulmonary artery stenosis persist?
- The effectiveness of surgical treatment and whether there are complications after surgery.
Recommendations for pulmonary valve replacement
American Heart Association Guidelines (2008)
Pulmonary valve replacement should be considered for severe pulmonary regurgitation and when clinical symptoms or decreased exercise capacity occur (Class I, Level of Evidence B).
Pulmonary valve replacement should be performed in the absence of symptoms in one of the following cases (Class IIa, Level of Evidence B/C):
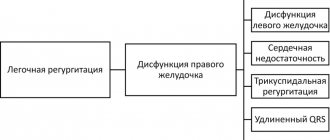
- moderate to severe right ventricular dysfunction.
- moderate to severe dilatation of the right ventricle.
- development of atrial or ventricular arrhythmia.
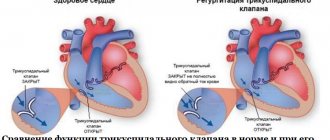
- moderate to severe tricuspid regurgitation.
Pulmonary valve replacement for right ventricular outflow tract obstruction should be performed in the following cases (Class IIa, Level of Evidence C):
— peak pressure gradient according to echocardiography >50 mm Hg.
- pressure in the right ventricle is more than 0.7 pressure in the left ventricle.
- progressive or severe dilatation of the right ventricle.
Canadian Heart Association Guidelines (2009)
Pulmonary regurgitation causing severe right ventricular dilatation (right ventricular ejection volume >170 ml/m2) (Class IIa, Level of Evidence C).
Moderate or severe right ventricular dysfunction (Class IIa, Level of Evidence C).
Tricuspid regurgitation, arrhythmias, or symptoms associated with decreased exercise intolerance (Class IIa, Level of Evidence C).
Residual pulmonary stenosis with a pressure of at least 2/3 of the systemic pressure (class IIa, level of evidence C).
European recommendations (2010)
Pulmonary valve replacement should be performed in symptomatic patients with severe pulmonary regurgitation or pulmonary stenosis (right ventricular systolic pressure >60 mmHg and tricuspid regurgitation velocity >3.5 m/s) (Class I, Level of Evidence C).
Pulmonary valve replacement should be considered in asymptomatic patients in one of the following cases (Class IIa, Level of Evidence C):
— objective decrease in tolerance to physical activity.
- progressive dilatation of the right ventricle.
- progressive systolic dysfunction of the right ventricle.
- progressive tricuspid regurgitation (moderate or severe).
— obstruction of the outflow tract of the right ventricle, systolic pressure in the right ventricle >80 mm Hg. and tricuspid regurgitation velocity >4.3 m/s).
- sustained atrial or ventricular arrhythmias
Surgery
The question of choosing the optimal conduit for reconstruction of the right ventricular outflow tract still remains open. Among the variety of conduits available, there is not a single conduit that meets the parameters of an ideal prosthesis. Being exposed to the aggressive influence of surrounding tissues, as a result of degenerative processes, the conduit eventually loses its original properties and its function is impaired [35].
Pulmonary homograft is the “gold standard” in reconstruction of the right ventricular outflow tract, but early calcification of the graft, high cost and limitations in the size of the conduit forced us to look for an alternative [36]. To solve this problem, a xenoconduit from the jugular vein of a bull, Contegra, was developed in 1999 and purchased in 2001. Over the past decade, the Contegra xenoconduit has been widely used by many surgeons around the world due to the availability of a range of sizes from 12 to 22 mm, relatively low cost, and low incidence of calcification [37]. T. Breymann et al. [38] reported a low reoperation rate in patients with Contegra compared with patients with implanted homografts after 4 years of follow-up. J. Brown et al. [40] described excellent early and mid-term results of right ventricular outflow tract reconstruction with a Contegra xenoconduit. The authors recommend using Contegra as the conduit of choice during operations to form the outflow tract from the right ventricle [37—39].
Despite encouraging initial results, many centers reported frequent distal xenoconduit stenoses. B. Meyns et al. [41] observed severe distal stenoses in 51% of patients with Contegra xenoconduit 2 years after surgery. V. Gober et al. [42] reported supravalvular stenoses requiring reintervention in 15.8% of Contegra patients with a mean follow-up of 18 months. In a meta-analysis [42] and a number of other works [43, 44], it was noted that the most common cause of conduit dysfunction developing during the first year after surgery is stenosis of the distal anastomosis caused by pseudointimal hyperplasia. This complication develops more often the younger the patient’s age and the smaller the diameter of the conduit [45]. In addition, in addition to conduit stenosis, some centers describe xenoconduit aneurysms, thrombosis, and endocarditis [26, 45].
Another type of conduit that is used in right ventricular outflow tract surgery is Dacron grafts with built-in biological valves. The main advantage of such conduits is a complete set of all sizes and a relatively low price. A. Corno et al. [46] believe that the disadvantages of such grafts are frequent stenosis due to the growth of pseudointima and calcification of the biological valve leaflets. In the early postoperative period, thrombosis may be observed in these conduits more often than in a homograft or Contegra, due to the structural features of the wall, so anticoagulant therapy should be selected more carefully in such patients. Despite these data, E. Belli et al. [47] note the absence of the need for reoperations when using Hancock up to 98% within 1 year and 81% within 5 years. The authors call the growth of pseudointima the main reason for the development of conduit stenosis. K. Vitanova et al. [36], comparing Hancock, Contegra and homografts, found that after 5 years there was no stenosis in 69.1±7.9% of patients for Hancock, 75.1±9.1% for Contegra and 85.4±5.6 % for homografts. Conduit valve failure was 91.7±4% for homografts, 74.6±9.1% for Contegra, and 86.9±7.4% for Hancock at 5 years. The authors cite the heterotopic position of the graft as the only risk factor for conduit dysfunction. In conclusion, the authors note that the terms of reprosthetics of Contegra, Hancock conduits and homografts are comparable.
Transcatheter interventions
Since its first procedure in 2000, percutaneous transcatheter pulmonary valve replacement has become widely accepted as a minimally invasive alternative for patients undergoing radical repair of tetralogy of Fallot [48]. Currently, the most used transcatheter valves have a number of disadvantages, such as stent fracture, limited size and the inability to install the system in the native outflow tract; this has served as an incentive for the production of new devices with a larger range of sizes and a more durable stent design. In table 2 shows the characteristics of valves that are being tested or are already available on the market.
Table 2. Characteristics of transcatheter pulmonary valves
Studies conducted after placement of transcatheter valves show good short- and medium-term results [7]. S. Virk et al. [49] showed in their study that after implantation of transcatheter valves, exercise tolerance increases and right ventricular function is restored, and the procedure is accompanied by a low rate of complications. There are currently no studies comparing transcatheter technologies and conduits for right ventricular outflow tract replacement; however, the functional state of the right ventricle, according to MRI, seems comparable [50, 51]. Despite the many positive qualities of transcatheter technologies, there is one important unresolved issue - infective endocarditis after valve placement. This was first noted in the review by J. Buber et al. [52], in which out of 155 patients, 5 were diagnosed with infective endocarditis. More recent studies have shown that the development of endocarditis with transcatheter intervention is 10-15% compared with open surgery, after which the incidence of infective endocarditis does not exceed 2% [53, 54]. The authors cite stent disposition, obstruction of the right ventricular outflow tract, and abrupt cessation of aspirin intake as the main risk factors [54].
Another unresolved issue in transcatheter surgery is the timing of valve implantation. Historically, the main goal of reintervention after radical repair of tetralogy of Fallot has been to avoid right ventricular dysfunction or dilatation (for late intervention) and the need for reoperation (for early intervention). Currently, the indications for endovascular pulmonary valve replacement are the same as for open surgery. However, some authors recommend performing the operation at an earlier stage, without waiting for the development of clinical symptoms. They install a valve of the maximum size acceptable for the patient, understanding that if it is dysfunctional, it is possible to avoid open surgery and implant a valve prosthesis using the transcatheter “valve-to-valve” technique [7, 54, 55].
Thus, modern methods and technologies of cardiac surgery provide a wide range of parameters for the prevention and correction of developed right ventricular dysfunction in patients after radical correction of tetralogy of Fallot. The rapid expansion and development of transcatheter surgery allows achieving satisfactory immediate and mid-term results comparable to those after repeated open interventions, exposing patients to less risk. At the same time, questions about clear criteria and timing for re-intervention remain controversial. Perhaps the wider introduction of MRI methods for cardiac diagnostics into everyday practice and careful monitoring of patients in the long-term postoperative period will make it possible to timely identify dysfunction of the right ventricle at an early stage and determine the exact scope of the necessary intervention.
Is it possible to diagnose tetralogy of Fallot in utero?
Diagnosis of this heart defect directly depends on the qualifications of the specialist performing ultrasound diagnostics during pregnancy, as well as on the level of the ultrasound machine.
When an expert-class ultrasound is performed by a high-category specialist, tetralogy of Fallot is detected in 95% of cases up to 22 weeks; in the third trimester of pregnancy, this defect is diagnosed in almost 100% of cases.
In addition, an important factor is genetic research, the so-called “genetic doubles and triples,” which are performed as screening on all pregnant women at 15-18 weeks. It has been proven that tetralogy of Fallot in 30% of cases is combined with other anomalies, most often chromosomal diseases (Down syndrome, Edwards syndrome, Patau syndrome, etc.).
Causes and risk factors
The exact cause of tetralogy of Fallot is not known. However, some studies suggest that the disease may be due to the interaction of several genetic and/or environmental factors (multifactorial). Therefore, researchers suspect that something may affect the genes of the developing fetus, causing this birth defect, but the exact nature of this trigger is unknown.
Some conditions that may increase the risk of having a baby with tetralogy of Fallot include:
- viral diseases;
- alcohol consumption;
- diabetes;
- poor nutrition;
- pregnancy over 40 years of age.
About 25 percent of infants with tetralogy of Fallot also have other birth defects not related to heart function or structure.
Complex heart surgeries abroad with Booking Health
Selecting a medical facility and cardiac surgeon can be a daunting task for someone without relevant experience or medical knowledge. Treatment of children requires special attention, since not all clinics have equipment for complex heart interventions in children. Most clinics of this level are located in Germany.
Certified medical tourism operator Booking Health offers patients assistance in choosing a medical facility and doctor, preparing for travel, performing surgery and organizing follow-up care. Booking Health provides the following services:
- Selecting a suitable clinic based on the annual qualification profile (cardiology, cardiac surgery, pediatrics)
- Preliminary communication with a cardiologist and surgeon
- Making an appointment or booking a room in the cardiology department
- Preliminary preparation of a treatment program (without repeating previously performed diagnostic examinations), explanation of its stages
- Ensuring favorable prices for treatment and related services, eliminating overpayments (savings up to 50%)
- Independent external control of all medical interventions
- Insurance against an increase in the cost of the treatment program in case of complications (200,000 euros, valid for 4 years)
- Assistance in purchasing and shipping medications
- Communication with the clinic and doctor after completion of treatment
- Control of invoices and return of unspent funds
- Organization of rehabilitation, follow-up examinations, maintenance therapy
- Services of the highest level: booking air tickets and hotels, organizing transfers, translation services
To schedule treatment for yourself or your child, leave a request on the Booking Health website. A patient manager or medical consultant will contact you as soon as possible to discuss all the details. The main goal of Booking Health is to help you improve and maintain your health.