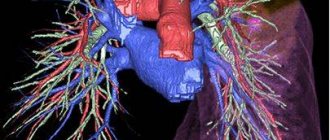
Pulmonary stenosis
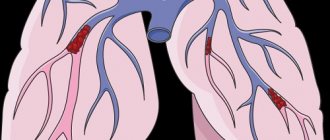
Pulmonary artery stenosis (narrowing) can be caused by isolated valvular, subvalvular or peripheral obstruction, and also occurs in combination with more severe congenital heart defects.
Pulmonary stenosis with an isolated valve occurs in 8-12% of cases of congenital heart defects in children. Subvalvular stenosis can result in a funnel-shaped appearance of the artery and is often found in children with a normal pulmonary valve. This condition may be caused by tetralogy of Fallot.
Peripheral narrowing of the pulmonary artery can lead to obstruction at the level of the main pulmonary artery, at the bifurcation, or in more distal branches. In some places, there is difficult patency, but stenosis can be caused by other congenital cardiac anomalies, for example, a ventricular septal defect or patent ductus arteriosus, etc.
Stenosis can be diagnosed in utero or shortly after birth. Critical narrowing of the pulmonary artery is considered potentially dangerous for newborns.
Ultrasound imaging of the heart during pregnancy may show isolated valve stenosis due to abnormal thickening or bicuspidity of the valve. Also, with the help of ultrasound, the doctor can detect stenosis caused by rubella infection during fetal development or accompanied by other congenital anomalies, such as tetralogy of Fallot or Noonan syndrome.
In infants, the condition is usually diagnosed by the presence of systolic murmurs while listening to the lungs with a phonendoscope.
Symptoms and diagnosis of pulmonary artery stenosis
Symptoms of stenosis depend on the severity of the pathology. The mild form may be asymptomatic; a more severe form of the pathology may cause the following symptoms:
- dyspnea
- chest pain
- fainting during exercise
- causeless fainting
Soft pulmonary systolic murmurs are heard when the child is lying down, but such murmurs can also be heard in healthy children and may be associated with physiological changes in the respiratory system.
Diagnosis of pulmonary artery stenosis may consist of the following procedures:
- echocardiography – allows you to confirm the presence of a valve defect;
- ECG - shows the presence of ventricular hypertrophy, right atrium and other abnormalities in the physiology of the heart;
- angiography allows you to confirm the diagnosis and is often prescribed to children with multiple cardiac anomalies;
- A chest x-ray allows the doctor to determine the enlargement of the right ventricle and atrium.
Treatment of pulmonary stenosis
Initial treatment of critical heart disease in a newborn includes general resuscitation and medications to dilate the ductus arteriosus. In the absence of pathological symptoms and the blood pressure in the right ventricle is less than 60 mm Hg. Art. The child is seen by a cardiologist every 1-2 years. These routine examinations include ECG, echocardiography and chest x-ray.
For infective endocarditis, surgical treatment is performed:
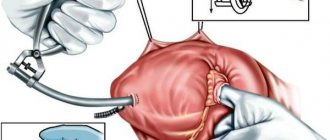
- in case of symptoms and blood pressure in the ventricle more than 60 mm Hg. Art. Cardiac catheterization is performed using valvotomy, which leads to the elimination of pulmonary valve insufficiency. If the procedure does not correct the problem, the pulmonary valve may need to be replaced;
- Percutaneous pulmonary valve implantation is an alternative to surgical valve correction and replacement of right ventricular dysfunction;
- Pulmonary artery balloon angioplasty, with or without placement of an expandable metal stent, can be used to treat the disease.
Introduction
Tetralogy of Fallot belongs to a group of common congenital heart defects; the frequency of this defect in newborns and infants is 5.6–14% of cases [1–3]. The natural course is unfavorable, so patients undergo surgical treatment in infancy. Despite the significant improvement in the immediate results of surgical treatment of tetralogy of Fallot, long-term results still cannot be called satisfactory [4, 5]. The main reason for the complicated course of the long-term period is dysfunction of the right ventricle due to long-term severe pulmonary regurgitation [6]. Currently, an adequate strategy for surgical treatment of right ventricular dysfunction is a current topic of discussion regarding congenital heart defects [7]. For many years, the only treatment for right ventricular dysfunction was to construct the right ventricular outflow tract with a xenograft or homograft, but with the development of endovascular surgery, transcatheter technologies have emerged as an alternative treatment option. This article reviews current problems and approaches to the treatment of right ventricular outflow tract dysfunction after radical correction of tetralogy of Fallot.
Data from EACTS, ECHSA and STS Congenital Database
In 2012, data from the European Congenital Database were published for the first time in EJCTS. The study included 6654 patients operated on for tetralogy of Fallot between 1999 and 2011. The study included 119 centers, 27 of which were outside Europe. In 57.5% of cases, transannular plastic surgery was performed, in 19.7% - radical correction without transannular plastic surgery, but with the use of ventriculotomy, in 18.2% - transatrial - transpulmonary correction without ventriculotomy. Thus, the rate of ventriculotomy for radical correction of tetralogy of Fallot was 77%, and overall hospital mortality was 2.58%. The study showed that after transannular plastic surgery the risk of complications in the early and late postoperative period is significantly higher than after using other types of reconstruction [8]. Similar data were obtained when analyzing the STS Database. The rate of ventriculotomy was 52% with an in-hospital mortality rate of 1.3% and a high rate of complications in the long-term period [9].
In conclusion of this analysis, the authors note the obvious uncertainty of the optimal and generally accepted strategy for surgical treatment of tetralogy of Fallot and the need for further modern research [9].
Modern strategy for surgical treatment of tetralogy of Fallot
The modern concept of surgical treatment of tetralogy of Fallot aims to minimize the neurological problems of the frequency of ventriculotomy and reoperations, the rate of perioperative mortality and the severity of the postoperative period [10]. In addition, in the long term, this concept should help to minimize the involvement primarily of pulmonary valve insufficiency and right ventricular dysfunction and arrhythmia.
Currently, this approach is becoming increasingly popular, and many large centers have adopted and are developing this strategy [11–13]. The advantages of this approach are the preservation of the intrinsic structures of the right ventricle, reduction of pulmonary regurgitation and right ventricular dysfunction [11]. As a rule, a Z score of the pulmonary ring of more than –3 allows almost all patients to undergo radical correction of this type without any significant problems [11, 12]. A fairly narrow retained ring may contribute to an increase in the residual gradient [11], but in some studies up to 30% of patients had an RV/LV ratio greater than 0.7 (0.7–0.9) without increasing mortality, and this had little effect on severity postoperative period [14]. Many studies have noted the fact of a decrease in dynamic obstruction of the outflow tract from the right ventricle after surgery, including due to the growth and development of the initially narrow valve ring of the pulmonary artery. There is also evidence that this approach does not affect the reoperation rate, which does not exceed 5% [11].
Modern studies assessing the strategy of preserving the native outflow tract from the right ventricle are rare, the immediate results are very optimistic, the long-term results are practically unstudied, therefore this area is the most relevant and promising for study in the surgical treatment of tetralogy of Fallot.
Pulmonary valve dysfunction
Surgical treatment of tetralogy of Fallot is aimed at closing the ventricular septal defect and eliminating obstruction of the right ventricular outflow tract. In the presence of severe hypoplasia of the pulmonary valve ring, massive infundibulectomy and transannular repair are required, which leads to the development of severe pulmonary regurgitation [15]. The latter leads to chronic volume overload of the right ventricle, leading to progressive dilatation and dysfunction of the right ventricle [6, 7, 15, 16]. Over time, patients have reduced exercise tolerance, develop atrial and ventricular arrhythmias, and also have a high risk of sudden cardiac death within 3–4 decades of life [17].
In the case of correction of tetralogy of Fallot with anomalies of the coronary arteries, the branches of which cross the outflow tract of the right ventricle, the only available option is conduit implantation. Over time, deformation and stenosis of the conduit occurs, which, due to pressure overload, leads to concentric hypertrophy of the right ventricle, increased diastolic pressure in its cavity and increased pressure in the right atrium [7]. Pressure overload and right ventricular hypertrophy are risk factors for poor outcome, including sustained ventricular tachycardia and sudden cardiac death in the long term after radical correction of tetralogy of Fallot [18].
Timing of pulmonary valve implantation
Currently, there are a large number of publications that note the connection between dilatation and dysfunction of the right ventricle in the long-term period with severe pulmonary regurgitation [6, 7, 15, 16, 19] (see figure). Particular attention is paid to the optimal timing of treatment of pulmonary regurgitation. Surgical treatment is indicated for patients with clinical symptoms, while relatively asymptomatic processes are not observed in generally accepted tactics. Proponents of early correction rely on the pathogenetic influence of residual regurgitation of systemic valves on the ventricles of the heart. Experience in studying the mechanism of action of chronic aortic insufficiency on the left ventricle shows that after a compensatory phase, which lasts for many years, a decompensation phase begins - irreversible changes in the myocardium develop, characterized by its dysfunction and even the formation of scars. Taking this into account, treatment of chronic pulmonary valve insufficiency should be carried out in the early stages, before the development of irreversible changes in the myocardium at the cellular level.
Outcomes of massive pulmonary regurgitation.
Right ventricular dysfunction
J. Therrien et al. [20] introduced the concept of “too late surgery” to restore or maintain normal contractility of the right ventricle, the function of which has deteriorated due to massive pulmonary regurgitation. These data were confirmed in a recent meta-analysis by Ferraz Cavalcanti et al. [21], who showed no increase in right ventricular ejection fraction after correction of pulmonary regurgitation. T. Geva et al. [22] also believe that a right ventricular ejection fraction of less than 45% is the main predictor of the development of permanent right ventricular dysfunction in the long-term postoperative period. However, there are studies that indicate an increase in ejection fraction after elimination of pulmonary regurgitation [23]. It has now been proven that right ventricular failure indirectly affects left ventricular function and the risk of sudden cardiac death. A large prospective multicenter study in which 873 patients underwent cardiac MRI to assess right ventricular function found that right ventricular dysfunction was a predictor of sudden cardiac death, sustained ventricular tachycardia combined with left ventricular dysfunction, atrial fibrillation, and right ventricular hypertrophy [ 18]. In this regard, surgical treatment should be carried out before the development of right ventricular dysfunction.
Right ventricular remodeling
One of the important topics in the last decade is the long-term preservation of right ventricular function and the identification of early predictors of right ventricular remodeling. With the development of MRI technology, it has become possible to determine the exact anatomy of the right ventricle and its function. MRI technologies have helped identify threshold values of right ventricular size at which correction of pulmonary regurgitation will lead to reverse remodeling of the right ventricle and restoration of its function. J. Therrien et al. proposed as threshold values the right ventricular end-diastolic volume index (EDV) of 170 ml/m2 or the end-systolic volume index (ESV) of 85 ml/m2, but later researchers proposed lower values of these indicators, leaning towards earlier surgical intervention (Table 1) [23-25]. Recently, greater attention has been paid to end-systolic volume as a more sensitive marker of preservation of right ventricular function and remodeling [26].
Table 1. Evolution of right ventricular size thresholds for correction of residual pulmonary regurgitation in tetralogy of Fallot
Indications for pulmonary valve replacement
In addition to the previously given parameters of the right ventricle - EDV, ESV and ejection fraction - you need to focus on the following factors: the ratio of EDV of the right ventricle should be 2 times higher than the EDV of the left ventricle, the ejection fraction of the left ventricle is less than 55%, aneurysm of the outflow tract from the right ventricle, QRS more than 160 m/s, stable tachyarrhythmia [30].
In case of pulmonary valve stenosis, the indication for surgical treatment is an increase in pressure in the right ventricle exceeding 2/3 of the systemic pressure [31]. Surgical treatment should begin with balloon dilatation of the valve and only if valvuloplasty fails, open surgery should be performed [31].
In case of combined pulmonary valve disease, indications for surgery are still a controversial topic. Most authors recommend performing surgery when symptoms of a dominant pulmonary valve lesion appear [31, 32]. However, Y. Kim and E. Ruckdesche [7[ advise using an individual approach to each patient, explaining this by earlier dysfunction of the right ventricle with combined lesions.
Pulmonary valve insufficiency
Pulmonary insufficiency occurs extremely rarely as a congenital anomaly. However, pathology often occurs as a complication after surgery or percutaneous angioplasty for pulmonary stenosis, as well as treatment for tetralogy of Fallot. Also, pulmonary valve insufficiency can develop as a result of a dilated pulmonary valve ring due to pulmonary hypertension or Marfan syndrome.
Significant pulmonary insufficiency can be caused by primary pulmonary hypertension, secondary pulmonary hypertension, infective endocarditis, rheumatic heart disease, carcinoid heart disease, Marfan syndrome and certain drugs, for example, methysergide, pergolide.
Pulmonary valve insufficiency is a serious pathology that is usually asymptomatic and is not accompanied by signs of right ventricular failure. With this pathology, a soft diastolic murmur is heard in the left upper region of the sternum and hypertrophy of the right ventricle is observed.
When diagnosing this pathology, the following studies are carried out:
- ECG;
- chest x-ray;
- color Doppler echocardiography;
- cardiac catheterization.
Treatment of pulmonary valve insufficiency
Pulmonary insufficiency usually does not require special treatment, but to monitor the pathology, it is necessary to conduct cardiograms every 1-3 years, depending on the severity and cause of the pathology. If symptoms or right ventricular dilatation are present, surgical pulmonary valve replacement is necessary. If severe right ventricular failure and pulmonary hypertension are detected, a heart transplant may be required.
Pulmonary insufficiency is usually well tolerated in childhood. Long-term studies have shown that pulmonary valve regurgitation can lead over time to progressive right ventricular dilatation, right ventricular dysfunction, exercise intolerance, gastric tachycardia, and, in extreme cases, sudden death.
Introductory part
Before heart surgery, a person has many questions. Some of them we ask the doctor, and some we cannot even formulate. When we understand what is happening to our body and what we can do to restore health, it is easier for us to endure all procedures.
Acquired heart valve defects
- these are diseases that are based on morphological and/or functional disorders of the valve apparatus (valve leaflets, annulus fibrosus, chordae, papillary muscles), developed as a result of acute or chronic diseases and injuries, disrupting the function of the valves and causing changes in intracardiac hemodynamics.
Valve defects can be congenital or acquired.
Congenital defects occur when the structures of the heart are formed incorrectly during intrauterine development; sometimes they do not make themselves felt until adulthood. Acquired defects arise due to rheumatism, infection, metabolic disorders (when calcium is deposited in the valves), trauma and other reasons.
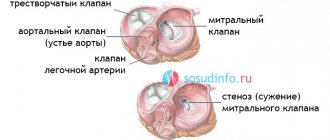
The main types of heart valve defects:
- mitral stenosis
- mitral valve insufficiency
- mitral valve prolapse
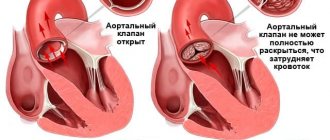
- aortic stenosis
- aortic valve insufficiency
- tricuspid stenosis
- tricupidal insufficiency
The normal functioning of the heart largely depends on the functioning of its valve apparatus.
Obstacles to the passage of blood cause overload, hypertrophy and expansion of the structures above the valve. Obstructed heart function disrupts the nutrition of the hypertrophied myocardium and leads to heart failure.
Etiology and pathogenesis
Etiology of stenosis
and combined rheumatic disease,
insufficiency
- usually rheumatic, rarely septic, atherosclerotic, traumatic, syphilitic.
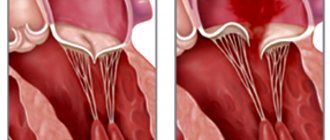
Stenosis is formed as a result of cicatricial fusion or cicatricial rigidity of the valve leaflets and subvalvular structures; valve insufficiency - due to their destruction, damage or scar deformation.
Failure
valve damage occurs due to destruction or damage to its valve flaps. Valve insufficiency is characterized by incomplete closure of the leaflets and occurs as a result of their wrinkling, shortening, perforation or expansion of the fibrous valve ring, deformation or separation of the chordae and papillary muscles. In some cases, valve insufficiency develops as a result of dysfunction of the valve apparatus, in particular the papillary muscles.
Often stenosis and insufficiency develop on one valve (the so-called combined defect
).
In addition, there are cases when the defects affect two or more valves - this is called combined
heart disease.
Affected valves form an obstacle to the passage of blood - anatomical in case of stenosis, dynamic in case of insufficiency. The latter is that although some of the blood passes through the hole, it returns back in the next phase of the cardiac cycle.
A “parasitic” volume is added to the effective volume, performing a pendulum-like movement on both sides of the affected valve. Significant valvular insufficiency is complicated by relative stenosis (due to increased blood volume). Obstruction to the passage of blood leads to overload, hypertrophy and expansion of the overlying chambers of the heart.
The expansion is more significant with valve insufficiency, when the overlying chamber is stretched by additional blood. With stenosis of the atrioventricular orifice, the filling of the underlying chamber is reduced (left ventricle with mitral stenosis, right ventricle with tricuspid stenosis); There is no hypertrophy or expansion of the ventricle.
With valve insufficiency, the filling of the corresponding ventricle is increased, the ventricle is dilated and hypertrophied. Difficulty in the functioning of the heart due to improper functioning of the valve and degeneration of the hypertrophied myocardium leads to the development of heart failure.
to the top of the page
Anatomy of the heart
A healthy heart is a strong, continuously working organ, about the size of a fist and weighing about half a kilogram.
In addition to maintaining steady, normal blood flow, it quickly adapts and adapts to the body's ever-changing needs.
For example, the heart pumps more blood when it is active and less when it is at rest. During the day, the heart produces an average of 60 to 90 beats per minute - 42 million beats per year!
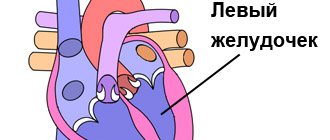
The heart is a two-way pump that circulates blood throughout the body. It consists of 4 chambers.
A muscular wall called the septum divides the heart into left and right halves. Each half has 2 chambers.
The upper chambers are called atria, the lower chambers are called ventricles. The right atrium receives all the blood returning from the upper and lower parts of the body.
Then, through the tricuspid valve, it sends it to the right ventricle, which in turn pumps blood through the pulmonary valve to the lungs.
In the lungs, the blood is enriched with oxygen and returns to the left atrium, which sends it through the mitral valve to the left ventricle.
The left ventricle pumps blood through the arteries through the aortic valve throughout the body, where it supplies the tissues with oxygen. Oxygen-depleted blood returns through the veins to the right atrium.
Four valves (tricuspid, pulmonary valve, mitral, aortic) act as a door between the chambers, opening in one direction.
These valves help blood move forward and prevent it from flowing in the opposite direction.
The petals of a healthy valve are thin, flexible tissue of perfect shape. They open and close when the heart contracts or relaxes.
Heart valves can become abnormal due to birth defects. They can become damaged or scarred due to rheumatic fever, infection, hereditary factors, age or heart attacks.
The mitral valves are most susceptible to such changes.
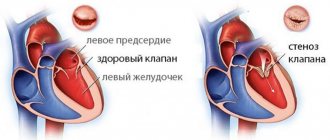
Regardless of the case, the heart valve can become stenotic (narrowed opening) or insufficient (does not close completely).
With valve stenosis, the heart must work harder to pump the required amount of blood through the narrowed opening.
Valve insufficiency causes blood to flow backwards through the valve after it closes. Once again, the heart has to work harder to pump enough blood for the body's needs to make up for the deficiency caused by the backflow of blood.
Both cases - stenosis and failure - force the heart to work harder to pump the required amount of blood. This extra work can weaken the heart, cause it to enlarge, and cause various diseases.
to the top of the page