Description
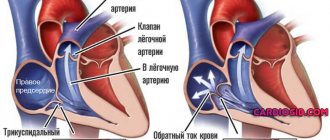
The main task of the tricuspid valve (TV) is to ensure one-way blood circulation in the right side of the heart.
Opening when the atria contract, it allows blood to flow into the right ventricle. By closing, the valve prevents blood from flowing into the atrium when the ventricles contract. What happens when TC is insufficient? The valve flaps do not close completely, allowing blood to flow in the opposite direction. This is called regurgitation, which causes the right atrium to become filled with blood and dilate. As a result, more blood flows from the RA into the right ventricle (RV) than normal, which also leads to expansion of the latter. In order to push an excessive amount of blood, the ventricle has to work non-stop, which is why its hypertrophy develops (thickening of the muscle layer). The situation worsens over time: the pancreas can no longer work at the desired pace and completely loses strength.
A condition called right ventricular heart failure occurs. Blood begins to stagnate wherever it can, especially in the lower extremities, kidneys, spleen, and liver.
Tricuspid valve insufficiency accounts for approximately 15-30% of all heart defects. I would also like to note that the isolated variant of TC deficiency is the exception rather than the rule. It is very rare and most often goes hand in hand with mitral heart defects.
To a minor extent, tricuspid regurgitation can also be observed in healthy people.
In advanced forms, the outcome may be disability and the following complications:
- atrial fibrillation;
- thrombosis and pulmonary embolism;
- cardiogenic cirrhosis of the liver due to prolonged venous stagnation of blood.
Heart valve defects and their treatment
Heart valve therapy includes several methods: 1) drug treatment; 2) valve reconstruction surgery; 3) valve replacement surgery; and 4) transcatheter valve replacement. Although drug therapy cannot eliminate valve dysfunction, it can relieve symptoms in many cases. If valve reconstruction or replacement becomes necessary, your cardiologist, surgeon, or interventional cardiologist will tell you about possible treatment options. The choice between replacement or reconstruction depends on a number of factors, including which valve is affected, the severity of the disease, and the presence of stenosis or regurgitation.
Your Cardiology Team
If you are undergoing heart valve reconstruction or replacement surgery, you will be cared for by a team of medical specialists who are committed to ensuring your safety and comfort before, during and after surgery. The following information describes the different health care providers you may see during your treatment.
Your general practitioner (GP) may be the first to detect symptoms of heart valve disease or a condition that may cause the valve to become damaged or malfunction. The therapist may prescribe special tests to confirm the diagnosis or refer you to an appropriate specialist.
A cardiologist is a doctor who specializes in heart disease. A cardiologist does not perform heart surgery, but usually performs diagnostic tests to determine the cause of heart problems and prescribe treatments to correct heart problems. Your cardiologist may prescribe medications and/or refer you to a cardiovascular surgeon.
A cardiovascular surgeon is a doctor who specializes in heart surgery, including reconstruction or replacement of diseased heart valves. The surgeon is the key decision maker in deciding the timing and best strategy, including the surgical technique and the choice of medical device for your valve case.
An interventional cardiologist (interventional cardiologist) is a doctor who has received additional specialized training to perform catheter-based procedures to treat heart disease. The interventional cardiologist works with the surgeon to make the right decision regarding the need for transcatheter aortic valve replacement.
An anesthesiologist is a doctor trained to administer sedation or general anesthesia (sleep) during surgery.
Intensive Care Unit Doctors and Nurses - In the Intensive Care Unit, the patient is closely monitored and maintained after most types of surgery. Together with your surgeon and/or cardiologist, intensive care unit doctors and nurses care for you during this time.
Approaches to surgical treatment of heart valve defects
Most heart surgeries are performed through an incision along the entire length of the chest, or breastbone. This incision is called a median sternotomy. Typically the healing process goes quite well, requiring about 6 weeks for the bone to fully recover.
In some patients, heart valve surgery can be performed using small, or minimally invasive, incisions. Smaller incisions offer patients many benefits. Preoperative studies, including coronary angiography, echocardiography, and, in many cases, a chest CT scan (computed axial tomography), help determine which patients may benefit from minimally invasive surgery. Surgical approaches that involve small incisions also involve the use of a heart-lung machine, as do sternotomy surgeries.
Transcatheter aortic valve replacement (TAVR): a less invasive alternative to open heart surgery
If a patient has been diagnosed with severe aortic stenosis but open-heart surgery carries moderate or high risk, another option is available: transcatheter aortic valve replacement (TAVR). This procedure is also called transcatheter aortic valve implantation (TAVI). This is a minimally invasive surgery that does not require open heart surgery.
| The transcatheter aortic valve replacement procedure allows you to install a new valve into your diseased aortic valve. The new valve moves the flaps of your diseased valve to the side. The frame is implanted into the leaflets of your diseased valve to ensure proper placement. |
This minimally invasive procedure is different from open heart surgery. In transcatheter aortic valve replacement, instead of cutting into the chest and completely removing the diseased valve, the valve is replaced using a catheter.
Transcatheter aortic valve replacement can be performed in several ways, but the most commonly used is transfemoral (through an incision in the thigh). Only the cardiology team can determine the best method, taking into account the person's health and other factors.
Valve replacement surgery
If a cardiovascular surgeon decides to replace a patient's heart valve, the first step is to remove the diseased valve (removing the valve and calcium deposits) and the second is to implant a prosthetic heart valve in its place. Prosthetic valves, used to replace diseased natural valves, are made from a variety of materials and sizes.
There are two broad categories of prosthetic heart valves that are used to replace diseased valves:
- Biological prostheses are made primarily from animal tissue, namely bovine (cow) pericardium (the strong sac surrounding the heart), pig aortic valve, or human valves taken from a postmortem donor.
- Mechanical valves are made of a synthetic material, predominantly carbon.
Bioprosthetic valves
There is a wide variety of bioprosthetic valves:
- Heterograft (or xenograft) is valve or pericardial tissue of animal origin suitable for use in medicine (for example, cattle (cows) or pigs).
- Homograft (or allograft) are human valves obtained from a posthumous donor.
- Autograft/self tissue is a healthy valve that has been moved from one location in the human body to another to replace a damaged valve (applies only to the pulmonary valve, which is used to replace the aortic valve).
A xenograft is a biological valve made from animal tissue. Thus, pericardial valve leaflets are usually made from bovine pericardium (the sac surrounding the heart) and sutured to a flexible or semi-rigid frame. In addition, biological valve prostheses are made from porcine valves. The valve is made from a porcine aortic valve and is usually sutured to a flexible or semi-rigid frame to create a “scaffold” valve. Otherwise, the natural root of the porcine aorta remains unchanged and acts as a framework in a “frameless” valve. Each flap is surrounded by a fabric ring (cuff) for sewing. Sutures are placed through the cuff to attach the valve to the heart.
| Biological valve prostheses |
Mechanical valves
Mechanical valve blades are made of a special type of carbon. Such valves usually have two leaflets. The leaflets open and close during the cardiac cycle, allowing blood to flow in one direction.
Criteria and selection of heart valves
The choice between a mechanical and biological valve prosthesis depends on an individual assessment of the benefits and risks of each valve, as well as the lifestyle, age and health status of each patient.
Bioprostheses do not require long-term use of drugs that suppress the natural ability of blood to clot (anticoagulants). This is important for those who cannot take anticoagulants due to a history of major bleeding (eg, gastrointestinal or genitourinary) or an increased risk of traumatic injury and bleeding associated with activity, sports, or advanced age. Bioprostheses usually last at least 10 years, and in some cases more than 30 years. If a patient under the age of 60 years is implanted with a bioprosthetic valve, there is a high probability that at some point the need for a replacement valve will arise, whereas most patients aged 70 years and older do not require this.
Mechanical valves are subject to little wear. However, they require daily use of anticoagulants, which may require dietary or lifestyle changes.
Often the decision to choose a biological or mechanical valve prosthesis is related to the patient's age, with older patients predominantly receiving bioprostheses. However, there is no consensus regarding the exact age at which a bioprosthesis may be preferable to a mechanical valve.
Valve reconstruction surgeries
If possible, it is preferable to reconstruct the patient's valve rather than replace it with a prosthesis. Typically, during valve reconstruction surgery, the surgeon adjusts the tissue or underlying structures of the mitral or tricuspid valves.
Almost all valve reconstruction procedures involve the installation of a ring or half-ring for annuloplasty. This is a tissue-covered device that is implanted around the circumference, or opening, of the mitral or tricuspid valve. It provides support to the patient's own valve and allows the leaflets to close more tightly, potentially reducing leakage through the valve. There are many different annuloplasty products available. The surgeon will choose the one that best suits your heart valve.
| Annuloplasty rings |
In addition to annuloplasty, mitral valve reconstruction often requires correction of problems with the leaflets or chordae, which attach the valve leaflets to the heart. If the leaks are due to mitral valve prolapse, fixation of the leaflets and chordae (and implantation of a ring or half-ring for annuloplasty) restores normal valve function.
Caring for the patient after heart valve surgery
The normal recovery period after standard heart valve surgery is four to eight weeks. Recovery may be faster if a small incision was made, such as in minimally invasive and transcatheter surgeries. During this time, patients gradually gain strength and resume normal daily activities. Regular examination by a cardiologist is of great importance. Please contact your health care provider by phone or in person if you have any questions or concerns about your health, especially if you experience any unusual symptoms or changes in your general health.
Nutrition and exercise
Two additional important aspects of recovery and overall wellness are a healthy diet and regular exercise. If your doctor has recommended a special diet, it is important to follow it. Healthy eating is an integral part of a healthy life. During the recovery period, eating nutrient-dense meals will provide your body with energy and can help speed up the healing process.
To improve the overall health of the cardiovascular system, it is recommended to combine a balanced diet with your doctor's recommendations for physical activity and weight control. Maintaining a regular exercise program is an important part of maintaining a healthy lifestyle. With the guidance of your doctor, you should gradually increase your physical activity and activity level. Before starting a new sporting activity, consult your doctor.
Anticoagulants - It is important to follow your doctor's directions for taking medications, especially if an anticoagulant has been prescribed. Anticoagulants, or anti-clotting drugs, suppress the blood's natural ability to clot. If you are prescribed anticoagulants, you will need periodic blood tests to evaluate your blood's ability to clot. The result of such a study will help your doctor determine the required dosage of the anticoagulant. Finding the correct dosage of the drug may take some time, but consistency and cooperation with your doctor are important. Sometimes it is possible to conduct the test yourself at home; ask your doctor about this. Check with your doctor about interactions with other drugs you are taking and about dietary restrictions while taking anticoagulants. Learn about all the signs that may indicate your dosage is too high.
Other Medical Information —Before having any dental procedures, including dental procedures, endoscopy, or surgery, tell your doctor if you have a prosthetic heart valve. Patients with a prosthetic valve are more susceptible to infections, which can lead to heart damage in the future. Therefore, you may need to take antibiotics before and after some medical procedures to reduce the risk of infection.
Also, when traveling for more than a few days, try to keep your diet and exercise levels as close to your normal as possible. Be sure to discuss all medications you take (including over-the-counter medications) with your doctor, and do not change the dosage unless specifically instructed to do so.
Possible reasons for the appearance
There are many reasons for the development of tricuspid insufficiency. The most common of them include:
- Rheumatism is an autoimmune inflammation that often affects the valves. It may occur 1-2 weeks after a sore throat (tonsillitis) caused by a special beta-hemolytic streptococcus. This is the most common cause not only of heart failure, but also of many other acquired heart defects in children.
- Infectious endocarditis is an acute pathology of the inner lining of the heart, which is sometimes called “injection drug addicts’ disease” due to the high risk of infection during unscrupulous injection. But it can also develop due to poor antiseptic treatment of the skin during an unprofessional procedure.
- Mitral valve (MV) defects - MV insufficiency or mitral orifice stenosis often occur as a consequence of tricuspid valve pathology.
- Diseases of the respiratory system - chronic obstructive pulmonary disease, bronchial asthma.
- Congenital heart defects - Ebstein's anomaly.
- Cardiac diseases - cardiomyopathies, myocardiosclerosis.
- Increased pulmonary artery pressure.
More rare causes of TC deficiency:
- Use of medications - some medications can have a destructive effect on the valves, for example, the migraine drug Methysergide or the diet pills Fenfluramine.
- Exposure to ionizing radiation —radiation therapy for the treatment of malignant tumors.
- Hereditary diseases manifested by a connective tissue defect are Ehlers-Danlos syndrome, Marfan syndrome, undifferentiated dysplasia.
- Carcinoid tumors. With neuroendocrine neoplasms located in the organs of the gastrointestinal tract, the tricuspid valve is often affected for unknown reasons.
- Rheumatic diseases - rheumatoid arthritis, systemic lupus erythematosus, systemic scleroderma, dermatomyositis.
- Whipple's disease is a very rare chronic intestinal infection that can be complicated by endocarditis.
The tricuspid valve (TV) and its pathology, compared with the aortic and mitral valves (MV), do not receive due attention from cardiologists and cardiac surgeons. As a result, there is a lack of research and scientific publications in this direction, including in our country. An accurate understanding of the mechanisms of development of functional insufficiency of the heart valve and the determination of treatment tactics for correcting the defect remain a completely unresolved problem in valvular heart surgery.
Functional insufficiency of the valve, as a rule, is understood as failure without structural changes in the elements of the valve against the background of dilatation and dysfunction of the right ventricle (RV), dysfunction of the left ventricle (LV), pulmonary hypertension, which occur with pathology of the valve, less often the aortic valve.
According to various authors [10, 22, 37], functional insufficiency of the ventricle occurs in 8–35% of those operated for MV or aortic valve defects.
As is known, tricuspid regurgitation (TR) serves as an independent prognostic factor for the progression of chronic heart failure and death after adequate correction of MV and/or aortic valve defects [11, 21, 25]. Thus, according to J. Nath et al. [47], the one-year survival rate for patients with grades II and III regurgitation was 78.9 and 63.9%, respectively, regardless of LV function, degree of pulmonary hypertension and age. At the same time, according to A. Calafiore et al. [16, 18], the 5-year survival rate of patients with grades II and III TR after isolated correction of mitral valve disease was 46%.
The etiological factor in the occurrence of functional tricuspid insufficiency (TN) is pathological processes causing defects of the left heart or pulmonary hypertension [10, 41, 51, 53, 55]. Most often these are rheumatic heart disease, ischemic mitral regurgitation, myxomatous degeneration, degenerative (atherosclerosis) valve disease, infective endocarditis of the left heart, dilated cardiomyopathy, pulmonary embolism. At the same time, a number of studies [59] do not find a reliable connection between the severity of MV defects and the severity of TN.
Most authors [9, 27, 51, 55] consider dilatation of the fibrous ring to be the main factor in the development of regurgitation. According to G. Dreyfus et al. [25], dilatation of the annulus fibrosus is a more objective indicator of the severity of TN than the degree of TR. One cannot but agree with this, since it is known that the degree of TR, determined by echocardiography, can vary depending on the pre- and afterload and contractility of the RV. It is believed that with a dilated ring, pronounced TR will manifest itself in one way or another over time in most cases. S.G. Sukhanov et al. [5] monitored the results of combined operations—MV annuloplasty and myocardial revascularization. They consider dilatation of the fibrous ring and LV ejection fraction to be prognostic factors for the occurrence of functional TN after these operations. L.A. Bokeria et al. [1], in addition, note a correlation between the diameter of the fibrous ring and the degree of TR.
In addition to, in fact, the expansion of the fibrous ring, there is evidence of a change in the spatial three-dimensional configuration of the ring towards its “flattening” [8]. T. Ton-Nu et al. [60] studied the three-dimensional configuration of the valve in individuals without TR and with valve regurgitation. In the group without regurgitation, the valve annulus has a clearly higher position of the anteroposterior segments (relative to the mediolateral ones) and an ellipsoidal shape. As functional TN develops, the valve ring “flattens” and takes on a more rounded shape. Similar data are provided by S. Fukuda et al. [29] on a smaller number of observations.
Other factors causing regurgitation include pancreatic dilatation and subsequent displacement of the papillary muscles, which leads to an increase in the annuloapical distance, excessive chordal tension and impaired coaptation of the leaflets. This condition in the English-language literature is referred to as “tethering” or “tenting” [9, 55]. In addition, according to some data, pancreatic dilatation increases the number of complications associated with valve defects [44].
M. Antunes et al. [10] described “restriction-dilatation syndrome” as one of the mechanisms for the development and aggravation of pancreatic dysfunction, and consequently TR. This mechanism represents a “vicious circle” when LV dysfunction through increased pressure and/or volume in the pulmonary circulation leads to RV dysfunction. This in turn exacerbates LV dysfunction through interventricular communication through the interventricular septum.
Another important element in the development of TN is pulmonary hypertension. M. De Bonis et al. [23] revealed a relationship between systolic pressure in the pulmonary artery and the degree of LV dysfunction. Similar data are provided by S. Fukuda et al. [28]. In another study by R. Ghanta et al. [33] found that patients whose pulmonary artery pressure after TC repair was 52 mmHg. and higher are more susceptible to relapse of TR. J. Nath et al. [47] noted that pulmonary artery pressure was more than 40 mmHg. aggravates TR and increases mortality.
According to G. Lin et al. [39], one of the reasons for the development of TN may be the implantation of endocardial electrodes for pacemakers and cardioverter-defibrillators.
Thus, understanding the mechanisms of development of TN helps in determining the main directions for surgical correction of the defect.
For a long time, under the influence of the publication of N. Braunwald et al. [15] it was believed that correction of TR after successful correction of left heart disease was not required and that TR regressed on its own. O. Yilmaz et al. [62] in their retrospective study showed that even grade III and higher TR regressed within 3 years of follow-up after isolated MV repair. On this basis, they believe that asymptomatic TR does not require mandatory correction.
However, there are reports that contradict this belief. According to A. Calafiore et al. [16, 18], the 5-year survival rate of patients with grades II and III TR after isolated correction of mitral valve disease was 45-46%, and with grade I or without regurgitation - 74.5-88.2%. It has also been shown that, despite a decrease in the degree of regurgitation in the early postoperative period in patients with uncorrected TN, it progresses in the long-term period. H. Song et al. [57] tracked the course of uncorrected stage I and II heart failure in 638 patients operated on for left heart defects. 7.7% of patients subsequently developed grade III or IV TR. K. Matsuyama et al. [41] analyzed the long-term results of correction of defects of the left side without interventions on the TC with regurgitation within the II degree. In 16% of patients, during a follow-up period of up to 8 years, TN progressed to grade III or higher. Preoperative grade II regurgitation, atrial fibrillation, and left atrial atriomegaly greater than 60 mm were identified as factors that worsen the course of TR in the long-term period.
A. Matsunaga et al. [40] presented the results of treatment of 124 patients with ischemic mitral regurgitation. At the same time, 30% of patients had severe TR and only 43% of them underwent annuloplasty. By the end of the observation, 49% of patients had regurgitation of at least grade III; in 74% of patients without regurgitation before surgery for more than 3 years, the appearance of regurgitation on the TV was noted. In patients with TR, both before and after surgery, a high ring diameter index/body surface area was detected.
There are different opinions when determining indications for correction of functional TN. Most surgeons have no doubt about the need to correct severe regurgitation of degrees III and IV [11, 17, 23, 49]. However, the issue of correcting grade I and II regurgitation remains unresolved. G. Dreyfus et al. [25], patients with a diameter of the valve annulus less than 7 cm, measured between the anteroseptal and anteroposterior commissures, underwent correction of only the mitral defect, and those with a diameter of 7 cm or more underwent correction of the mitral defect and annuloplasty of the valve. The degree of regurgitation before surgery in both groups was on average less than I. In the long-term period, aggravation of TR by more than two degrees was observed in 48% of patients who did not undergo annuloplasty, and only in 2% of those who received annuloplasty. As a result, they concluded that focusing on the diameter of the tricuspid annulus when determining indications for TN correction gives the best result.
N. Van de Veire et al. [61] concluded that performing annuloplasty in patients with dilation of the annulus fibrosus of more than 40 mm, according to echocardiography, without significant regurgitation, prevents further progression of TN and promotes reverse remodeling of the RV. In patients with grade I and II TR and dilatation of the annulus fibrosus, who did not undergo annuloplasty, progression of TR and pancreatic dilatation were observed. Other authors [56] also suggest performing TB annuloplasty in patients with moderate regurgitation, if there is dilatation of the annulus fibrosus of more than 40 mm or pulmonary hypertension. V. Chan et al. [21] indicate that grades III and IV TR are associated with increased mortality in the postoperative period. Performing annuloplasty of the heart failure prevents the progression of failure and improves the functional class of heart failure of patients, but does not affect mid-term survival. The presence of TR in patients with grade III and higher in combination with dilatation of the fibrous ring of more than 30 mm are factors that determine further progression of TR. V.A. Ivanov [3] also considers regurgitation of degree II and higher and dilation of the annulus fibrosus more than 40 mm (according to transesophageal echocardiography) as an indication for correction of functional insufficiency of the TC.
There is a certain difference in the guidelines for the correction of TC defects proposed by the American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC).
ACC/AHA approved indications for tricuspid repair include the following:
— MV repair is indicated for severe TR in patients with MV defect requiring surgery (level of evidence B);
— Trunk replacement or repair is appropriate in patients with primary severe symptomatic TR (level of evidence C);
— TV prosthetics are advisable in cases of severe TR and altered valves in cases of ineffectiveness of plastic surgery (level of evidence C);
— Trunk annuloplasty is possible in patients with less than grade IV TR during mitral valve surgery if there is pulmonary hypertension or dilatation of the valve annulus fibrosus (level of evidence: C);
— Trunk repair or replacement is not indicated for patients with asymptomatic isolated TR and systolic pressure in the pulmonary artery less than 60 mmHg. (level of evidence C);
— prosthetics or TC repair are not indicated for patients with primary TR less than grade III (level of evidence C).
According to the guidelines for the correction of tricuspid defects adopted by the ESC, intervention on the tricuspid is indicated in the following cases:
— patients with severe TR during correction of left heart defects;
— patients with severe primary TR with clinical manifestations that are not amenable to drug therapy even in the absence of ventricular dysfunction;
- patients with severe tricuspid stenosis (with or without TR) that is not amenable to drug therapy;
- patients with severe tricuspid stenosis (with or without TR) undergoing interventions on the left side of the heart;
— patients with moderate organic TR during correction of left heart defects;
— patients with secondary moderate TR and annular dilation of more than 40 mm during correction of left heart defects;
- patients with severe symptomatic TR with corrected defects of the left side, in the absence of myocardial dysfunction, valves, RV dysfunction and pulmonary hypertension;
— patients with severe isolated low-symptomatic TR with progressive dilatation of the pancreas and an increase in its dysfunction [43].
The most common methods of surgical correction of functional TN are support ring annuloplasty, proposed by A. Carpentier in 1971, and suture annuloplasty, proposed by NG De Vega in 1973, and their modifications [6, 19, 24].
I.I. Skopin et al. [4] identified 3 types of TC structure and put forward the position that the choice of the type of annuloplasty should be made taking into account the anatomical structure of the TC.
According to some data [14, 48, 49, 59], annuloplasty with a support ring provides a more durable effect in the long term and higher survival.
In particular, according to P. McCarthy et al.[42], 1 month after De Vega annuloplasty, patients with grade III and IV regurgitation accounted for 13.6%, and after 8 years - 33%. After plastic surgery with a support ring, these figures were 15.2 and 17%, respectively. Despite the low rate of reoperation (3% over 8 years of follow-up), the authors note high postoperative mortality after reoperation. Mortality was 8%, and 5- and 8-year survival rates were 65 and 50%, respectively. F. Filsoufi et al. [27] reported good short-term results after using support rings in a three-dimensional configuration and did not observe a significant increase in the degree of regurgitation.
G. Gatti et al. [31, 32] presented results using an elastic polytetrafluoroethylene strip for annuloplasty. Mortality was 5.7%, 4-year actuarial survival was 91.7%. In the mid-term period, 89.8% of patients had TR less than grade II and satisfactory functional status. A. Calafiore et al. [17] proposed a method of annuloplasty with a standard 50-mm strip without taking into account body surface area and reported satisfactory short-term results. After such plastic surgery, the diameter of the tricuspid foramen approached the N25 meter.
J. Bernal et al. [11] used De Vega suture annuloplasty and its modifications to correct functional TN. Mortality was 8.1%, survival at 6.8 years was 23.3%. The actuarial 12-year survival rate was 50.5%. Positive dynamics of echocardiographic parameters were noted in the form of a decrease in the size of the pancreas and a decrease in pressure in the pulmonary artery. During the observation period, TR was absent or grade I in 50.6% of patients, grade II in 44.8%. The degree of residual TR did not affect mortality and the rate of re-operation on the valve in the long-term period. Preoperative severe TR was associated with a higher rate of reoperation. Reoperation was required in 3.1% of patients with functional TN. The mortality rate after reoperation was 18.5%. A. Calafiore et al. [18] when performing annuloplasty according to De Vega, they reported a 5-year survival rate of 74.5% and progression of TR to grades III, IV in 5% of patients; while the mortality rate was 5.5%. S.S. Dobrotin et al. [2] also note satisfactory mid-term results after De Vega annuloplasty. A. Sarraj et al. [54] reported their use of adjustable segmental TK annuloplasty, very similar to the technique proposed by N.M. Amosov in 1972, with satisfactory mid-term results.
R. Ghanta et al. [33] did not reveal statistically significant differences in mid-term results between suture bicuspidalization and ring annuloplasty; both methods, according to the authors, give a good, lasting effect.
O. Alfieri et al. [7] in 2002 presented the “clover technique” they used to correct post-traumatic TR. Subsequently, E. Castedo et al. [20] described a similar technique in patients with recurrent TN, calling it the edge-to-edge technique. Y. Lai et al. [38] used this technique as an additional procedure in cases of inadequate correction of insufficiency with ring annuloplasty and Key repair and obtained satisfactory results. M. De Bonis et al. [23] also presented results using the “clover technique”. They used it in addition to annuloplasty for prolapse of the valves and for their tension, in 97% of cases due to displacement of the papillary muscles, in 3% in isolation. In the mid-term period, regurgitation was absent or corresponded to grade I in 87.7% of patients; there were no manifestations of TC stenosis.
G. Dreyfus et al. [26] for adequate correction of severe TR in patients with impaired coaptation due to displacement of the papillary muscles, proposed enlarging the anterior leaflet with a patch from the autopericardium, followed by implantation of a support ring. This intervention was performed in patients with a coaptation depth of more than 8 mm. During follow-up periods from 6 to 20 months, residual regurgitation was no more than grade I. F. Roshanali et al. [52] compared groups of patients with severe TR who underwent annuloplasty and annuloplasty in combination with leaflet augmentation using an autopericardial patch if the coaptation depth was greater than 8 mm or the coaptation depth area was greater than 16 mm2. After one year, residual regurgitation in the groups that underwent De Vega annuloplasty and ring annuloplasty was 28 and 14%, respectively, and in the groups with De Vega plastic surgery combined with leaflet augmentation and ring annuloplasty and leaflet augmentation - 10 and 8%, respectively.
U. Kappert et al. [35] proposed a different approach for correcting TN due to severe dilatation of the pancreas - resection of the free wall of the pancreas in combination with ring annuloplasty. After one year of observation, TR was not detected.
R. Moraca et al. [46], when analyzing the results of prosthetics and TC repair, did not reveal any differences in the postoperative and long-term periods. In conclusion, the authors indicate that in patients at risk of relapse of TR, valve replacement is preferable as a more reliable and radical intervention to reduce insufficiency. C. Park et al. [50] provide similar data; they noted only a trend toward better long-term survival in patients with valve repair. Moreover, there were no deaths in patients who underwent TC replacement in addition to correction of left heart disease. The authors also believe that in cases with severe TR and RV dysfunction, preference should be given to valve replacement. Other authors [13] identify TC replacement as a prognostic factor for increased mortality.
Despite numerous reports of good results of correction of functional TR, in some cases relapse or progression of TR is observed.
P. McCarthy et al. [42] identified a high preoperative degree of TR, LV dysfunction, the presence of an endocardial lead, atrial fibrillation, and types of annuloplasty other than ring annuloplasty as independent prognostic factors determining the progression of TR. M. De Bonis et al. [23] presented data on correction of TR in patients with dilated cardiomyopathy and mitral regurgitation. After 1.8 years of follow-up, 12% of patients had grade III or IV regurgitation, and 18% of patients with preoperative grade II or lower regurgitation subsequently progressed to two grades or higher. Severe regurgitation at the time of discharge and preoperative pancreatic dysfunction are considered as prognostic factors for the progression of TR to grades III and IV. G. Gatti et al. [32] reported that patients with preoperative regurgitation greater than grade II, RV shortening fraction less than 35%, and a permanent pacemaker tended to have recurrent TR. R. Ghanta et al. [33] found that factors such as pulmonary artery pressure after TC repair of 52 mmHg. and higher, as well as a high preoperative degree of regurgitation, determine the tendency to relapse of TR.
In a prospective study by Y. Kim et al. [36] RV dysfunction was assessed as an independent prognostic factor for the unfavorable course of TN after correction of the defect. A sensitive indicator of RV dysfunction is RV end-systolic area; Thus, with an area of less than 20 cm2, a lower incidence of cardiovascular complications in the mid-term period was noted. Similar data were presented by C. Park et al. [50].
S. Fukuda et al. [28] identified a preoperative coaptation depth (“tethering height”) of more than 0.51 cm and a “tethering area” of more than 0.8 cm2, early postoperative LV ejection fraction of less than 36.6% as echocardiographic prognostic factors relapse or persistence of TR a year or more after surgery. They also found a correlation between RV pressure and the degree of TR, and a relationship between RV pressure and LV ejection fraction at follow-up periods of more than a year. The authors obtained similar data for the early postoperative period [30].
S. Min et al. [45] in their work identified a tethering volume of more than 1.68 ml and an anteroposterior ring size of more than 36 mm as preoperative echocardiographic prognostic factors for residual TN, and pulmonary artery pressure as postoperative factors.
H. Je et al. [34] showed that age, rheumatic etiology of mitral valve disease and failure to perform the Maze procedure are predictive factors for the progression of grade II TR. Similar data were presented by H. Song et al. [57]. J. Stulak et al. [58] also compared a group of patients with successful Maze procedure and correction of mitral valve disease with a group in which the rhythm was not restored. The authors concluded that a successful Maze procedure prevents the progression of TN. In patients with sinus rhythm after surgery, the degree of TN decreased in 42% of cases, progressed in 9%, and in patients with atrial fibrillation - in 36 and 45%, respectively.
C. Yoon et al. [63] found that B-natriuretic peptide levels greater than 200 pg/ml are an unfavorable prognostic factor in patients with severe TN.
According to various data, mortality after correction of TN in combination with correction of left heart defects ranges from 0 to 18%, but most authors cite figures of 4.4-8.1%. According to J. Bernal et al. [12], the mortality rate during reoperations after previously performed TB repair was 33.8%, the rate of developmental complications was 64.9%. Actuarial survival at 10 years after reoperation was 40%, and at 26 years it was 11.8%. In other works, the authors [11, 42] cite mortality after repeated operations at 18.5–37%.
Thus, functional TN is a rather serious problem that accompanies left heart defects and aggravates their course. Severe TR should be corrected without a doubt. However, frequent unsatisfactory long-term results of uncorrected regurgitation of degrees I and II may necessitate more active surgical tactics. Other parameters need to be considered as additional or equivalent to the degree of regurgitation when determining indications for correction of the defect. It is still not completely clear which annuloplasty technique is the operation of choice, although there is a tendency towards ring annuloplasty methods. The progression of TR after correction in patients with dilated pancreas, who apparently require the use of other surgical techniques than isolated annuloplasty, also remains a problem.
Types of pathology
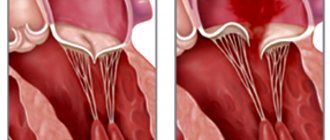
There are two types of TC deficiency:
- Organic , which occurs as a result of morphological changes in the valve leaflets (wrinkling, fibrosis, degeneration) with rheumatism, endocarditis, connective tissue diseases, etc.
- The functional form is three times more common than the organic form. In her case, the valve structure is not damaged. Failure occurs due to increased pressure in the right ventricle, which causes the valve fibrous framework to expand and the leaflets cannot close completely. This type of tricuspid valve defect is observed in combination with mitral heart defects and other pathologies: with pulmonary hypertension, severe bronchial asthma (i.e., with conditions leading to overload and increased pressure in the RV).
Symptoms of tricuspid insufficiency
The main clinical picture is drawn by signs of chronic heart failure (CHF):
- difficulty breathing (shortness of breath), which worsens with physical activity;
- rapid onset of fatigue;
- drowsiness;
- aching chest pain;
- tachycardia;
- swelling in the legs, especially in the evenings;
- heaviness or aching pain in the right side under the rib due to an enlarged liver;
- bluish coloration of the lips and tip of the nose;
- pulsation of dilated neck veins.
In later stages, heart rhythm disturbances such as atrial fibrillation often occur. Patients begin to experience discomfort in the chest, high frequency and irregular pulse, dizziness, a feeling of lightheadedness, and nausea. Due to a sharp decrease in blood pressure, they may even faint.
Atrial fibrillation is one of the most unfavorable and dangerous complications of TC insufficiency, as it quite often causes ischemic strokes.
How does heart failure with a defect differ from a condition of other origin? Even with extremely pronounced phenomena of blood stagnation in the internal organs, people can feel completely healthy, not experience any unpleasant sensations and, moreover, can tolerate physical activity perfectly.
Degrees of tricuspid valve insufficiency
To objectively assess the progress of the defect, the volume of regurgitation (backflow of blood) is taken into account according to the data in the table below.
| Degree of regurgitation | Echo-CG sign | Symptoms |
| Tricuspid insufficiency 1st degree | Minimal and barely noticeable blood flow without any hemodynamic disturbances | Can be observed in healthy people. There are absolutely no symptoms |
| Tricuspid insufficiency 2nd degree | Regurgitation 2 cm from the TC | Due to compensation of hemodynamic disorders, it may be accompanied by slight shortness of breath during intense physical work. |
| Tricuspid valve insufficiency 3rd degree | Regurgitation at a distance of more than 2 cm from the TC | Shortness of breath appears with normal exertion, increased heart rate, slight heaviness in the legs and swelling in the evenings |
| 4th degree TC insufficiency | Regurgitation occupying almost the entire cavity of the RA | Detailed picture of CHF: difficulty breathing, pain in the heart, swelling, heaviness in the right side, etc. |
Publications in the media
Tricuspid regurgitation is the inability of the right atrioventricular valve to effectively prevent the backflow of blood from the right ventricle into the right atrium during ventricular systole.
Frequency • According to autopsies, tricuspid valve defects are found in 15–30% of patients with rheumatic heart disease • Tricuspid insufficiency makes up 85% of tricuspid valve defects • According to echocardiography, grade I tricuspid insufficiency can be detected in almost all healthy people.
Etiology • Primary tricuspid insufficiency with congenital anomalies (Ebstein anomalies, anomalies in the number of leaflets, in combination with an atrial septal defect or with corrected transposition of the main ones) - 50%, cardiac trauma, carcinoid syndrome (due to the formation of deforming fibrous plaques in the valve leaflets), myxomatous degeneration or rheumatic valve damage, endocarditis of drug addicts (isolated damage to the tricuspid valve - 40% of cases), connective tissue dysplasia syndromes (usually tricuspid valve prolapse with Marfan or Ehlers-Danlos syndrome) • Secondary tricuspid insufficiency due to dilatation of the fibrous ring with high pulmonary hypertension complicates 90% all mitral valve defects (of which 95% are mitral valve stenoses) and 90% of cases of dilated cardiomyopathy • Secondary tricuspid regurgitation due to ischemic dysfunction or rupture of papillary muscles during right ventricular infarction.
Pathophysiology • With primary failure, the load on the volume of the right parts of the heart increases, the size of their cavities increases, and blood stagnates in the systemic circulation • In some cases, with dilatation of the right atrium, thrombosis occurs, as well as supraventricular cardiac arrhythmias (Ebstein’s anomaly is often combined with Wolff’s syndrome -Parkinson-White) • The addition of secondary insufficiency in mitral defects complicated by high pulmonary hypertension is usually accompanied by unloading of the pulmonary circulation and some improvement in hemodynamics without significant stagnation in the systemic circulation and a decrease in stroke volume • As the defect progresses, cardiac output decreases more significantly • When the pressure in the right atrium increases by more than 10 mm Hg. stagnation develops in the systemic circulation • There are four degrees of defect: I - a barely detectable reverse flow of blood, II - a reverse flow is determined at a distance of 2 cm from the valve, III - a regurgitant stream is determined at a distance of more than 2 cm from the valve, IV - regurgitation is determined at a large extent of the cavity of the right atrium.
Clinical picture and diagnosis • Complaints •• In infants, severe heart failure and cyanosis are usually detected, at older ages - shortness of breath, increased fatigue, cyanosis and symptoms of right ventricular failure • In 25% of cases, the initial manifestation is supraventricular tachycardia as a manifestation of Wolff-Parkinson syndrome –White or atrial fibrillation during their dilatation • Complaints caused by concomitant conditions in secondary insufficiency (pain syndrome during right ventricular infarction, symptoms of mitral valve defects, etc.).
• Peripheral symptoms •• Pulsation of the jugular veins •• Pulsation along the left edge of the sternum, increasing with inspiration •• Palpable pulsation of the right atrium and pulmonary artery in ventricular systole •• Pulsation of the liver.
• Valve symptoms •• Systolic tremor •• Weakening of the 1st tone •• Strengthening of the pulmonary component of the 2nd tone •• 3rd tone •• Sounds of flapping sail with valve prolapse •• Pansystolic murmur (especially with increased afterload of the right ventricle) above the xiphoid process of the sternum, often musical or resembling a horn, the intensity of which increases with inspiration (Rivero-Corvalho sign) or with pressure on the liver •• In severe tricuspid regurgitation, a low-frequency protodiastolic or mesodiastolic murmur is sometimes heard.
• Symptoms of insufficiency in the systemic circulation •• Expansion of the size of the heart to the right •• Swelling of the neck veins •• Enlargement of the liver •• Plesch's symptom - swelling of the neck veins with slight compression of the liver during palpation •• Edema •• Ascites.
• Symptoms of the underlying disease (Marfan syndrome, infective endocarditis, MI, PE).
Special studies • ECG •• Signs of hypertrophy and overload of the right heart •• -Wave and paroxysms of tachycardia from the AV node in Wolff–Parkinson–White syndrome •• Atrial fibrillation and flutter •• AV block with Ebstein anomaly or cleft septal cusp valve • Jugular venogram : pronounced V waves, the height of which correlates with the severity of tricuspid regurgitation. • X-ray of the chest organs •• Bulging of the arches of the right ventricle and right atrium •• Expansion of the shadows of the vena cava •• In case of secondary insufficiency - strengthening of the pulmonary pattern, expansion and lack of structure of the roots of the lungs, Kerley B lines, bulging of the arches of the left parts of the heart and pulmonary artery.
• EchoCG •• Increase in the size of the cavities and hypertrophy of the walls of the right heart •• Expansion of the superior vena cava •• Expansion of the diameter of the fibrous annulus of the valve with a secondary defect •• Deformation of the valvular apparatus in rheumatism •• Signs of other valvular or congenital anomalies •• Displacement of the fibrous annulus of the valve and “atrialization” of the right ventricle, as well as an increase in the amplitude of movement of the leaflets with Ebstein’s anomaly •• Thickening and deformation of the leaflets with carcinoid syndrome •• The presence of vegetations or leaflet defects with infective endocarditis •• Registration of the regurgitant flow in Doppler mode and determination of the severity of regurgitation in relation to the area jet to the area of the right atrium (I degree - the ratio does not exceed 1:3, II degree - 2:3, III degree - exceeds 2:3) •• Based on the maximum speed of the regurgitant jet (v), the systolic pressure in the pulmonary artery (SPAP) is calculated : SDPA = SDPV (in the absence of pulmonary stenosis); SDPV = 4v2 + CVP, where SDPV is the systolic pressure in the right ventricle •• Transesophageal echocardiography is performed in all patients to exclude right atrial thrombosis and vegetations, even with normal heart rhythm.
• Catheterization of the right heart •• On the pressure curve in the right atrium - a pronounced wave V and a steep Y-decline •• In severe failure - wave A and the X-decline are smoothed, so the pressure curve in the right atrium is similar to that in the right ventricle •• In case of isolated insufficiency - acceleration of AV blood flow during early diastolic filling •• The presence of a high end-diastolic AV pressure gradient indicates concomitant tricuspid valve stenosis •• Measurement of pressure in the right ventricle, right atrium and pulmonary artery at rest and against the background of tests with oxygen and aminophylline to determine the reversibility of pulmonary arterial hypertension and the prognosis of surgical treatment •• With an increase in systolic pressure in the right ventricle more than 60 mm Hg. Tricuspid insufficiency is almost always caused by mitral valve defects.
• Right ventriculography •• Registration of regurgitant flow •• Assessment of the topography of the right parts of the heart in Ebstein anomaly and exclusion of associated anomalies (transposition of the great vessels, ASD, etc.).
• Coronary angiography •• Performed in the presence of episodes of angina and positive results of stress testing, as well as in all women over 45 years of age, men over 40 years of age, and all candidates for multivalve reconstruction •• 14% of patients with tricuspid valve disease are diagnosed with CAD.
TREATMENT • Conservative treatment •• In the absence of pulmonary hypertension, even severe insufficiency can usually be treated with diuretics and venous vasodilators (nitrates orally and in the form of patches, for severe refractory tricuspid insufficiency - ACE inhibitors) •• The dose is selected depending on CVP, diuresis and the severity of edema •• The effectiveness of cardiac glycosides in sinus rhythm is low •• For refractory tricuspid regurgitation and severe right ventricular dysfunction, IV inotropes are indicated (preferably dobutamine) •• For pulmonary hypertension, the most beneficial effect is a decrease in pressure in the pulmonary artery: in some cases, diuretics are effective and vasodilators, but they should be used with caution because the range of acceptable values of cardiac filling is narrowed, and the ability to increase cardiac output in response to a decrease in peripheral vascular resistance is limited •• Prevention of infective endocarditis is recommended in all cases of primary tricuspid regurgitation, and in cases of secondary tricuspid regurgitation, the need for prophylaxis , apparently not •• With infective endocarditis, hemodynamic complications and embolism are better tolerated than with endocarditis of the left heart, so antibiotic therapy before surgery can be carried out longer (in some cases, it is possible to do without surgery at all).
• Surgical treatment •• Indications ••• III–IV degree of tricuspid insufficiency with pronounced clinical manifestations ••• Hemodynamically significant defects in combination with II or greater degree of relative tricuspid insufficiency ••• III–IV degree of asymptomatic defect with gross deformation of the leaflets or valve device •• Contraindications ••• Severe concomitant pathology that threatens the patient’s life ••• Terminal stage of circulatory failure ••• Negative results of tests with aminophylline and oxygen ••• The activity of the rheumatic process is not considered a contraindication to surgical treatment •• Methods of surgical treatment •• • The Kay-Boyd and DeVega suture methods or the support-ring Carpentier method of annuloplasty are used for primary defects and intact valve apparatus ••• In case of gross morphological changes in the valve, infective endocarditis, Ebstein’s anomaly and the ineffectiveness of previous annuloplasty, valve replacement is performed with biological artificial valves (30% operations on the tricuspid valve) ••• An ambiguous attitude towards mechanical valves in the tricuspid position is caused by increased thrombus formation in the right parts of the heart, apparently due to the low concentration of PgI2, which has an antithrombotic effect (this Pg is synthesized in the lungs and enters the bloodstream only in left side of the heart).
Specific complications • PE • Secondary infective endocarditis • Thrombosis of the prosthesis • Degenerative changes in the biological prosthesis and the need for re-prosthesis • AV block.
Prognosis • In the natural course of the defect, the prognosis is almost entirely determined by concomitant conditions, for example, the severity of damage to the mitral and aortic valves •• The addition of a secondary tricuspid defect worsens the prognosis •• With traumatic insufficiency, patients who survive the acute period usually tolerate the defect relatively easily for 5–10 years, after which rapid progression of symptoms occurs •• Among patients with moderate tricuspid regurgitation after surgical correction of concomitant severe mitral valve disease, 66% of patients subsequently require correction of progressive tricuspid regurgitation • Prognosis for surgical treatment •• Hospital mortality - 14.1%.
Synonyms • Right atrioventricular valve insufficiency • Tricuspid valve insufficiency. Abbreviations • PSPA - systolic pressure in the pulmonary artery • SDPV - systolic pressure in the right ventricle.
ICD-10 • I07.1 Tricuspid insufficiency
Diagnostics: Ultrasound criteria and other methods
When examining a person with tricuspid regurgitation, I should look for the following signs:
- increased and diffuse pulsation in the epigastric region, which occurs due to pronounced thickening and expansion of the right ventricle;
- enlarged and pulsating liver;
- a very characteristic symptom of right ventricular CHF is hepatojugular reflux (when I put pressure on the liver, the jugular veins begin to swell greatly);
- When listening to the heart, you may encounter a prolonged systolic murmur on the xiphoid process at the lower edge of the sternum (this cartilage is located in the epigastric region).
My main instrumental method for diagnosing tricuspid insufficiency is echocardiography (Echo-CG, ultrasound of the heart). Her main criteria for the final verdict:
- regurgitation of blood into the right atrium;
- the width of the regurgitant jet is more than 7 mm;
- the area of the regurgitation opening is more than 40 mm2;
- expansion of cavities and thickening of the muscle layer of the pancreas and RA;
- increased pulsation of the dilated inferior vena cava.
Also, one of the criteria for the presence of the disease is reverse blood flow in the hepatic veins. On the ECG film, I quite often find signs of overload of the right side of the heart, namely:
- hypertrophy of the RA and RV: high pointed P wave in leads II, III, aVF (the so-called “P-pulmonale”), high R waves in leads I, II, deep S waves in leads V5, V6;
- incomplete blockade of the right bundle branch;
- atrial fibrillation (atrial fibrillation).
To better track rhythm disturbances, I conduct Holter (24-hour) ECG monitoring, since many arrhythmias are attack-like (paroxysmal) and may not be detected on a regular cardiogram.
Tricuspid valve insufficiency
DEFINITION, ETIOLOGY AND PATHOGENESIStop
Pathological reflux of blood from the right ventricle into the right atrium due to a violation of the closure of the tricuspid valve. Causes: organic - rheumatic disease, infective endocarditis, carcinoid syndrome, Marfan syndrome, Fabry disease, Whipple disease, tricuspid valve prolapse, rheumatoid arthritis, SLE, congenital defects (Ebstein syndrome, etc.), papillary muscle dysfunction, drugs (methysergide, fenfluramine ); functional (most often with an acquired defect) - expansion of the ring with an anatomically normal valve, a change in the geometry of the right ventricle most often caused by mitral valve disease, as well as pulmonary hypertension, right ventricular infarction, congenital heart disease (for example, pulmonary stenosis).
CLINICAL PICTURE up
Symptoms of mitral valve disease usually predominate, accompanied by tricuspid valve disease.
1. Subjective symptoms: decreased exercise tolerance, weakness, heaviness and distension in the right hypochondrium.
2. Objective signs: pulsation of significantly dilated jugular veins, Plesh’s symptom; in patients with severe insufficiency, pulsation of the vessels of the head and neck appears, less often, pulsation of the eyeballs, right ventricle, and liver; in the later stages of the defect - generalized edema of the subcutaneous tissue, ascites and yellowish-cyanotic coloration of the integument; pansystolic murmur, increasing with deep inspiration, as well as diastolic murmur (with severe insufficiency).
DIAGNOSTICStop
Additional research methods
1. ECG: P pulmonale, signs of right ventricular hypertrophy and often incomplete blockade of the right bundle branch; usually atrial fibrillation.
2. X-ray of the chest organs: in case of functional failure - cardiomegaly with expansion of the shadow of the right atrium, effusion in the pleural cavity and expansion of the azygos vein may appear; with severe insufficiency - enlargement of the right ventricle.
3. Echocardiography: assessment of the structure, degree of valve insufficiency and systolic pressure in the right ventricle (pressure >55 mm Hg suggests a secondary cause of the defect); Severe tricuspid valve regurgitation with normal valve structure may occur when systolic pulmonary artery pressure is ≥55 mmHg. Art.; failure with systolic pressure in the pulmonary artery <40 mm Hg. Art. rather indicates a pathology in the valve structure; Many healthy individuals have clinically insignificant deficiency.
TREATMENT to the top
1. Tricuspid valve insufficiency in combination with mitral disease:
1) isolated correction of mitral valve stenosis can significantly reduce the degree of functional insufficiency of the tricuspid valve;
2) surgery of the tricuspid valve → with significant insufficiency or expansion of the ring (≥40 mm or >21 mm/m2) and the need for surgery on the mitral valve;
3) in patients with severe tricuspid regurgitation after intervention on the left heart valve if they have complaints or progressive dilatation of the right ventricle or decreased function, without decreased function of the left heart valves, severe dysfunction of the right or left ventricle and without severe vascular damage to the lungs → consider the possibility of surgical treatment.
2. Severe primary isolated tricuspid insufficiency with subjective symptoms and without severe right ventricular failure: valve repair, if impossible → valve replacement.
3. Severe isolated tricuspid insufficiency, asymptomatic or with mild symptoms, but with progressive dilatation of the right ventricle or deterioration of its function: consider surgical treatment.
4. In combination with conduction abnormalities: placement of an epicardial pacing lead during valve replacement surgery.
FORECAST to top
Patients with severe tricuspid insufficiency, regardless of its cause, have a poor long-term prognosis due to increasing right ventricular dysfunction and stagnation of blood in the veins of the systemic circulation.
Treatment: methods and indications
The primary task of doctors is to eliminate the cause of valve failure.
Drug therapy has several directions:
- combating CHF and slowing its progress as much as possible;
- prevention and treatment of heart rhythm disorders;
- prevention of thrombosis.
I use the following medications to treat heart failure:
- beta-blockers - “Bisoprolol”, “Metoprolol”;
- ACE inhibitors - Perindopril, Lisinopril;
- aldosterone antagonists, or potassium-sparing diuretics - Spironolactone.
For edema, I use more powerful diuretics - Torasemide, Indapamide. If too much fluid accumulates in the cavities (thoracic, abdominal) or pericardial sac, I consult with surgeons about pumping it out. Depending on the cavity from which excess is removed, the following procedures are distinguished: pleural puncture, laparocentesis, pericardial puncture.
For the treatment of arrhythmias, antiarrhythmic drugs are prescribed - Amiodarone, Propafenone. To prevent thrombus formation and avoid pulmonary embolism or stroke in patients with atrial fibrillation, I use anticoagulants - Warfarin, Dabigatran, Rivaroxaban.