How to notice atmospheric pressure?
Although gas molecules are odorless and colorless, they constantly interact with the receptors of our skin, compress all objects from all sides, fill voids, and their rapid movement in the horizontal direction, called wind, can knock us off our feet. It is possible to prove that atmospheric pressure exists using simple experiments.
Experiment 1 – “Sippy Cup”
Pour water into the glass to the brim. Cover it with a piece of thick paper and, holding the paper with your palm, quickly turn the glass upside down. Remove your palm. Water will not spill out of the glass, since the atmosphere presses on the paper from below.
Explanation: the phrase “a column of atmospheric air presses on us,” sometimes used, including in school textbooks, is incorrect. It is pronounced by association with the pressure force acting from a solid body. This force acts on bodies located below, and does not act on bodies on the side or, especially, on top of a given body. The pressure of a liquid or gas is another matter.
According to Pascal's law, pressure is transmitted not only to points at the bottom of the vessel, but also to points on the walls and lid. The forces of hydrostatic and atmospheric pressure act perpendicular to the arbitrarily oriented surface of the body in contact with the medium and can have any direction.
The air pressing on the paper from the bottom of a filled glass is proof of the failure of such an association. Interestingly, if the glass is only half filled with water, the remaining air will press with the same force as the outside air, and the paper will not hold the water (and air) in the glass.
Experiment 2 – “Dry out of the water”
Place a coin or metal button on a flat plate and pour in water. The coin will be under water. Our task is to catch the coin with your bare hands without getting them wet.
Light the paper inside a dry glass and, when the air warms up, tip the glass onto a plate next to the coin so that the coin does not end up under the glass. You won't have to wait long. The paper in the glass will immediately go out and the air will begin to cool. As it cools, the water will be drawn into the glass and soon all will gather there, exposing the bottom of the plate.
Explanation: when the air in the glass heated up, it expanded, like all heated bodies, and the excess of its new volume came out of the glass. When the remaining air began to cool, it became insufficient to exert the same pressure in a cold state, to balance the external pressure of the atmosphere. Now the water under the glass experiences less pressure on every centimeter of its surface than in the open part of the plate. It is not surprising that it is driven under the glass, squeezed there by excess pressure from the outside air. The water is pressed in by the air!
On the same topic, look at the experiment of the Galileo program.
What to do if the weather pressure changes. Doctors' recommendations
Depending on what condition you are suffering from, hypotension or hypertension, the list of recommendations from doctors is strikingly different.
If the patient complains of symptoms characteristic of low blood pressure, then when the weather changes it is necessary:
- spend more time outdoors;
- engage in active sports (jogging, cycling, swimming);
- drink plenty of fluids;
- drink caffeine-containing drinks (strong tea, coffee);
- for an emergency increase in blood pressure, take tinctures of lemongrass, ginseng, and eleutherococcus.
For people prone to hypertension, doctors strongly recommend adhering to the following rules:
- avoid physical and emotional fatigue;
- avoid walking in the heat and sun;
- Constantly ventilate the room, use air conditioning if possible;
- adhere to proper nutrition, excluding salty, spicy, spicy and fatty foods;
- Do not drink coffee or other drinks containing caffeine.
In any case, you should establish a daily routine, get more rest, avoid stress and eat right. And in order to help your body in a timely manner, it is important to control your blood pressure using special devices.
Why don't we feel atmospheric pressure?
Knowing that 1 m3 of air at a temperature of 0° at sea level weighs 1.3 kg, it is easy to calculate that on the roof of a house, having an area of, for example, 100 m², the atmosphere presses with a force of 107 N, which corresponds to the weight of a body weighing 1000 tons. However the roof of the house does not fall in.
The area of the back of a person lying on the beach is obviously greater than 0.2 m2; Consequently, the atmosphere presses on a person’s back with a force greater than 20,000 N, which corresponds to a pebble weighing 2 tons. However, a person does not feel any pressure from above at all.
The “Dry Out of the Water” experiment also shows us evidence of internal pressure balancing the external pressure of the atmosphere.
We do not feel air pressure because atmospheric pressure is evenly distributed on all sides and because inside us there is the same air and liquid pressure, and the body's adaptive abilities constantly balance internal pressure, adjusting it to changes in atmospheric pressure. But adaptations occur only within a short interval.
If people live for a long time at high altitude, then their body adapts to both less oxygen and lower pressure. The highest mountain settlements in the world:
- La Rinconada (Peru) – 5100 m;
- El Alto (Bolivia) – 4150 m;
- Potosi (Bolivia) – 4090 m;
- Lhasa (Tibet) – 3650 m;
- Namche Bazaar (Nepal) – 3450 m;
- in Russia it is Kurush (Dagestan) - 2600 m.
Gold mining village of La Rinconada-Ananey, 5100 m. Author: IJISCAY
But fish living in the depths of the ocean are accustomed to higher pressure, and their body is not able to quickly adapt. Their body has adapted to it, and its internal pressure is much higher than 1 atm. Therefore, when they are taken out of the depths, they explode due to high internal pressure. The same would happen to a person in airless space (in space).
Film on the topic “Atmospheric pressure and human well-being.”
How does weather affect human blood pressure? What to do if the pressure changes due to the weather?
Weather changes are perceived by most people as a natural phenomenon. Yes, your mood may spoil on a cloudy and rainy day, nothing more. But there is a category of people who, when the atmospheric pressure changes, feel a significant deterioration in their overall health: headaches, dizziness, loss of strength, aching joints. These and other symptoms significantly complicate the life of a person suffering from weather dependence. But knowing how atmospheric pressure affects the pressure in the blood vessels, you can understand what measures to take to prevent health problems.
From the history of the discovery of knowledge about weight, air pressure and the invention of the barometer
E. Torricelli , figured out how to measure atmospheric pressure . Together with V. Viviani , a young student of Galileo, he conducted experiments to measure it. Torricelli was also one of Galileo's last students, and based on his guesses, he proved that air has weight and exerts pressure.
Evangelista Torricelli and his barometer. Author: Saperaud~commonswiki
Torricelli was the first to openly oppose Aristotle's dogmas. Speaking about the pump, he stated that
“First of all, the water rises after the piston not at all because “nature is afraid of emptiness,” it’s just that the water is driven into the pump by the pressure that the air exerts on the surface of the river. There is no air in the pump pipe, under the piston, so water enters it until the weight of the water column in the pump pipe balances the external air pressure.”
But he proved it a little later. The experiment he proposed was carried out in 1643. In this experiment, a glass tube about 1 m long was used, sealed at one end. It was filled with mercury and, having closed it with a finger (so that the mercury did not spill out ahead of time), turned it over, and lowered it into a wide cup with mercury.
Some of the mercury poured out of the tube, and a vacuum formed in its upper part (the first real void discovered on Earth - the Torricelli void ). In this case, the height of the mercury column in the tube turned out to be approximately 760 mm (if measured from the level of mercury in the cup). The air pressed on the mercury of the cup and did not allow it to flow out of the tube.
The scientist also guessed that atmospheric pressure is associated with weather changes. Observing the height of the mercury column in the tube, Torricelli noticed that atmospheric pressure was not constant and depended on “warmth or cold.” The column in the tube lowered and then rose, indicating the desired division of the scale. That is why millimeter of mercury (mmHg) is taken as one of the units of pressure. Gravity in Greek is "baros", and Torricelli's device began to be called a barometer .
Operating principle of the Torricelli barometer
Almost simultaneously with Torricelli, another famous scientist of that time, Descartes, . He explained why perfume does not flow out of a bottle with a hole in the bottom when the lid is closed, but does flow out when it is open, precisely because of the difference in air pressure on different surface areas. When the bottle cap is closed, the surface tension of the water at the small opening is able to hold the liquid in the bottle. When the lid is open, it is overcome by the force of air pressure and the perfume begins to flow out. Descartes hypothesized that with height the air becomes thinner, which means its pressure should also decrease.
After Torricelli’s experiments, Descartes instructed the talented French mathematician and physicist Blaise Pascal to test his guess - whether it was true that pressure decreases with height. To do this, he had to climb the mountains with Torricelli's pipe. The descending column of mercury at the height of Mount Puy de Dome confirmed the hypotheses of Torricelli and Descartes.
Pascal concluded:
“the laws of fluid pressure, known since the time of the glorious Archimedes and developed by the Dutchman Simeon Stevin , are in many ways valid for air.”
Air pressure is not noticed by a person, because according to the laws of pressure in liquids and gases, it is directed both to the sides and down.
Meteopathy
If the pressure on the weather fluctuates, you feel unwell, emotional and physical disorders, this condition is usually called meteopathy.
Various factors influence susceptibility to this condition:
- genetic predisposition;
- age;
- personal qualities;
- presence of chronic health problems.
Manifestations of this syndrome can knock a person out of the usual rhythm of life for several days.
At the same time, in addition to physical ailments (dizziness, surges in blood pressure, changes in body temperature, weakness, numbness of the limbs, allergy attacks, etc.), a whole range of psychological reactions is observed:
- apathy;
- depression;
- tearfulness;
- irritability;
- increased anxiety;
- panic attacks.
How is atmospheric pressure measured?
The Torricelli barometer is still used today. This simple device helps determine the approximate altitude above sea level. Climbers take it with them high into the mountains. A barometer is a mandatory instrument in the cockpit of every aircraft, be it an airplane or an Earth satellite. Nowadays, his “brothers” go down to the bottom of the seas. From altimeters they turned into depth gauges.
Over more than three centuries, barometers have changed: they have become automatic, self-recording, and have learned to control other mechanisms.
Mercury barometer measures atmospheric pressure with the greatest accuracy
Old mercury barometers.
Author: GianniG46 At meteorological stations, atmospheric air pressure is measured by the same mercury barometers , since they have the greatest accuracy. They work on the same principle as Torricelli's invention.
When measuring pressure, corrections are made for temperature, since as temperatures rise, mercury and the barometer scale expand. In practice, they use a ready-made correction table, which immediately gives the desired value.
Membrane barometers
Diaphragm pressure gauges are also used to measure atmospheric pressure . The simplest membrane pressure gauge is shown schematically in Fig. 1.
Rice. 1. Membrane barometer
A thin elastic plate-membrane 1 hermetically closes the box 2 , from which part of the air is pumped out. 3 is connected to the membrane , turning around O at an angle depending on the degree of deflection of the membrane, which in turn depends on the difference in the measured air pressure force outside the box and inside the box.
Such pressure gauges are called aneroid barometers . They are calibrated and verified using a mercury barometer. They are less accurate, but more convenient to use because they do not contain mercury. When determining pressure with an aneroid, three corrections are made (to the scale, to the temperature, and additionally to the device), specified in the device certificate. An aneroid can only give reliable readings if it is subjected to careful testing from time to time.
Aneroid barometer. Image by Wolfgang Eckert from Pixabay
The aneroid can be calibrated directly to the height of the atmosphere. Such aneroids are called altimeters ; or altimeters , they are used in airliners and allow the pilot to control the flight altitude.
Bulova B-11 altimeter, from a fighter aircraft. Author: Dosimeter
To continuously record changes in atmospheric pressure, a recording device is used - a barograph . The receiving part of the barograph is several small aneroid boxes connected to each other.
Other devices
Hypsothermometer
(
hypsometer
,
thermobarometer
,
barothermometer
) - a device for measuring atmospheric pressure by the temperature of a boiling liquid (usually water). It is more accurate than the aneroid.
Consists of a boiler and a thermometer with a scale divided into 0°.01. This device is usually used in expeditionary conditions for barometric leveling.
Stormglass is a chemical or crystalline barometer consisting of a glass flask or ampoule filled with an alcohol solution in which camphor, ammonia and potassium nitrate are dissolved in certain proportions.
This chemical barometer was actively used during his sea voyages by the English hydrograph and meteorologist, Vice Admiral Robert Fitzroy , who carefully described the behavior of the barometer; this description is still used today. Therefore, stormglass is also called the “Fitzroy Barometer”. In 1831–1836 _ Fitzroy led the oceanographic expedition on HMS Beagle, which included Charles Darwin .
In spring and autumn, a sharp drop in barometer readings portends windy weather. In summer, in extreme heat, it warns of a thunderstorm. In winter, especially after prolonged frosts, a rapid drop in the mercury column indicates an upcoming change in wind direction, accompanied by thaw and rain. On the contrary, an increase in the mercury column during prolonged frosts foreshadows snowfall.
Patterns in changes in atmospheric pressure and how to use this knowledge
Almost the entire mass of the Earth's atmosphere is concentrated in a layer up to approximately 50 km high. Upon reaching an altitude of 50 km, the acceleration due to gravity decreases by only 1.5% compared to the acceleration at sea level; therefore, we can assume that within the entire 50-kilometer layer of the atmosphere, the acceleration of gravity remains equal to g = 9.8 m/s2.
Representing atmospheric air as a continuous medium, we, of course, must not forget that in reality it is a gas. Pressure is a statistical quantity expressed through the square of the speed of their chaotic movement averaged over many molecules. The force of pressure on any real or mentally selected area in a gas is due to the chaotic bombardment of this area by many molecules.
Pressure decreases with altitude and increases when descending into deep mines. The reason is the rarefaction of air (decrease in density) with ascent and compaction with descent, because it is attracted by the earth and the bulk of its mass is concentrated around it. In the lower troposphere, pressure decreases with altitude by approximately 1 mm for every 10.5 m. This makes it possible to determine the height of a place using a barometer-altimeter.
How does atmospheric pressure change with altitude?
In fact, this pattern is observed only up to a height of 1 km. The distance in meters that one must rise or fall in order for the atmospheric pressure to change by 1 mb is called pressure level . The pressure level at an altitude from 0 to 1 km is 10.5 m, from 1 to 2 km – 11.9 m, at an altitude of 2-3 km the pressure level is 13.5 km. The pressure level depends on the temperature. It is greater in warm air. The barometric formula is described more precisely here: https://ru.wikipedia.org/wiki/
In practice, special tables are often used that allow more or less approximately obtaining data on heights. But to solve problems that do not require high accuracy, you can also use the average value. You can estimate the pressure from the difference in height, and calculate the height from the difference in pressure.
Problem 1
Climbers climb a mountain whose height is 5100 m. At the foot of the mountain, the pressure is 720 mm Hg. Art. What pressure will there be at the top?
Solution:
When ascending 10.5 m, the pressure decreases by 1 mmHg. Art.
1) Find out how many mm. rt. Art. The pressure will decrease when climbing this mountain. 5100:10.5=486 (at 486 mmHg)
2) Find out what the pressure will be at the top. 720-486=234 (mm Hg)
Answer: At the top there will be a pressure of 234 mmHg. Art.
Problem 2
Determine at what altitude the plane flies if the pressure outside is 450 mm Hg. Art., and at the surface of the Earth 750 mm Hg. Art.
1) Determine the difference in pressure. 750-450=300 mm Hg. Art. - the plane rose 10.5 meters so many times.
2) Find out how many meters the plane rose. 10.5 X 300 = 3150 (m)
Answer: the plane is at an altitude of 3150 m.
Problem 3
At the foot of the hill, the barometer shows pressure - 761 mm Hg. Art., and at the top - 761 mm Hg. Art. What is the height of the hill?
The problem is solved according to the same principle as the previous one.
1) 761-750=11 (mm Hg)
2) 11 X 10.5 = 115.5 (m)
Answer: the height of the hill is 115.5 m.
§ 1 Weight of air
In this lesson we will learn whether air has weight, what atmospheric pressure is, and we will reveal the causes of atmospheric pressure.
Weight is the force with which the body acts on a support or suspension due to gravity.
The force of gravity acts on air, therefore air has weight.
The weight of a body located on a horizontal support or vertical suspension is calculated by the formula P = mg. To determine the weight of air, you need to know its mass.
In front of us are a pencil and a glass of water. How to determine the mass of these bodies?
It is known that scales are used to measure the mass of bodies.
We put a pencil on one pan of the scale and weights on the other, so we determine the mass of the solid.
To determine the mass of water, we weigh the mass of a glass with water and without water, the difference between them will show us the mass of water.
You can apply the same method to determine the mass of air. Let's use a device called an “air-weighing ball” (Fig. a). It is a thick-walled glass ball with a stopper and a tube with a clamp. First you need to measure the mass of this ball along with the air, then pump the air through the tube using a pump (Fig. b), close it with a clamp and weigh it again. The mass of the ball will be significantly less (Fig. c).
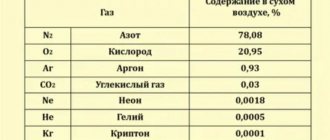
Experiments have established that the mass of air with a volume of 1 m3 at a temperature of 0 ° C and normal atmospheric pressure is equal to 1.29 kg. Then the weight of air with a volume of 1 m3 is 12.9 N.
Atmospheric pressure is constantly changing
Air density depends on temperature, and temperature is the main reason for changes in air pressure. The pressure of warm air is less than that of cold air. This is explained by the fact that when heated, air, like all objects, expands, its volume increases and it flows into the upper layers in place of less heated air, which leads to a decrease in pressure near the earth's surface.
On climatic and synoptic maps, points with the same pressure indicators, normalized to sea level, are connected by isolines called isobars . Isobars can be closed or open. A system of closed isobars with low pressure in the center (H) is called a pressure minimum , or a cyclone . A system of closed isobars with increased pressure in the center (B) is called a pressure maximum , or anticyclone . Unclosed systems of isobars - baric ridge, trough and saddle .
All pressure areas are divided into two groups: permanent and seasonal (retain characteristic pressure features during a certain period of the year).
Pressure belts on Earth
Pressure on Earth is distributed zonally. In a generalized form, this zoning is represented in the form of belts:
- at the equator there is a low pressure belt - an equatorial depression;
- to the south and north of the equator to 30-40° latitude - a belt of high pressure;
- at 60-70° N. and Yu. w. – low pressure belts;
- Subpolar regions – low pressure.
Atmospheric pressure belts on Earth
In fact, the real picture of pressure distribution on the earth's surface is much more complex.
Constant pressure regions
The equatorial belt of low pressure remains constant, only shifting its axis following the Sun. In July it moves to the Northern Hemisphere at 15-20° N. sh., in December - to Yuzhnoye, at 5° south. w. In winter, a continuous belt of high pressure appears over the ocean and over land. In summer, high pressure remains over the oceans, and a thermal depression and low pressure form over land. The pressure maxima of Antarctica and Greenland are also constant.
Above the ice-free oceans and warm currents of the temperate zone, both in winter and summer, pressure minima are clearly expressed:
- Icelandic;
- Aleutian.
Seasonal pressure areas
30-40° latitude
Only in winter is there really a belt of high pressure here. In summer, it becomes low over the mainland, but over the oceans, which warm up slowly, the pressure remains high and even increases. In other words, baric maxima persist here throughout the year only over the oceans:
- North Atlantic;
- North Pacific;
- South Atlantic;
- South Pacific;
- South Indian.
Temperate and subpolar
In the temperate and subpolar latitudes of the northern hemisphere, where oceans and continents alternate, the pressure over land and water is different, especially in winter. Over land there is a minimum in summer, and a maximum in winter. In summer, pressure is low throughout the entire zone. In winter, pressure is high over cooled continents, and seasonal pressure maxima occur here:
- Asian, centered over Mongolia;
- North American (Canadian).
Daily fluctuations in atmospheric pressure
There is also a daily fluctuation in pressure. There is one maximum at night, and one minimum during the day. Twice a day, in the morning and in the evening, it increases and decreases the same number of times, after midnight and after noon.
The change in pressure during the day is associated with air temperature and depends on its changes. Annual changes depend on the heating of continents and oceans in the summer and their cooling in winter. In summer, an area of low pressure is created on land, and an area of high pressure is created over the ocean.
The minimum value of atmospheric pressure - 641.3 mm Hg or 854 mb - was recorded over the Pacific Ocean in Hurricane Nancy, and the maximum - 815.85 mm Hg. or 1087 MB – in Turukhansk in winter. The maximum pressure in Russia was recorded in the Krasnoyarsk Territory in 1968 – 870 mm Hg. Art.
All pressure systems have a great influence on air currents, weather and climate over large areas. We'll talk about the winds they cause next time.
§ 5 Brief summary of the lesson
IMPORTANT TO REMEMBER:
The force of gravity acts on air, therefore air has weight.
Air with a volume of 1 m3 at a temperature of 0 ° C and normal atmospheric pressure has a mass of 1.29 kg and has a weight of 12.9 N.
The pressure of the Earth's air shell on the Earth's surface and bodies on Earth is called atmospheric pressure.
The atmosphere extends for thousands of kilometers. Due to pressure, the lower layers of air are compressed more strongly than the upper layers, that is, the density of air decreases with height.
The reason for the existence of the atmosphere is the effect of the Earth's gravitational force on air molecules and the continuous random movement of air molecules.
Test to consolidate learned material
Test on the topic: “Atmospheric pressure”
Sources:
- Tomilin A. N., Terebinskaya N. V. Why nothing? Essays. /L., “Det. lit.”, 1975.
- Ya. I. Perelman. Entertaining tasks and experiments. - M.: “Children's Literature”, 1972.
- Physical Geography: Reference. aid for preparation. dept. universities/G. V. Volodina, I. V. Dushina, S. G. Lyubushkina and others; Ed. K.V. Pashkanga - M.: Higher. school, 1991.
- Tarasov L.V. Atmosphere of our planet. - M.: FIZMATLIT, 2012.
- Savtsov T.M. General geography: Textbook. aid for students higher ped. textbook institutions - M.: Publishing House, 2003
- Dronov V.P. Geography. 5-6 grades: Textbook/V. P. Dronov, L. E. Savelyeva. 5th ed., stereotype. - M.: Bustard, 2015.
- Geography grades 5-6: textbook. for general education institutions / A. I. Alekseev, E. K. Lipkina, V. V. Nikolina, etc.; Edited by A. I. Alekseev. - M.: Education, 2012.
List of used literature:
- Volkov V.A. Lesson developments in physics: 7th grade. – 3rd ed. – M.: VAKO, 2009. – 368 p.
- Volkov V.A. Physics tests: grades 7-9. – M.: VAKO, 2009. – 224 p. – (Workshop of a physics teacher).
- Kirik L.A. Physics -7. Multi-level independent and control work. M.: Ilexa, 2008. – 192 p.
- Testing and measuring materials. Physics: 7th grade / Comp. Zorin N.I. – M.: VAKO, 2012. – 80 p.
- Maron A.E., Maron E.A. Physics. 7 Didactic materials. – M.: Bustard, 2010. – 128 p.
- Peryshkin A.V. Physics. 7th grade - M.: Bustard, 2011.
- Tikhomirova S.A. Physics in proverbs and sayings, poetry and prose, fairy tales and anecdotes. Teacher's manual. – M.: New School, 2002. – 144 p.
- I'm going to physics class: 7th grade. Part III: Teacher's Book. – M.: Publishing House “First of September”, 2002. – 272 p.