It branches like a tree, first into large branches (trunks), then into smaller branches and twigs, and is conventionally divided into several parts or sections:
- 1. The ascending aorta is the area from the aortic valve to the brachiocephalic trunk.
- 2. The aortic arch is a short section from which all the vessels supplying the arms and head (brachiocephalic arteries) depart. They anatomically form an arch connecting the ascending and descending aorta.
- 3. The descending (thoracic) aorta begins from the mouth of the left subclavian artery and continues to the diaphragm.
- 4. Below the diaphragm and before the bifurcation of the aorta (bifurcation) is the abdominal aorta.
Dividing the aorta into sections is very important for assessing risk and choosing optimal treatment tactics in patients with aortic aneurysms.
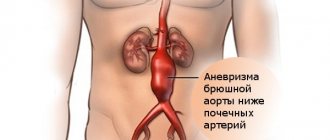
An aortic aneurysm is an area of local expansion.
Causes of aortic enlargement
Congenital systemic connective tissue diseases: Marfan syndrome, Ehlers-Danlos syndrome, caused by genetic changes in which the aortic wall has an abnormal structure, can cause the development of an aneurysm.
Acquired diseases that cause aneurysmal changes in the aortic wall: most often this is atherosclerosis. About 80% of all complicated aortic aneurysms are aneurysms caused by an atherosclerotic process, which leads to weakening of the vessel wall and the inability to withstand normal blood pressure, and as a result, to its expansion.
Less commonly, an aortic aneurysm develops in inflammatory diseases caused by external agents (syphilis, fungal infection, tuberculosis) or in autoimmune diseases (nonspecific aortoarteritis).
What have we learned about veins and arteries?
The veins and arteries of the human body are the basis of the vascular system. They have different structures because they must perform different functions. The artery carries oxygenated blood to the body's organs directly from the heart. To ensure the movement of blood, myocardial contractions are used, so the movement becomes intense and can reach a speed of 25 cm/sec.
In the veins, blood moves directly from the organs themselves to the heart. It is poor in oxygen, but it contains a lot of carbon dioxide and other decay products. The movement of the venous occurs due to the structure of the vessel. That is why the speed here is an order of magnitude lower. At the same time, the percentage of venous blood is 64%, while arterial blood accounts for only 14%.
Symptoms of aortic aneurysm
Unfortunately, the diagnosis of aortic aneurysm cannot always be established during the “cold period” (before complications develop), since this disease is usually asymptomatic. Most often, it is discovered accidentally during fluorography, ultrasound or tomography studies performed in connection with other diseases. Treatment of an aneurysm of the ascending aorta before complications develop is much safer for the patient, therefore, in the early diagnosis of an aortic aneurysm, routine medical examination is important.
It is worth noting that every hundredth patient who died suddenly dies from aortic dissection.
Complaints usually appear when the aneurysm begins to stratify or, enlarging, compresses surrounding organs and tissues. Pain or dysfunction of those organs located in the area of the aneurysm appears. At first, this is not of a bright nature and, therefore, does not alarm either the patient or the doctor.
However, the pain intensifies as these deadly complications of an aortic aneurysm develop—it is some of the most severe pain a person can experience. It is localized in the chest if the aneurysm is located in the ascending, descending sections or in its arch, or in the abdomen if it formed in the abdominal section. Characterized by severe weakness, pallor, and often the person loses consciousness.
Impaired blood supply to organs located in the area of aneurysm rupture or aortic dissection (brain or spinal cord, kidneys, intestines, upper or lower extremities) leads to loss of function of these organs, and a large volume of blood loss during aortic rupture represents the most serious danger. To save a life, minutes count. If early surgical treatment is not available, the first-day mortality rate for aortic dissection is 1% per hour (one person in a hundred dies every hour). Within the first 24 hours, 33% of patients die from aortic dissection, 50% of patients within 48 hours and 75% within two weeks. Only early surgical intervention makes it possible to save a significant proportion of patients.
What is blood pressure
It is blood pressure that ordinary people often hear about, which is why they pay the greatest attention to it. Indeed, arteries are important vessels, the normal functioning of which is vital for humans. Normal blood pressure indicates that the blood can supply oxygen and nutrients to the entire body.
All blood vessels are divided into veins and arteries. When blood passes through them, pressure is created. Blood pressure appears as a result of the work of red fluid passing through the arterial vessels, and its indicators depend on the degree of elasticity of these vessels. It is known that the muscles of our heart contract at least 60 times per minute during normal life. This indicator is necessary for the entire body to be successfully supplied with nutrient fluid.
When measuring blood pressure, systolic and diastolic values are recorded. The first indicator is systolic blood pressure. Its norm is considered to be 120 mmHg. Art. For diastolic pressure, the norm is 80 mmHg. Art. When an increase in pressure occurs, systole is recorded. As the pressure decreases and the heart muscle relaxes, diastolic blood pressure is recorded.
Diagnosis of aortic aneurysm
In the diagnosis of aortic aneurysms, the so-called imaging techniques (ultrasound, MRI, CT, AG) are of greatest importance. In the ascending aorta, its arch and in the abdominal section, an aneurysm can be detected using ultrasound methods (ultrasound). To diagnose an aneurysm of the descending (thoracic) aorta, X-ray methods (x-ray, computed tomography) are required. To establish a final diagnosis and select a treatment method, contrast research methods are performed. Currently, the optimal diagnostic method, which provides the most complete information about the location, extent, diameter of the aneurysm and its relationship to nearby organs, is multislice computed tomography - aortography.
Treatment methods for aortic aneurysm
The main method of treating an aneurysm of any part of the aorta is surgical. The point of the method is to replace the dilated section of the aorta in order to prevent its further stretching and rupture. To replace the aorta, two methods are used - the endovascular (intravascular) method using a special intravascular prosthesis (stent graft), and open surgery - aortic replacement.
Each method has its own indications, and each of them has its own advantages and disadvantages.
The advantages of the surgical method lie in its versatility, that is, the ability to correct all disorders associated with an aortic aneurysm, regardless of the department and nature of the lesion. For example, in case of an aneurysm of the ascending aorta and damage to the aortic valve, aortic and aortic valve replacement is performed in combination with coronary bypass surgery.
To perform surgery on the ascending aorta and its arch, it is necessary to use artificial circulation, systemic hypothermia, and often complete circulatory arrest.
Indications for surgical treatment
- transverse size of the aneurysm,
- aneurysm growth rate;
- the formation of complications of this disease.
For each section of the aorta, there is a cutoff limit for the transverse size of the aorta, after which the risk of aortic rupture statistically significantly increases. Thus, for the ascending and abdominal aorta, the transverse diameter of the aneurysm is 5 cm dangerous in terms of rupture, for the thoracic aorta - 6 cm. If the diameter of the aneurysm increases by more than 6 mm in 6 months, then this is also an indication for surgery. Also threatening in terms of rupture and dissection of the aorta are the saccular form of the aneurysm and expansion of the aorta, which is smaller than the diameter that is the indication for surgery, but accompanied by pain at the site of expansion and dysfunction of the presenting organs. Dissections and completed ruptures of aneurysms are absolute indications for emergency surgery.
The importance of veins in the human circulatory system
A feature of the veins is the transfer of red liquid that is not yet saturated with oxygen. They have less strong walls than the largest artery in the body. Accordingly, unlike arterial walls, venous walls experience less pressure. However, among the veins there is also one rather large one. The largest vein reaches a diameter of 2.5 centimeters. Small veins are usually called venules.
The pulmonary vein carries blood that is already saturated with oxygen. Each vein has internal valves that prevent backflow. A person experiencing valve dysfunction may develop varicose veins.
Types of open surgical operations for aortic aneurysms:
- Bentalla-De Bono operation (replacement of the ascending aorta with a valve-containing conduit with a mechanical prosthetic aortic valve);
- David's operation (replacement of the ascending aorta while preserving the native aortic valve);
- Supracoronary aortic replacement;
- Prosthetics of the ascending aorta and its arch (Borst technique, the use of oblique aggressive anastomosis and other techniques);
- Thoracic aortic replacement;
- Abdominal aortic replacement.
The problem of surgical treatment of diseases of the aortic root (CA) has been studied in our country for a long time. Despite the fact that more than 40 years ago academician. E.N. Meshalkin performed the first open aortic commissurotomy in Russia; it continues to be relevant [3].
It is difficult to find in the literature a complete description of the anatomy of the coronary artery, considered from the point of view of a practicing surgeon.
The purpose of this work is to summarize knowledge in this area with practical tips and recommendations for surgeons.
Fibrous framework of the heart
The fibrous rings of the mitral (MV) and aortic valves (AV) are closely connected with the fibrous frame of the CA through the membranous part of the interventricular septum (IVS), forming a single fibrous frame of the heart. If you look at the planes, it turns out that the tricuspid valve (TV) is shifted slightly to the apex relative to the MV, and the AC, together with the structures surrounding it, is sort of wedged between them, being in the center of the heart (see Fig. 1 on the color insert).
Figure 1. Fibrous framework of the heart [10]. MK - mitral valve; TC - tricuspid valve; PLV - pulmonary valve; L - left coronary cusp; R - right coronary cusp; N - non-coronary cusp. Just above and in front of the AC is the pulmonary valve. In the axial plane, the AC is located at an angle of 30° [26]. Thus, blood ejection from the left ventricle (LV) does not occur upward, but at an angle of 45° or more relative to the frontal plane [44].
The CA is adjacent directly to the wall of the right atrium, at a short distance it comes into contact with the IVS and “rests” on the right ventricle (RV). The convexity in the IVS, which is formed due to the abutment of the CA into it, forms the anterosuperior wall of the LV (Fig. 2, a on the color insert).
Figure 2. Cross-section of the heart [39]. a — fibrous structures of the anterior and superior wall of the LV; b — structures of the lower wall of the LV, the dotted line shows three levels of the aortic root. LV - left ventricle; LA - left atrium; Ao - aorta. Most of the structures of the LV wall are formed from muscle tissue, but the superomedial part consists of connective tissue and forms the membranous portion of the IVS. Next, it is adjacent to a fibrous area passing between the anterior leaflet of the MV and the AC, thus forming the posterior wall of the LV.
Aortic root and aortic valve leaflets
The coronary artery is the LV outflow tract, consisting of supporting structures for fixation of the valve leaflets and serving as a bridge between the LV and the aorta. The term “arterial root” was first used by Henle, displacing the previous term “aortic annulus” [45]. The reason for this was the difference between the functional and anatomical structure of the coronary artery.
When studying the CA as a locking element of the LV outflow tract, conclusions were drawn about the need to study this structure as a single anatomical and functional complex [2, 4, 9, 16].
To better understand this structure, it is necessary to identify the lower and upper boundaries of the coronary artery, which correspond to the area of fixation of the valve leaflets and the sinotubular junction. Thus, for simplicity, the KA is represented as a cylinder, the walls of which are formed by the sinuses of Valsalva [29] (see Fig. 2, b; 3 on the color plate).
Figure 3. Schematic representation of the aortic root [10]. Considering that the widest area is at the level of the sinuses, and the size at the level of the valves can be 20% wider than in the region of the sinotubular junction [25], the shape of the coronary artery is more correctly characterized as an onion. After numerous studies (echocardiography, intraoperative measurements, autopsy results), summarized by N. Borst et al. [15], it was determined that the average diameter of the coronary artery in the widest area is 31 mm, but it can change along its length both in healthy people and with the development of a pathological process [25, 33]. The average diameter of the aortic orifice in adults is approximately 2.3 ± 0.3 cm, and the area of the valve opening is 4.6 ± 1.1 cm2 [8].
There is still no consensus on how best to view CA structures [12], and an even bigger question is the presence of the so-called annulus
, which is interpreted differently [32, 42].
W. McAlpine [28] in 1975 expressed the following opinion: “ Annulus
translated from Latin as ring, i.e. circle. In this case, this word can only be addressed to the fibrous base of the AC and to the pulmonary valve, which in reality constitute only a segment, not a circle, but an oval. The term "annulus" is used to describe the four fibrous structures that are connected by the four valves of the heart."
Another author [16], studying macro- and microscopic preparations of the coronary artery, describes the annulus as “the border between the upper and middle third of the aortic root” [16]. At the same time, most surgeons believe that fibrous structures remaining after removal of the valve leaflets should be called annulus [32, 46]. Another opinion is based on the identification of a “virtual” ring, the circle of which is formed by connecting the most proximal points of each valve [43] (see Fig. 3, color plate). Thus, one should not try to isolate any one “annulus”, but use all the proposed “rings” to establish a more accurate spatial perception of the coronary artery as an integral structure of the heart.
To understand the function of the coronary artery, it is necessary to separate two functional and anatomical transitions: the first - between the LV and the coronary artery, the second - between the coronary artery and the aorta, respectively.
The ventricle-aortic junction is localized in the area of transition of the muscular structures of the ventricle into the fibro-elastic tissue of the coronary artery. However, this transition is not a ring; it has a wave-like shape, which is determined by the method of fastening the AK flaps in this area (see Fig. 4 on the color insert).
Figure 4. Aortic root [39]. Small arrows indicate the orifices of the coronary arteries, large arrows indicate the attachment points of the leaflets and the sinotubular ridge, * the triangle of Henle is indicated, the dotted line indicates the fibrous aortomitral junction. The valves are attached to the fibrous tissue of the coronary artery in the form of elongated crescents. It is worth noting that this place is a border zone in which the voltage from the blood flow to the surrounding structures changes. All underlying structures are subject to tension at the time of contraction of the LV walls, and the overlying ones contain the pressure that increases from the flow of blood into the aorta.
The area between the attachment of two adjacent valves, limited from below by a “virtual” ring, forms triangles, which are called triangles of Henle (see Fig. 4 on the color plate) [9]. They are fibromuscular components of the coronary artery, providing a unified geometry of the structure, and allow the sinuses of Valsalva to function relatively independently of each other [41].
The sinotubular junction is the most distal zone of the coronary artery (see Fig. 2, b; 3 on color plate). The valves of the AC at this level are connected to the integral component of the AC. Any expansion at this level leads to the development of AC insufficiency, and stenosis of this zone is called supra-aortic [40]. From an anatomical point of view, the sinotubular junction is the outlet from the coronary artery.
Considering that the AC is shaped not like a cylinder, but an onion, and that the line of attachment of the valves does not follow a geometric ring, but has a crown shape, when a mechanical valve is implanted into the AC position, it is fixed to the fibrous ring, which naturally leads to natural deformation forms of KA, indirectly affecting biomechanics. When implanting frameless grafts, some of the stitches are stitched behind the fibrous ring, and some are fixed to the myocardium in the area of the ventricular-aortic junction, so as not to deform the bioprosthesis, which does not have a rigid frame [8].
In a narrow sense, the AV is sometimes understood as the structure of the fibrous framework of the AV with obturator elements included in it—the leaflets [37]. AC valves consist of a body (the main load-bearing part), a coaptation surface (closing) and a base [18]. Each leaflet has several folding surfaces that face the aorta and a smooth surface that faces the ventricle. A triangular thickening in the central part of the coaptation zone is called Arantzi's node.
The valves divide the KA into sub- and supravalvular KA components. The subvalvular elements are similar in their morphological structure to the ventricle, and the supravalval elements are similar to the aorta. It is worth noting that the similarity is transitive, i.e. Only the dominant morphological structures are histologically noted, but in each of these areas there are also opposing structures.
The valve flaps are a locking element that prevents blood from flowing in the opposite direction. Morphologically, they consist of dense bundles of collagen, which allows them to ensure their functional properties. Between the ventricular and aortic surfaces of the valves of the aortic valve, 5 layers are distinguished: ventricular, radial cancellous, fibrous and arterial [24]. The arterial layer contains in most cases collagen fibers oriented circumferentially in the form of bundles, cords and a small amount of elastic fibers [35, 36], which allows the leaflets to compress during load reduction [17]. In the coaptation zone, collagen fibers are located at an angle of 125° relative to the aortic wall [43], in the body - at an angle of 45° from the fibrous ring. This orientation of the bundles is necessary to redistribute the pressure load during diastole from the leaflets to the sinuses and fibrous frame [30].
In a relaxed state, the structure of the valves is in a state of contraction, and during loading they straighten. When maximum straightening is achieved, the extensibility of the valves is reduced to zero. The ability to stretch manifests itself with minimal loads of blood flow on the valve [36].
Sinuses of Valsalva
The sinuses of Valsalva are dilated areas of the coronary artery, limited by the valves and sinotubular junction [33]. At the base level they are separated from each other by the triangles of Henle. Their main role is the redistribution of blood tension during diastole and the establishment of an equilibrium position of the valves in systole [14].
The sinuses of Valsalva serve as a transition link between the ventricle and the aorta, as a result of which both origins are present in their histological structure. The muscular elements of the LV provide only support to the base of the coronary artery, mainly at the level of the right coronary sinus.
The sinuses of Valsalva consist only of intima and media in the proximal part, with adventitia added at the level of the sinotubular junction. The inner layer of the sinuses is covered, like the vessels, with endothelium. The middle layer has circular connective tissue structures (collagen, elastin), as well as smooth muscle cells. The adventitia consists predominantly of type I collagen [36].
In the general structure, it is worth noting that the media of the sinuses of Valsalva is thickened due to an increase in the number of collagen fibers. At the same time, in the direction from the ventricle to the aorta, there is an increase in the number of elastic fibers and a decrease in collagen fibers. This change leads to an increase in functionality, since collagen is “responsible” for the strength and rigidity of the structure, and elastin is for the ability to stretch and “extinguish” excess tension in the aorta during systole.
There are three functionally identical sinuses, although from an anatomical point of view there are differences between them. The coronary arteries depart from two of them, which is why they are called the left and right coronary sinuses, while the third is usually called the non-coronary sinus.
(see Fig. 5 on the color insert).
Figure 5. Relationship between the aortic root and the conduction system of the heart [10]. AB - atrioventricular; n. His - bundle of His. If you look at anatomical atlases, you can see a different name, which is based on the topography of the sinuses when looking at the heart from above. They will be called anterior (for right coronary), left posterior (for left coronary) and right posterior (for non-coronary). However, in surgical practice it is more convenient to call them coronary and non-coronary sinuses, as this greatly facilitates navigation. Normally and with various anomalies of coronary artery development, the coronary sinuses will be adjacent to the pulmonary valve; this is called lining the aortic sinuses. In isolated cases, the coronary arteries may arise from the “unlined” aortic sinus [23, 26]. The sinuses are also unequal in size: the largest is the non-coronary sinus, and the smallest is the left coronary sinus [13]. The size of the sinuses of Valsalva must be taken into account when planning the Jakub operation, which involves prosthetics of the latter.
As noted earlier, the CA is located in the center of the heart and is connected to all its structures (Fig. 6).
Figure 6. Diagram of aortic root topography. LCL—left coronary cusp; NCL—non-coronary cusp; ACL—right coronary cusp; IVS - interventricular septum; CFT - central fibrous body With the development of aneurysms involving the sinuses of Valsalva, rupture of one of the sinuses and the formation of an anastomosis with various cavities of the heart are possible. A non-coronary sinus rupture opens into the right or left atrium, and a left coronary sinus rupture can pierce into the left atrium or pericardial cavity. In most cases, rupture of the right coronary sinus occurs, which leads to perforation into the right atrium or into the outflow tract of the RV [22] (see Fig. 1, color plate). Due to the anatomical proximity of the coronary artery and the right atrium, it is possible to easily form an anastomosis between the paraprosthetic space of the coronary artery and the right atrium, which is used in the Bentall-de Bono operation to prevent paraprosthetic hematomas [6].
The triangle formed by the left coronary and non-coronary sinuses is part of a large area along the path of connection between the AC and MV. When it is removed, a window is formed connected to the transverse sinus, which is located between the posterior surface of the coronary artery and the anterior wall of the left atrium [42]. The triangle formed by the right coronary and non-coronary sinuses is closely connected with the membranous part of the IVS. Its removal creates a window between the LV exit and the right side of the transverse sinus. The third triangle, which separates the two coronary sinuses, is the least extensive of the three. To mark the exit site when removing this triangle, it is necessary to resect the free-standing muscular portion of the pancreatic outflow tract. This creates a “window” between the subaortic portion of the LV outflow tract and the level of tissues that separate the coronary artery from the pulmonary artery.
The triangles of Henle are fibrous or fibromuscular components of the coronary artery and are located between adjacent segments of the annulus fibrosus and the two leaflets of the coronary artery [9]. Collagen fibers are located in the outer layer of these structures, which provides the supporting properties of the valve apparatus of the coronary artery [7, 43].
The triangle between the left coronary and non-coronary sinuses forms part of the aortomitral velum, which is histologically represented by connective tissue and is equivalent to the valves of the mitral valve. The triangle between the non-coronary and right coronary sinuses connects to the membranous part of the IVS, which also consists of connective tissue. In contrast, the intercoronary triangle is supported by both connective and muscle tissue [10, 46]. Recent studies show that the triangles of Henle have a complex cytoskeleton and are capable of contraction due to the presence of vementin, desmin and smooth α-myosin chains in their structure. This helps reduce the load on the coronary artery and redistribute it to most of the heart structures [20].
It is necessary to clearly understand the spatial relationships of the heart structures with the coronary artery in order to understand the essence of the techniques for expanding the “narrow” aortic root (see Fig. 6). The annulus fibrosus is considered “narrow” if its diameter is less than 21 mm and the patient’s body surface area is more than 1.7 m2 [1, 27].
The most commonly used method of expansion of the coronary artery according to Nicks (the useful perimeter of the AC ring increases by 4.0-4.5 mm), which involves dissecting the coronary artery through the middle of the non-coronary cusp to the base of the anterior mitral valve leaflet. The Manouguian technique involves transannular dissection through the lateral commissure and the mitral-aortic contact zone with a transition to the initial portion of the anterior valvular leaflet, with possible opening of the roof of the left atrium (the method allows you to expand the aortic root by 7-8 mm). The most aggressive technique for expanding the coronary artery is aortoventriculoplasty according to Konno, i.e. longitudinal transannular dissection of the coronary artery and IVS through the outflow tract of the pancreas, which makes it possible to increase the size of the fibrous ring of the coronary artery by 40-50% and implant prostheses larger than 21 mm in all patients [3].
The continuity of the mitral-aortic contact is of great importance for surgical practice. With decalcification of the AC and the anterior mitral leaflet, perforation of the latter can easily occur. In this case, the defect in the anterior mitral valve leaflet can be sutured with 5/0 prolene thread.
Adjacent to the zone of mitral-aortic contact is a section of the membranous septum, which actually separates the LV outflow tract for a short distance from the cavity of the right atrium and the RV. In the presence of high perimembranous IVS defects of small diameter, they can be sutured from the transaortic approach with several U-shaped sutures on spacers with a puncture on the fibrous ring of the AC above the leaflets. Similarly, the proximity of the AC must be taken into account during operations on the MV. Thus, when placing sutures on the fibrous annulus of the mitral valve in the area of its anterolateral commissure, care must be taken not to capture the non-coronary valve of the aortic valve in the suture, which will lead to iatrogenic aortic insufficiency [8]. With significant dilatation of the fibrous ring of the AV, it is possible to replace the MV with a transaortic approach.
Relationship with the conduction system of the heart
It is necessary to clearly understand the relationship of the coronary artery structures with the conduction system of the heart, because suturing tissue in the area of passage of the His bundle will inevitably lead to cardiac arrhythmia.
The atrioventricular node is located in the wall of the right atrium, slightly distal to the coronary artery. Next, the bundle of His goes to the central fibrous body, located at the level of the triangle formed by the right coronary and non-coronary sinuses (see Fig. 5, color inset) and formed from the connection of this triangle with the membranous part of the IVS. Having reached the central fibrous body, the His bundle pierces it, resulting in isolation of the conduction system. Located on the anterior surface of the crest of the muscular septum, the His bundle gives off legs: the left one spreads out like a fan on the wall of the LV, while the right one penetrates the muscular septum and emerges on the surface medial to the papillary muscles.
When applying sutures to the fibrous ring, especially after its decalcification, on the one hand, for reliable fixation, it is necessary to capture more tissue in the suture, and on the other hand, the risk of injury to the conductive tract increases. Thus, it becomes clear that it is inappropriate to apply deep sutures in the area between the right coronary and non-coronary sinuses, as well as gross decalcification in this area. Such manipulations can lead to conduction disturbances up to complete transverse blockade in the postoperative period. In a number of situations, in the area of the non-coronary sinus, for reliable support of the prosthesis, valve-fixing sutures can be placed on the outer wall of the aorta.
Bicuspid aortic valve
The prevalence of bicuspid AK in the population is 1–2.5% [21]. The morphology of AC valves can vary significantly; they can be either the same or different [11].
In some cases, when three valves of the AC are formed, the valves fuse during embryogenesis and the AC takes on the appearance of a bicuspid one. In this case, most often during the audit it is stated that there are three normally located sinuses of Valsalva in the presence of two valves.
The formation of triangles of Henle also depends on the structure of the valves and the number of sinuses of Valsalva. When two valves of AC are formed, the height of the triangles of Henle is less than with TC. This circumstance reduces the mobility of the valves [40]. In case of anomaly, the valves are called anterior and posterior or left and right (according to their location). Anteroposterior are found in 79% of cases, and the coronary arteries in this case arise from one (anterior) sinus [19]. With the left-right arrangement of the valves, the coronary arteries arise from both sinuses.
In most cases, bicuspid aortic valve does not have specific symptoms for humans, but compared with the normal tricuspid valve, it is more likely to calcify and lead to the formation of aneurysms of the ascending aorta [31].
According to the literature, it can be stated that bicuspid aortic valve is one of the reasons for the formation of aneurysms and dissections of the ascending aorta. The question of the surgeon's tactics when detecting a bicuspid aortic valve intraoperatively during surgery for an aneurysm of the ascending aorta remains unresolved. If the bicuspid valve is healthy and the patient’s age at the time of surgery is over 60 years, it is logical to preserve the native valve, since its service life will be longer than the patient’s life expectancy. For patients under 50 years of age, the question of the advisability of preserving a bicuspid aortic valve or its reimplantation into a coronary artery prosthesis is currently open.
Thus, the KA is, as it were, the center of the heart, being in close relationship with its structures. To plan surgical tactics and prevent intraoperative complications, the surgeon must clearly understand the features of the anatomy and syntopy of the coronary artery. Our further research will be aimed at studying the anatomy of the coronary artery in the anatomical theater in order to optimize the surgical technologies used in the pathology of this area.
Endovascular interventions
They allow you to dramatically reduce the amount of surgical trauma, shorten hospitalization periods and reduce the inevitable suffering of the patient associated with surgical approaches. One of the main disadvantages of the method is the need for repeated interventions.
Types of endovascular operations for aortic aneurysm:
- implantation of a stent graft into the abdominal aorta,
- implantation of a stent graft into the ascending (thoracic) aorta.
The most modern method of treating aortic aneurysm is a hybrid method, which allows achieving optimal treatment results with the least surgical trauma.
Hybrid surgeries combine the advantages of open and endovascular interventions.
To prevent the development of aortic aneurysms, the most important thing is the need to control risk factors, namely arterial hypertension. In addition to arterial hypertension, the most significant risk factors are age (over 55 years), male gender, smoking, the presence of aneurysms in direct relatives, and high cholesterol levels.
Why does high blood pressure occur?
High (high) blood pressure is commonly called hypertension. When pumping power becomes excessive, the human body becomes overwhelmed by its own blood. The body is unable to function normally, which can have harmful consequences. Hypertension is common among residents of developed countries where there is a stressful standard of living.
The older a person gets, the higher the risk of developing hypertension. Even young people sometimes experience high blood pressure. Vessels may experience stress due to a large release of hormones (adrenaline and norepinephrine). Problems with high blood pressure occur in those who like to drink beer, causing damage to the kidneys. The problem with hypertension is that it almost always goes silent. People who lead a sedentary lifestyle, gain excess weight, or consume a lot of alcohol and salty foods often do not even suspect that they are developing hypertension. Meanwhile, the higher the pressure, the higher the risk of vascular damage. The most terrible consequences of hypertension are stroke, the development of heart or kidney failure, and myocardial infarction.