Alena Yakimenko, Anastasia Sveshnikova, Elena Artemenko, Mikhail Panteleev “Nature” No. 2, 2014
About the authors
| Alena Olegovna Yakimenko ( |
| Anastasia Nikitichna Sveshnikova ( |
| Elena Olegovna Artemenko - Researcher at the Center for Physics and Physics of the Russian Academy of Sciences. He studies the reorganization of the platelet cytoskeleton during activation and the mechanisms leading to the formation of procoagulant activated platelets. |
| Mikhail Alexandrovich Panteleev — Doctor of Physics and Mathematics, Professor, Head of the Laboratory of Molecular Mechanisms of Hemostasis, TsTPFCP RAS. Winner of the award named after. R.V. Khokhlova (2002) and the European Academy Prize (2007). Area of scientific interests: mechanisms of regulation of hemostasis and thrombosis, biochemistry and biophysics of blood coagulation, mathematical modeling of biological systems. |
The most important role of platelets in a living organism was discovered by the Italian physician and pathologist Giulio Bizzocero, who in 1882 conducted a series of brilliant experiments with only a light microscope at his disposal. Today we have much more measuring instruments and computers that perform complex mathematical calculations, but many questions remain open. It is known that platelets play a key role in stopping bleeding from a wound (hemostasis) and dangerous closure of a healthy vessel (thrombosis). However, it is still unclear how exactly the hemostatic system functions. What reasons lead to its switching from protecting the body to the development of life-threatening pathologies? What is the role of platelets in the regulation of hemostasis and thrombosis? We don’t know why platelets are so complex, and we don’t understand the entire sequence of events that ensure the formation of a blood clot at the site of damage, and experimental data bring with them new mysteries.
Structure
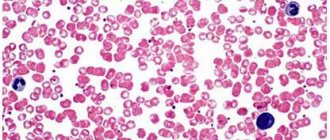
Rice. 1.
Micrograph of non-activated platelets []
Platelets (from the Greek θρομβοζ - 'clot' and κυτοζ - 'cell') are specialized non-nuclear blood cells, having the shape of a disk with a diameter of about 3 microns and a thickness of about 0.5 microns (Fig. 1). They are formed during the fragmentation of large bone marrow cells—megakaryocytes—and circulate in the bloodstream at a concentration of 200–400 thousand cells per 1 μl of blood. Platelets live in the bloodstream for an average of 5–9 days, and then are destroyed in the spleen and liver.
The structure of a platelet is quite complex. Externally, it is limited by a bilipid layer of the membrane, numerous invaginations of which (an open tubular system) provide a reserve surface for changing shape (Fig. 2). It supports it and at the same time allows for significant changes in the cytoskeleton (framework) of the cell. Inside there are the endoplasmic reticulum (a repository of calcium ions necessary for signaling and the platelet performing its functions) and mitochondria (organelles that ensure respiration). The cytosol contains granules containing substances that spill out into the extracellular space upon cell activation (transition to a new state). Dense granules contain nucleotides (ATP, ADP, GTP, GDP), serotonin, calcium ions in high concentrations, α-granules contain various proteins (including blood clotting factors), and lysosomes contain some enzymes (collagenase, elastase and etc.).
Rice.
2. Scheme of the platelet structure []
After platelet activation, a negatively charged lipid, phosphatidylserine, appears on the outer surface of its membrane. Some coagulation factors bind to it with the help of calcium ions, forming special complexes. They greatly accelerate the reactions leading to gelation of blood plasma at the site of injury (this process is called plasma hemostasis). In other words, phosphatidylserine provides a procoagulant function of platelets that promotes plasma hemostasis.
Why is the lifespan of these blood cells so short-lived (red blood cells, for example, live three to four months), because normally, in the absence of serious damage to blood vessels, they practically do not work? Why do they look like disks? Why does a platelet need mitochondria if its energy expenditure is extremely modest? Why did nature need to accelerate plasma coagulation reactions on cell membranes? Why do α-granules contain coagulation proteins, which are also found in blood plasma? These are just some of the questions that do not yet have clear answers.
What does platelet aggregation disorder lead to?
If the aggregation process is disrupted, this can lead to negative consequences. So, if the activity of colorless blood cells is increased, this can lead to a stroke or heart attack. And if platelet production is reduced, this can lead to large blood loss: a person suffers from frequent bleeding that does not stop for a long time, leading to exhaustion and anemia.
It is in order to prevent these consequences that platelet aggregation analysis is performed. Indications for testing are:
- Frequent bleeding (nasal, uterine)
- Poorly healing wounds.
- Bruises that appear from the slightest injury.
- Constant swelling.
Activation
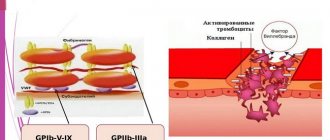
To perform their main function - repairing damage in the vessel wall - platelets must enter an active state. Like most cells in our body, this process proceeds according to the following scheme: signal - receptor - intracellular signal - amplifier - regulator - response (Fig. 3). The signal for activation is the appearance in the bloodstream of an agonist - a special signaling molecule, which should appear only when necessary and bind to a specific molecule that penetrates the platelet membrane (receptor). The agonist interacts with one “tail” of the receptor, protruding from the outside, and this leads to a change in the other, on the cytosol side, where the next signaling molecule, the secondary messenger, appears. It triggers the synthesis of several more messengers, which, in turn, triggers several more, and so the signal propagates in the cytosol and is amplified through a cascade of intracellular reactions, which ultimately leads to a complex platelet response. It is important that in the platelet there are special regulatory systems that modulate the concentrations of intracellular messengers at different stages of activation, so that, for example, there is no reaction to trace amounts of the agonist.
Rice. 3.
Platelet activation scheme
How is this scheme implemented in our body? In blood vessels, platelets are pushed out of the main flow by red blood cells and move along the walls, conducting a kind of monitoring of their condition. One of the first signals for platelet activation is collagen, the main protein of connective tissue, exposed when a vessel is damaged. Having detected collagen, they bind to it through special receptors, simultaneously becoming activated and firmly attaching to the site of damage. The interaction of a platelet with collagen leads to the launch of the mentioned intracellular signaling cascade and the appearance of a secondary messenger in the cytosol - inositol triphosphate (IP3). This small water-soluble molecule is able to move quickly in the cytosol and serves as a signal for the release of calcium ions from intracellular stores. And an increase in its intracellular concentration can lead to various responses of the platelet: splashing out the contents of granules (secretion), changing shape, attaching to the vessel wall (adhesion), bonding with other platelets (aggregation), and the appearance of procoagulant activity (Fig. 4). After the circulatory system has already recognized the damage to the vessel, three more natural platelet activators appear in the blood - thrombin, ADP and thromboxane A2. The thrombin protein is formed from its precursor, prothrombin, in the blood plasma, but in large quantities - already on the membranes of activated platelets. When their dense granules are secreted, large amounts of ADP (a small molecule that primarily performs energy functions in cells) are released, and much less ADP is released from damaged endothelial cells lining the inner surface of blood vessels. Thromboxane A2 is synthesized from arachidonic acid, located in the membranes of activated platelets. The binding of these three activators to their receptors on the platelet membrane leads, as in the case of collagen, to the appearance of IP3 in the cytosol and an increase in the calcium concentration in it (Fig. 4). Thus, all three soluble activators and collagen act through the same pathway, but induce different platelet responses. For example, thromboxane A2 provokes the release of dense granules, but ADP does not. Activation separately by collagen or thrombin causes all of the above responses simultaneously, and together leads to the appearance of a group of procoagulant platelets and the synthesis of thrombin on their membranes. Apparently, there are still insufficiently studied differences in the signaling triggered by different agonists. To prevent accidental activation from turning a platelet into a real “bomb”, carried in the bloodstream and triggering the entire coagulation system, intact endothelial cells in the body constantly secrete prostacyclin and nitric oxide, which block cell activation, preventing an increase in calcium concentration in them.
Rice. 4.
Scheme of the main pathways of platelet activation and its responses:
ADP
- adenosine diphosphate,
IP3
- inositol triphosphate,
ER
- endoplasmic reticulum
Signaling is one of the most complex and poorly understood areas in platelet research. There are many questions about the structure of each receptor and signaling pathway, and the simplest of them is: why are there so many activators at all?
conclusions
A subgroup of patients with arterial hypertension living in China who have a reduced platelet count and elevated blood homocysteine levels have the highest risk of stroke, but this risk is reduced by 73% with the use of FA.
If these findings are confirmed, reduced platelet counts and elevated blood homocysteine levels could serve as biomarkers to identify individuals at high risk of stroke who may benefit from FC treatment particularly.
Cytoskeleton and shape change
The platelet cytosol is permeated by a three-dimensional network of water-insoluble protein threads (filaments), which forms the cytoskeleton. The filaments consist of polymerized actin protein and ensure that the platelet changes shape when activated. In addition, just below the plasma membrane is a membrane skeleton associated with the cytoplasmic “tails” of some receptors. It consists of short actin filaments connected to each other using special proteins. The membrane skeleton not only supports the plasma membrane, regulating the contours of the cell, and stabilizes it, preventing fragmentation, but also regulates the distribution of receptors attached to it in the plane of the membrane. It is also suggested that it plays an important role in the regulation of various intracellular events that are triggered upon activation.
Rice. 5.
Scanning electron micrographs of the process of spreading an activated platelet (
a–d
) over the surface []
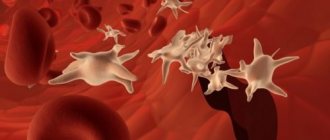
Interestingly, the cytoskeleton is a dynamic structure, thanks to which a platelet can not only change shape, but also grow “tentacles” (filopodia). With their help, it spreads over the surface of the damaged vessel (Fig. 5) and more easily adheres to other platelets (Fig. 6). Relatively recently, it was discovered that upon strong activation (by thrombin alone or together with collagen), platelets are divided into two groups (subpopulations), very different in properties and even shape, which suggests a fundamentally different organization of the cytoskeleton in them. Some of them (“regular” activated) have the appearance of amoebas - lumps with filopodia, others (procoagulant, since there is a lot of phosphatidylserine on the outer surface of their membrane) - balls without “tentacles”. The data obtained in our laboratory indicate that some membrane receptors responsible for binding cells to the surface and to each other are unequally attached to the cytoskeleton in platelets from the two subpopulations. This means that they can interact differently with the damaged vascular wall and with each other in the forming blood clot.
The sequence of processes during the restructuring of the platelet cytoskeleton has generally been studied quite little, but here is a new question: why do some cells become “amoebas” when activated, and others become “balls”?
Explanation of the platelet aggregation test
In the platelet aggregation test, indicators of 25–75% indicate good hematopoiesis. This means that tissues and organs are normally supplied with oxygen, and there are no blood clots.
Platelet rate
| Age | Indicator, x 10^9/l |
| Newborn | 100–420 |
| Child under one year old | 160–320 |
| 1–4 years | 150–300 |
| 15–18 years old | 180–340 |
| Men over 18 years old | 180–400 |
| Women over 18 years old | 150–380 |