The use of angiotensin receptor blockers in the treatment of arterial hypertension
What is the advantage of angiotensin receptor blockers over other classes of antihypertensive drugs, in particular over ACE inhibitors? What is the comparative effectiveness of various angiotensin receptor blockers?
Based on a representative sample (1993), it can be stated that the prevalence of arterial hypertension (AH) in Russia is 39.2% among men and 41.1% among women. At the same time, only 58.9% of women and 37.1% of men know about the presence of the disease; 46.7% and 21.6% receive treatment (including effective treatment - 17.5% and 5.7%), respectively (first report experts of the Scientific Society for the Study of Arterial Hypertension, the All-Russian Scientific Society of Cardiologists, the Interdepartmental Council on Cardiovascular Diseases, 2000). The management of patients with hypertension is currently regulated by the recommendations of experts from the World Health Organization (WHO) and the International Society of Arterial Hypertension (ISH) (WHO-SOG recommendations, 1999) and the National Guidelines for the diagnosis and treatment of arterial hypertension developed on this basis (All-Russian Scientific Society of Cardiology, Arterial Hypertension Section, 2001). According to these recommendations, the goal of hypertension treatment is to reduce the overall risk of cardiovascular morbidity and mortality, which involves reducing blood pressure to the target level (less than 140/90 mm Hg), as well as correcting all identified risk factors (for example, adequate treatment of hypercholesterolemia , diabetes mellitus). Since course treatment of hypertension is ineffective (in most cases, hypertension cannot be cured), the patient must receive individually selected antihypertensive therapy constantly.
For long-term treatment of hypertension, β-blockers, diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, calcium antagonists, and α-blockers are currently used. As is known, in the pathogenesis of arterial hypertension, angiotensin II plays a crucial role, causing vasoconstriction, stimulation of aldosterone synthesis and its release, sodium reabsorption in the kidneys, cardiac muscle growth, proliferation of smooth muscle cells of blood vessels, increased peripheral noradrenergic activity and a number of other effects. Therefore, the most promising in the drug correction of hypertension are currently considered to be angiotensin-converting enzyme (ACE) inhibitors, which prevent the transition of angiotensin I to angiotensin II, and angiotensin receptor blockers. Widely used ACE inhibitors, although highly effective, have a number of side effects (including cough, angioedema) due to their effect on the metabolism of bradykinin and substance P [4].
Angiotensin receptor blockers have a number of advantages over ACE inhibitors - they more specifically and effectively suppress the cardiovascular effects of activation of the renin-angiotensin system. Currently, this “youngest” group of antihypertensive drugs (the first of them is losartan, synthesized in 1988) is represented by a number of drugs that differ slightly from each other in their mechanism of action and pharmacokinetic properties.
Based on their chemical structure, they distinguish between biphenyl tetrazole derivatives (losartan, irbesartan, candesartan), non-biphenyl non-tetrazole compounds (eprosartan, telmisartan) and non-heterocyclic compounds (valsartan); depending on the presence of the active metabolite - prodrugs (losartan, candesartan) and active medicinal substances (valsartan, irbesartan, telmisartan, eprosartan); depending on the type of antagonism with angiotensin II - competitive antagonists (losartan, eprosartan) and non-competitive (valsartan, irbesartan, candesartan, telmisartan). The main characteristics of various angiotensin receptor blockers are given in table. 1.
The hypotensive effect of angiotensin receptor blockers is primarily associated with the suppression of the vasoconstrictor effect of angiotensin II, realized through receptors in the walls of blood vessels. In addition, blockade of angiotensin II receptors leads to a decrease in the secretion of aldosterone, a decrease in the reabsorption of sodium and water in the proximal segment of the renal tubules.
Stimulation of type 2 angiotensin receptors may play a certain role in the hypotensive effect when the level of angiotensin II is elevated (due to blockade of type 1 receptors). It is assumed that stimulation of angiotensin II type II receptors can lead to vasodilation and suppression of proliferative processes.
At the same time, electrophysiological studies in animals have shown that angiotensin II, by activating presynaptic angiotensin receptors of noradrenergic neurons of the sympathetic nervous system, increases the release of norepinephrine. When studying the effect of various angiotensin receptor antagonists (valsartan, irbesartan, losartan, eprosartan) on the sympathetic release stimulated in decerebrate normotensive rats by spinal cord irritation, an inhibitory effect was noted only for eprosartan [6]. Thus, in clinical practice, eprosartan (teveten) is the only drug in its group that can block both presynaptic receptors and angiotensin receptors in blood vessels in therapeutic doses.
Angiotensin receptor blockers, used in therapeutic doses, on average reduce systolic blood pressure by 10-20 mmHg. Art. and diastolic - by 10-15 mm Hg. Art., as shown in a large number of studies. The maximum reduction in blood pressure is achieved in most patients after 3-4 weeks of treatment.
As an example, here are several clinical studies on the effectiveness of eprosartan. An 8-week, double-blind, placebo-controlled, randomized, clinical (243 patients with mild to moderate hypertension) study of eprosartan (teveten at a dose of 600 mg once daily) showed that the drug lowered blood pressure significantly more effectively than placebo [3 ]: in the eprosartan group, systolic blood pressure decreased by 6 mmHg. Art., diastolic - by 7.5 mm Hg. Art.; the difference compared to the results in the placebo group was statistically significant. Therapy was considered effective if diastolic pressure in the sitting position decreased to 90 mmHg. Art. or the decrease in diastolic blood pressure from the initial level was 10 mm Hg. Art. and more. In the eprosartan group, therapy was effective in 42% of patients, in the placebo group - in 21%.
The relationship between the dose of eprosartan and the level of blood pressure reduction was assessed in a multicenter, double-blind, parallel, placebo-controlled study that included 364 patients with a baseline diastolic blood pressure of 95-114 mm Hg. Art. The effectiveness of eprosartan therapy was assessed at doses of 400, 600, 800, 1200 mg once a day compared with placebo; the duration of treatment was 8 weeks. According to the results obtained, the optimal initial dose of the drug was 600 mg per day [10].
In a 13-week, double-blind, placebo-controlled, parallel-group study [4], 243 patients received eprosartan at a daily dose of 400–800 mg once or twice daily. The dose of the drug was adjusted during the first 9 weeks until the optimal hypotensive effect was achieved, after which drug therapy at an effective dose continued for another 4 weeks. The hypotensive effect of eprosartan was once again confirmed (diastolic blood pressure decreased in the treatment group by an average of 9 mm Hg versus 4 mm Hg in the placebo group), and the therapeutic effect was the same when taking the drug once or twice a day. Therapy with eprosartan (once daily) was effective in 46.8% of cases.
A number of studies have shown that angiotensin receptor blockers are at least as effective as ACE inhibitors (Table 2). For example, in a 26-week, double-blind, clinical (528 patients aged 21-78 years with mild to moderate hypertension) study [2], therapy with eprosartan at a dose of 400-600 mg per day was more effective than treatment with enalapril in dose 5-20 mg per day. There were more patients in whom antihypertensive therapy was found to be effective in the eprosartan group (81.7%) compared to the enalapril group (73.4%). When analyzing the results obtained, it turned out that in the subgroup of elderly patients, the frequency of cases of “response to treatment” was the same as in young patients [1]. Similar results were obtained in another study devoted to a comparative assessment of the hypotensive effects of eprosartan and enalapril in mild to moderate arterial hypertension [7].
The comparative effectiveness of eprosartan (400-800 mg per day in two divided doses) and enalapril (10-40 mg per day in one dose) in severe arterial hypertension was studied in a 10-week double-blind study involving 118 patients (78% of them in over 65 years of age) [8]. The dose was titrated every two weeks; If necessary, hydrochlorothiazide (hypothiazide 25 mg per day) was added to therapy. Therapy with eprosartan led to a more significant reduction in systolic and diastolic blood pressure compared with enalapril; additional prescription of diuretics was required in both groups in almost the same number of patients (39% of patients in the eprosartan group, 37% in the enalapril group). Thus, compared with enalapril, eprosartan is more effective in reducing elevated systolic blood pressure in severe arterial hypertension.
A number of studies have assessed the effectiveness of various angiotensin receptor blockers. For example, an 8-week study [5] included 567 patients with mild to moderate hypertension (Table 3). Therapy with irbesartan at a dose of 300 mg per day was slightly more effective than treatment with losartan at a dose of 100 mg per day; the proportion of patients who responded to treatment was 52% and 42%, respectively. In a 4-week, double-blind, randomized, clinical trial involving 60 patients, eprosartan (600 mg once daily) was found to be more effective than losartan (50 mg once daily). Patients in whom therapy was considered effective were 73% in the eprosartan group and 53% in the losartan group [9].
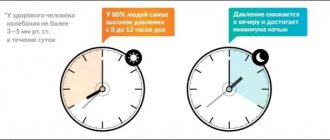
The most important requirement for modern antihypertensive drugs is a high duration of exposure, allowing control of blood pressure for 24 hours. To assess the severity and duration of the antihypertensive effect of long-acting drugs, the US Food and Drug Administration (FDA) proposed in 1988-1990 . use the “trough:peak” (T/P) coefficient, that is, the ratio between the smallest decrease in systolic or diastolic pressure at the end of the interdose interval and its maximum decrease at the height of the drug’s effect. Antihypertensive therapy seems optimal, in which there are no significant fluctuations in blood pressure during the day, that is, this coefficient should tend to unity, or 100%. According to FDA recommendations, the final:peak ratio should be at least 50%; this means that modern antihypertensive drugs should provide a reduction in blood pressure 24 hours after administration by at least 50% of the reduction in values during the period of maximum hypotensive effect. This allows for effective blood pressure control between doses of the drug; low fluctuations in blood pressure help reduce damage to the vascular wall, and consequently, the frequency of cardiovascular complications of arterial hypertension decreases.
The T/P ratio values for various angiotensin receptor blockers are presented in table. 4.
Using ambulatory blood pressure monitoring, it has been shown that a single dose of angiotensin receptor blockers provides control of blood pressure levels throughout the day, including in the morning, when the risk of developing vascular accidents (myocardial infarction and stroke) is especially high; Only losartan in some cases has to be used twice a day. The highest values of the T/P ratio (i.e., the longest duration of effective antihypertensive action) were found when using eprosartan, irbesartan and candesartan.
The high effectiveness of angiotensin receptor blockers is combined with good tolerability. According to data obtained from placebo-controlled clinical trials, the frequency of side effects during therapy with drugs of this group does not differ from this indicator in the placebo group. In particular, the incidence of side effects during therapy with losartan is 15.3% versus 15.5% in the placebo group, and during therapy with valsartan - 15.7% versus 14.5%; the frequency of side effects during therapy with eprosartan is given in table. 5. It is very important that drugs in this group, unlike ACE inhibitors, do not cause or worsen cough. Thus, angiotensin receptor blockers are quite safe; Contraindications to their use are only pregnancy, hyperkalemia and bilateral renal artery stenosis.
According to the National Guidelines for the Diagnosis and Treatment of Arterial Hypertension (2001), the absolute indication for the use of angiotensin receptor blockers is intolerance to ACE inhibitors (cough when using them), and the relative indication is congestive heart failure. The last recommendation is due to the fact that, as shown by Pitt B. et al. (1997), losartan can increase life expectancy in patients with chronic heart failure.
It should be noted, however, that inhibition of both the sympathoadrenal and angiotensin-aldosterone systems by eprosartan leads to a significant decrease in systolic blood pressure, therefore the use of this drug is promising for isolated systolic hypertension, arterial hypertension after a stroke, obesity, stress-induced, metabolic, alcoholic hypertension ( Kobalava Zh. D., Moiseev V. S., 2000).
Literature
- Argenziano L., Trimarco B. Effect of eprosartan and enalapril in the treatment of elderly hypertensive patients: subgroup analysis of a 26-week, double-blind, multicentre study. Eprosartan Multinational Study Group. Curr Med Res Opin 1999; 15(1):9-14.
- Gavras I., Gavras H. Effects of eprosartan versus enalapril in hypertensive patients on the renin-angiotensin-aldosterone system and safety parameters: results from a 26-week, double-blind, multicentre study. Eprosartan Multinational Study Group. Curr Med Res Opin 1999; 15(1):15-24.
- Gradman A.H., Gray J., Maggiacomo F., Punzi H., White WB Assessment of once-daily eprosartan, an angiotensin II antagonist, in patients with systemic hypertension. Eprosartan Study Group. Clin Ther, 1999; 21(3):442-453.
- Hedner T., Himmelmann A., for the Eprosartan Multinational Study Group. The efficacy and tolerance of once and twice daily doses of eprosartan in essential hypertension. J Hypertens, 1999; 17:129-136.
- Kassler-Taub K., Littlejohn T., Elliot W. et al., for the Irbesartan Study Investigators. Comparative efficacy of two angiotensin II receptor antagonists, irbesartan and losartan, in mild to moderate hypertension. Am J Hypertens. 1998; 11:445-53.
- Ohlstein EH, Brooks DP, Feuerstein GZ, Ruffolo RR Inhibition of sympathetic outflow by the angiotensin II receptor antagonist, eprosartan, but not by losartan, valsartan or irbesartan: relationship to differences in prejunctional angiotensin II receptor blockade. Pharmacology, 1997; 55(5):244-51.
- Oparil S. Eprosartan versus enalapril in hypertensive patients with angiotensin-converting enzyme inhibitor cough. Curr Ther Res, 1999; 60(1):1-14.
- Ponticelli C., for the Eprosartan Study Group. Comparison of the efficacy of eprosartan and enalapril in patients with severe hypertension. Am J Hypertens, 1997; 10:128A.
- Puig JG, Mateos F., Buno A., Ortega R., Rodriguez F., Dal-Re. R. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens, 1999; 17(7):1033-1039.
- Weber M. Clinical efficacy of eprosartan. Pharmacotherapy. 1999; 19(4, part 2):95–101.
- Zanchetti A., Omboni S., Di Biago C. Candesartan cilexetil and enalapril are of equivalent efficacy in patients with mild to moderate hypertension. J Hum Hypertens 1997; 11 (suppl 2):57-9.
Sartans in the treatment of atrial fibrillation
During long-term treatment of a patient with atrial fibrillation, the choice of rhythm control strategy does not affect the long-term prognosis, although it improves the quality of life of patients by reducing the severity of symptoms [19, 20]. Antiarrhythmic therapy is often poorly tolerated; radiofrequency treatment methods are available to a limited number of patients, so the search for safe pharmacological agents that can influence the course of atrial fibrillation and the prognosis of patients continues. The range of these drugs also includes sartans. Increased tissue levels of angiotensin-converting enzyme and increased expression of angiotensin receptors in patients with atrial fibrillation have been proven [21]. Activation of the RAAS is widely involved in the progression of chronic HF and may contribute to the occurrence of atrial fibrillation. Angiotensin II, causing proliferation of fibroblasts and reducing the activity of collagenases, is a powerful activator of myocardial fibrosis processes [22, 23]. Blockade of the RAAS caused by ARA II or ACE inhibitors leads to a slowdown in the processes of atrial fibrosis, a decrease in pressure in the left atrium, and a decrease in ectopic atrial activity [24–26]. In addition, the direct hemodynamic effect of drugs by reducing afterload may play a role in the prevention of atrial fibrillation.
An analysis of randomized trials provided evidence that ACE inhibitors and sartans can reduce the incidence of new attacks of atrial fibrillation compared with placebo. Advantages in relation to the risk of atrial fibrillation in people with systolic HF compared with placebo were demonstrated for enalapril in the SOLVD study, for valsartan in the Val-HeFT study, but these studies were conducted quite a long time ago, when the standards of treatment of patients with HF differed significantly from modern ones , did not include b-blockers. This was the basis for doubts that sartans would have additional benefits for the prevention of paroxysms of atrial fibrillation when prescribed along with adequate therapy for HF.
These doubts were dispelled after the results of the CHARM study were published. In patients with symptomatic HF receiving modern therapy, the administration of candesartan led to a 19% reduction in the relative risk of developing atrial fibrillation compared with placebo (RR 0.812; 95% CI 0.662–0.998; p = 0.048) [26]. In the subgroup of patients with reduced EF, there was also a significant reduction in the risk of atrial fibrillation by 22%. A meta-analysis of studies devoted to this problem demonstrated that the greater the degree of reduction in ejection fraction, the greater the protective effect on the risk of developing atrial fibrillation that drugs that block the RAAS have [28].
In addition to reducing the risk of new episodes of atrial fibrillation, sartans can prevent relapses in the paroxysmal form of the disease. Sartans and ACE inhibitors may have a direct antiarrhythmic effect, since angiotensin II is able to directly participate in the process of electrical remodeling of the atria even in the absence of HF. Thus, a decrease in the refractory period of the atria, observed in the experiment against the background of frequent stimulation of the atria, can be prevented by the administration of drugs that suppress the activity of the RAAS. Prescribing irbesartan in addition to amiodarone 3 weeks before planned cardioversion to patients with persistent atrial fibrillation reduces the likelihood of recurrent paroxysms of atrial fibrillation compared with amiodarone therapy without irbesartan (17% vs 37%, p = 0.008) [29]. The maximum effect of sartans was observed during the first 2 months of treatment, which confirms the role of blockade of the effects of angiotensin II in relation to the processes of electrical remodeling of the atria in the early period after cardioversion.
Further research is required to evaluate the benefits of one class over another in preventing the development of arrhythmias, as well as the role of drugs affecting the RAAS in the treatment of atrial fibrillation.
Contraindications for use
The medicine has been tested on different categories of patients. Its quality has been confirmed by pharmacologists. After the data received, it was revealed that it is prohibited for use by the following groups of people:
- hypersensitivity, intolerance to 1 of the components of the composition;
- pregnancy, breastfeeding.
The active substance penetrates the placental barrier and into breast milk, affecting the fetus and newborn. Possible slowdown in development and dysfunction of internal organs. If an unplanned conception occurs, stop taking the pills.
There is a group of patients to whom the drug can be used, but with caution. The doctor regulates the dosage, so the risk of negative effects is eliminated. Control of the number of tablets is indicated for patients with the following conditions:
- minors under 18 years of age;
- the total volume of circulating blood is below the norm for gender;
- renal failure in the stage of decompensation;
- cirrhosis, malignant degeneration of the liver;
- cholelithiasis, other pathologies leading to blockage of the bile ducts;
- taking medications aimed at preserving potassium in the body.
If at the initial stage of treatment no contraindications were identified, but side effects developed, the drug was immediately discontinued. You will not develop an addiction to it, but complications will arise.
Antihypertensive efficacy of candesartan and other ARBs
A special meta-analysis was devoted to the comparative effectiveness of candesartan and losartan, which included 14 studies (8 on hypertension and 6 on HF) [11]. Its secondary objective was to examine the comparative cost-effectiveness of both drugs. All studies involving hypertensive patients directly compared candesartan and losartan. The difference between the blood pressure values was -1.96 mm Hg. Art. (95% CI -2.40 to -1.51) for DBP and -3.00 mmHg. Art. (95% CI -3.79 to -2.22) for SBP in favor of candesartan. These differences were determined using a Markov model that estimates the cost of 1 year of quality life; the analysis demonstrated the economic feasibility of using candesartan.
Is it possible to develop malignant neoplasms?
Some medications aimed at lowering blood pressure cause the development of malignant tumors. Cancer usually forms in the lung tissue. The first sign of pathology is the presence of a cough. This is a side effect that occurs with ACE inhibitors. Therefore, it is difficult to recognize a complication or cancer in the early stages.
Studies have been conducted to determine the presence or absence of cell malignancy when using sartans. The following recent research findings have been identified:
- absence of the slightest percentage of development of malignant cells;
- reducing the risk of neoplasms of a benign or malignant nature.
Research on cancer is still not closed. Some antihypertensive drugs have this effect. The risks of cancer are minimal. If a patient with arterial hypertension does not take medications, there is a possibility of a heart attack or stroke. When using ARBs, people's lives are prolonged and their quality is improved.
Retinopathy in diabetes mellitus types 1 and 2
DIRECT-prevent 1 and DIRECT-protect 1 studies
The DIRECT (DIabetic Retinopathy Candesartan Trials) study was conducted to study the effectiveness of candesartan for the prevention (DIRECT-prevent 1) and slowdown of progression (DIRECT-protect 1) of diabetic retinopathy (DR) in type 1 diabetes [6, 18]. Patients aged 18–55 years with type 1 diabetes, normotension and normoalbuminuria, without DR were included in DIRECT-prevent 1 (710 - in the candesartan group, 710 - in the placebo group), patients with DR - in DIRECT-protect 1 (1905 - to the candesartan group, 954 to the placebo group) and were prospectively randomized to treatment with candesartan 16 mg once a day or placebo. After 1 month the dose of candesartan was increased to 32 mg. Primary endpoints are incidence and progression of DR: at least a 2-point increase or a 3-point increase, respectively, on the DR scale. The occurrence of DR was observed in 178 (25%) participants in the candesartan group versus 217 (31%) in the placebo group, RR 0.82 (95% CI 0.67–1.00, p=0.0508). Progression of DR occurred in 127 (13%) participants in the candesartan group versus 124 (13%) in the placebo group, RR 1.02 (95% CI 0.80–1.31, p=0.85) for the DIRECT-protect group 1. In a post-hoc analysis, for an increase in DR score of at least 3 points, the RR was 0.65 (95% CI 0.48–0.87, p=0.0034), this risk reduction remained significant after adjustment by baseline characteristics - RR 0.71, 95% CI 0.53–0.95, p=0.046. At the end of the study, the odds of having a lower DR score were higher among those taking candesartan in both DIRECT-prevent 1 (RR 1.16, 95% CI 1.05–1.30, p=0.0048) and DIRECT-protect 1 (RR 1.12, 95% CI 1.01–1.25, p=0.0264).
Migraine
A prospective, randomized, double-blind, crossover study evaluated the efficacy of candesartan in 60 patients with migraine [6, 22]. It was found that taking candesartan at a dose of 16 mg/day reduced the average number of days with headache and migraine compared with those when taking placebo (13.6 versus 18.5 days, respectively, with headache, p = 0.001; 9.0 versus 12 .6 days, respectively, with migraine, p=0.001). The use of candesartan significantly reduced the severity of headaches, as well as the number of sick days for this reason. The response rate to candesartan, defined as a reduction in the number of migraine days by 50% or more, reached 40.4%, and to placebo - 3.5% (p = 0.001). The incidence of side effects with candesartan was comparable to that with placebo.
Nephroprotective potential of sartans
Decreased proteinuria is associated with slower progression of chronic kidney disease. Enough data have been accumulated indicating that both sartans and ACE inhibitors can have a positive effect on the functional state of the kidneys. Renoprotective properties are also inherent in the class of calcium channel antagonists. Are there advantages to prescribing one class of drugs over another? Several large randomized studies have convincingly demonstrated that sartans are effective in preventing the progression of kidney damage.
The IDNT study examined the properties of irbesartan in 1715 patients with type 2 diabetes mellitus and nephropathy [30]. The effects of the drug at a dose of 300 mg were compared with the effects of amlodipine 10 mg and placebo for 2.6 years. The rate of endpoint achievement with irbesartan was overall 20% lower than in the placebo group and 23% lower than in the amlodipine group. At the same time, the risk of doubling the initial level of creatinine was lower than in these groups by 33 and 37%, respectively, and the risk of developing end-stage chronic renal failure was 23%. The nephroprotective effect of irbesartan in the IDNT study, as well as losartan in the RENAAL study, did not depend on blood pressure levels.
In the IRMA-2 study, a significant reduction in the incidence of microalbuminuria, an independent risk factor for cardiovascular diseases, was recorded in patients with hypertension and type 2 diabetes mellitus. The reduction in the risk of progression of diabetic nephropathy was observed regardless of the antihypertensive effect of the drug.
R. Kunz et al., having analyzed the results of 59 studies (6181 patients) comparing the nephroprotective potential of ACE inhibitors, sartans and calcium channel antagonists in people with chronic kidney disease, came to the conclusion that ARB II and ACE inhibitors were equally effective in reducing proteinuria, and when compared with calcium receptor antagonists, the advantage was on the side of ARA II [31]. Sartans reduced proteinuria regardless of its severity and cause of development.
Division by generation
Advertising:
There are 2 generations, each of which includes a list of medications. Their division is based on the quality of receptor blocking. The data is described in the table.
| Generation | List of drugs | Effect on receptors, additional effects |
| 1 | Losartan, Valsartan, Candesartan, Irbesartan | Block angiotensin 1. Reduce blood pressure, the amount of lipoproteins and cholesterol in blood vessels, protect against kidney and heart tissue |
| 2 | Telmisartan | Suppress all AT receptors and peroxisome activators. Eliminate atherosclerosis, reduce lipoproteins and glucose. Stimulates the pancreas. Relieves the inflammatory reaction |
Groups of 1st and 2nd generations are similar in blood pressure regulation. Their difference is insignificant and cannot be detected using instrumental examinations. If the patient does not have diabetes mellitus or atherosclerosis, a 1st generation drug is preferable.
It is often impossible to find the main name of the drug in a pharmacy. However, there are trade names that correspond in chemical composition and mechanism of action. The following classification of medicines is distinguished:
- Valsartan: Valsacor, Tareg;
- Losartan: Bloktran, Zisacar, Lozap, Larista, Renicard;
- Irbesartan: Aprovel, Firmasta;
- Candesartan: Angiakand, Giposart, Candecor, Ordiss;
- Olmesartan: Cardosal.
If a patient has side effects to one of the prescribed drugs, it is prohibited to independently replace it with another substance. Complications arise. This makes you feel worse and leads to an excessive decrease in blood pressure.
Advertising:
Drug interactions
Advertising:
The ARB group of drugs is prescribed with other drugs, since they rarely enter into chemical interactions without interfering with the therapeutic effect. It is recommended to take it together with other medications aimed at improving the patient’s well-being with cardiovascular pathologies and diabetes.
However, when selecting the dosage, it is taken into account that when combining antihypertensive substances, the pressure will decrease even more. To eliminate the risk of systemic hypotension and syncope, adjust the dose of both drugs used.
There is a group of drugs that can change the condition of the cardiovascular system, liver, and kidneys when combined with sartans. The risk of this action is minimal, but periodic laboratory tests of blood and urine are required. Such means include:
- non-steroidal anti-inflammatory drugs;
- diuretics with no potassium removal effect;
- anticoagulants;
- medicines containing potassium.
These medications may cause side effects. For example, if you take Heparin along with Valsartan, the blood will become excessively thin. The patient may experience sudden bleeding from minor bruises.