According to classical concepts, the renin-angiotensin system (RAS) plays a key role in the regulation of blood pressure and water-electrolyte balance. Research in recent decades has shown the great importance of increased RAS activity in the formation and progression of arterial hypertension (AH), heart failure (HF), chronic kidney disease (CKD), and systemic atherosclerosis [24, 52, 66]. In addition, the RAS is directly involved in the processes of tissue growth and differentiation, modulation of inflammation and apoptosis, as well as potentiation of the synthesis and secretion of a number of neurohumoral substances [39]. The main conductor that provides almost all known effects of RAS is angiotensin II. The latter realizes its tonic influences through stimulation of specific receptors. It has been established that activation of AT1 and AT2 receptors leads to opposite results. AT1 receptors cause a vasoconstrictor effect, stimulate the release of vasopressin, aldosterone, endothelin, norepinephrine, and corticotropin-releasing factor. The physiological role of AT3, AT4 and ATx receptors continues to be studied.
In vitro and in vivo studies have found that angiotensin II promotes the accumulation of collagen matrix, the production of cytokines, adhesion molecules, activation of multiple intracellular signaling cascades through stimulation of mitogen-activated protein kinase, tyrosine kinase and various transcription factors [41].
Numerous studies have confirmed the involvement of RAS activation in cardiac remodeling processes. Thus, great importance is attached to the participation of angiotensin II in the formation of pathological hypertrophy of the left ventricle (LV), which is associated not only with an increase in myocardial mass, but is also associated with qualitative changes in the cardiomyocyte and the accumulation of extracellular collagen matrix [1, 2]. Angiotensin II directly promotes increased expression of genes of the fetal phenotype [22], such as the genes of β-myosin heavy chains, skeletal α-actin, and atrial natriuretic factor. An increase in the expression of fetal isoforms of contractile proteins leads to an increase in LV mass [27], followed by a decrease first in the relaxation and then in the total pumping function of the heart [21]. In addition, angiotensin II promotes the expression of immediate-early or fetal genes, such as jun B, βgr-1, c-myc, c-fos, c-jun, which are responsible for the intensity of intracellular protein synthesis [5]. Although the role of activation of these genes is not completely clear, many researchers associate an increase in their expression with disruption of the intracellular signaling cascade and activation of the fetal type of metabolism [82].
It has been established that angiotensin II can also play a central role in the processes of arterial remodeling, intensification of oxidative stress and apoptosis [81]. In addition, angiotensin II may be involved in the formation and progression of arterial hypertension [56, 63], heart failure [75], atherosclerotic vascular damage [7, 20], diabetic and nondiabetic nephropathies [8], angiopathy in diabetes mellitus [8, 9], eclampsia in pregnancy, Alzheimer’s disease and many other diseases [65, 68].
It should be noted that the adverse effect of angiotensin II on the progression of cardiovascular diseases occurs regardless of its vasopressor effect [6]. However, the participation of most molecular and cellular mechanisms of RAS in the progression of cardiovascular diseases has been confirmed in experimental studies or in vitro. In this regard, the clinical and prognostic significance of many of them remains to be established [52, 53].
Thus, angiotensin II appears to be a central link in a complex cascade of RAS activation, which has a negative impact on the structural and functional characteristics of the cardiovascular system. However, renin secretion is the first and most important step in increasing the synthesis of angiotensin I, angiotensin II and other products of the RAS cascade in general [57]. Moreover, the implementation of all subsequent effects of the RAS is modulated by the influence of renin on specific receptors [36, 44]. The latter are present not only in the mesangial tissue of the kidneys, as previously assumed, but also in the subendothelium of arteries, including renal and coronary ones [4]. Renin has a high affinity for forming a specific bond with its own receptors. Renin bound to the receptor induces a series of intracellular processes, the result of which is an increase in the formation of angiotensin II [43]. It should be noted that the described type of receptor has the ability to bind prorenin with the subsequent implementation of processes of activation of angiotensin II synthesis. It has now been established that prorenin is a powerful predictor of microvascular complications in diabetes mellitus, although the mechanism underlying this process is not completely clear [23]. In this regard, limiting the activity of RAS components is considered as an effective method of drug intervention in the progression of cardiovascular diseases.
It should be noted that in recent years, pharmacological control over the activity of the RAS has been carried out in the direction of limiting the production of angiotensin II through inhibition of the angiotensin-converting enzyme, blockade of angiotensin II and aldosterone receptors, as well as by limiting renin secretion, mainly through the use of beta-blockers [11, 61, 74]. At the same time, numerous studies have shown that adequate reduction in RAS activity is postulated rather than actually achieved. It has been found that the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor antagonists (ARAs) is often associated with activation of alternative pathways of RAS activation. Thus, for ACEIs this is associated with an increase in the activity of tissue chymases and proteases, as well as the secretion of renin and aldosterone, and for ARAs - with an increase in the synthesis of angiotensin II and aldosterone without a corresponding increase in the pool of endogenous bradykinin [3, 45]. In a clinical sense, this phenomenon manifests itself in the so-called escape phenomenon of the antihypertensive and organoprotective effects of RAS blockers during their long-term use. Attempts to overcome this phenomenon include the use of combinations “ACEI + ARB”, “ACEI + beta-blocker”, “ACEI + spironolactone (eplerenone)”. The emergence of direct renin inhibitors (DRIs), which help reduce the secretion of the latter and limit the intensity of angiotensin II production, began to be considered as a possible way to achieve more complete control over the activity of the RAS and overcome the escape phenomenon [10].
Cyrenes - a new class of antihypertensive drugs
The first PIRs (enalkirene, remikiren, zankirene) were synthesized in the mid-70s of the last century [30], and clinical results regarding their use in healthy volunteers and patients with hypertension have become available since the late 80s [54]. However, researchers have encountered a number of difficulties, mainly associated with the extremely low bioavailability of PIR in the gastrointestinal tract (less than 2), short half-life and low stability of the components in tablet form, which significantly limited the potential therapeutic potential of cyrenes in general [50, 59 ]. In this regard, for quite a long time, cyrenes were not considered as a promising class of antihypertensive drugs, especially since the 90s of the last century were the heyday of ACEIs, and the end of the millennium - ARAs. The first success for kirenes came only after the synthesis of CGP 60536, a non-peptide small-molecule renin inhibitor suitable for oral administration, called aliskiren [78]. To date, the drug has passed all stages of clinical trials and has been recommended for the treatment of hypertension in the USA and EU countries since April 2007 [78].
Every year there are more than 15 million new cases of myocardial infarction (MI) worldwide. Long-term consequences of MI appear after many months and years. According to the American Heart Association (2004), within 6 years after an MI, despite optimal treatment, 18% of men and 35% of women suffer a recurrent MI, 7% of men and 6% of women die suddenly, 22% of men and 46% of women become disabled due to the development of severe heart failure, and 30–40% of patients develop left ventricular (LV) dysfunction.
Activation of circulating and local (myocardial) neurohumoral systems plays an important role in the pathogenesis of MI and its complications. In the early stages of myocardial infarction, an increased release of neurohumoral vasoconstrictors (primarily catecholamines, angiotensin II [AII] and endothelin) contributes to the development of coronary spasm, leading to expansion of the infarction zone, the occurrence of acute heart failure (AHF) and life-threatening cardiac arrhythmias. Neurohumoral activation during MI is initially compensatory in nature to maintain adequate pumping function of the heart in response to hemodynamic overload and a decrease in the mass of the functioning myocardium, but subsequently it can become maladaptive. Increased activity of neurohumoral systems that persists for a long time leads to the development of LV remodeling, manifested by abnormal myocardial rigidity, decreased coronary reserve, impaired diastolic and systolic functions of the LV, dilatation of its cavity and the appearance of symptoms of chronic heart failure (CHF). Most neurohumoral changes are mediated through vasoconstrictor and vasodilator reactions. The first are realized through the sympathetic-adrenal (SAS), renin-angiotensin-aldosterone system (RAAS), vasopressin, antidiuretic hormone (ADH), serotonin, endothelin, thromboxane A2; the second - through the kallikrein-kinin system, the natriuretic peptide (NUP) system, prostaglandins I2 and E2, endothelium-dependent relaxing factor, adrenomedullin, etc.
Correction of the activity of neurohumoral systems in patients in the early and late stages of myocardial infarction is one of the main directions in the treatment of the disease and the prevention of its complications. Currently, β-adrenergic and angiotensin receptor blockers, angiotensin-converting enzyme inhibitors (ACEIs) and aldosterone antagonists are used for this purpose. Several new groups of drugs are also at various stages of clinical trials (renin inhibitors, vasopeptidase blockers, NUPs, vasopressin and endothelin receptor antagonists).
Beta blockers (BAB)
BABs reduce myocardial oxygen demand, improve coronary blood flow, helping to reduce ischemia and limit the size of the necrosis zone. According to the results of a meta-analysis of 22 randomized studies that included more than 25 thousand patients (H. Dargie, 2001), it was revealed that long-term use of beta blockers led to a reduction in overall mortality by 23%, sudden death by 26%, and the number of recurrent myocardial infarctions by 41%. , cases of atrial fibrillation/flutter by 59% and severe ventricular arrhythmias by 70%.
In the early stages of MI, atenolol and metoprolol have been studied in more detail, with long-term use - carvedilol, metoprolol and propranolol. Preference is given to selective beta blockers, but there is reason to believe that a beneficial effect in myocardial infarction is characteristic of all drugs of this class, except for those with intrinsic sympathomimetic activity [1].
Based on the results of the COMMIT/CCS-2 study, the American College of Cardiology does not recommend intravenous beta blockers in patients with myocardial infarction, unless blood pressure (BP) control is required [2]. European (ESC) and Russian experts suggest wider use of intravenous beta blockers in patients with tachycardia, arterial hypertension (AH) and in cases of recurrent pain syndrome [1, 3]. Experts agree that oral beta blockers, in the absence of contraindications, should be started in all patients from the first day of MI and continued indefinitely, stopping treatment only if serious side effects occur.
The greatest effect from taking β-blockers is observed in patients with reduced LV contractile function, as well as in the presence of electrical instability of the myocardium. The use of β-blockers is contraindicated in the development of cardiogenic shock, severe obstructive pulmonary disease in the acute stage, and allergic reactions. In the presence of relative contraindications, such as diabetes mellitus and obstructive pulmonary diseases outside of exacerbation, as well as in patients with severely impaired LV contractility, treatment with beta-blockers should be carried out very carefully, starting with minimal doses.
ACE inhibitors
ACE inhibitors inhibit the conversion of angiotensin I into a powerful vasoconstrictor AII, reduce the release of norepinephrine from neuron endings and the secretion of ADH, as well as aldosterone; increase the formation of bradykinin and the level of circulating NUPs, have a variety of hemodynamic effects: reduce vascular resistance and normalize diastolic filling of the LV due to regression of its hypertrophy. ACEIs reduce platelet aggregation, have a positive effect on the rheological properties of blood and endothelial dysfunction, and have anti-inflammatory, antiarrhythmic, anti-ischemic and antianginal effects.
ACE inhibitors are of great practical importance in the treatment of acute heart failure, as well as as a means of preventing heart failure in patients who have had a myocardial infarction. The studies CONSENSUS II, CATS, SMILE, GISSI-3, ISIS-4, PRACTICAL, CCS-I and FAMIS were devoted to the early prescription of ACE inhibitors (from the first day of MI).
The CONSENSUS II study, which studied the use of enalapril intravenously and then orally from the first day of MI, was stopped early due to an unreliable increase in mortality by 9% in the main group due to the more frequent development of arterial hypotension. However, in patients with large-focal MI, enalapril reduced the processes of LV remodeling, improved the prognosis of life, and significantly reduced the incidence of complications [4].
In the ISIS-4 study, after 5 weeks of treatment in the captopril group, a significant reduction in mortality by 7% was noted - mainly in patients with anterior localization of myocardial infarction and over 70 years of age [5].
In the SMILE study, in patients with anterior MI without prior thrombolytic therapy (TLT) who received zofenopril, after 6 weeks of treatment there was a non-significant reduction in overall mortality by 25%, mortality from HF by 31%, and sudden death by 63%. The risk of developing severe CHF was significantly reduced by 46%. After one year of observation, a significant decrease in overall mortality was 29%. The greatest effectiveness of treatment was observed in cases of repeated MI, as well as in patients with hypertension and diabetes mellitus [6].
In the GISSI-3 study, mortality in the group of MI patients receiving lisinopril was significantly lower by 11% after 6 weeks [7]. The effectiveness of early administration of lisinopril has also been confirmed in patients with MI after TLT [8].
Early addition of fosinopril to therapy in patients with anterior MI who underwent TLT led to a significant reduction in mortality and the incidence of severe HF by 36%, and the improvement in prognosis was independent of the effect on LV remodeling [9].
Late administration of ACE inhibitors (from the third day of MI) was studied in the SAVE, TRACE, AIRE and PREAMI studies. In the SAVE study, patients with asymptomatic LV dysfunction were treated with escalating doses of captopril. A significant reduction in mortality was revealed by 21%, the risk of developing severe CHF by 37%, and recurrent MI by 25% [10].
When ramipril was prescribed, starting from the 3rd–10th day of the disease, in patients with signs of HF in the acute period of MI, a significant reduction in mortality by 27% was revealed, while a greater effect was observed in patients over 65 years of age and with concomitant hypertension [11]. Similar work by domestic authors also revealed a positive effect of ramipril therapy on hemodynamics and LV contractile function in patients with MI complicated by HF [12].
The PREAMI study demonstrated the effectiveness of perindopril in reducing the processes of LV remodeling and reducing the incidence of CHF in elderly patients after MI [13].
A number of studies have been devoted to comparing the effectiveness of ACEIs in patients with MI. The PRACTICAL study noted greater effectiveness of enalapril compared with captopril in terms of the effect on mortality and global LV myocardial contractility after 3 months of treatment [14]. In the work of N.B. Sidorenkova et al. (1999) revealed a more pronounced antianginal and antiarrhythmic activity of fosinopril compared to enalapril when administered early to patients with anterior myocardial infarction [15].
A meta-analysis of large studies showed that the use of ACE inhibitors leads to a reduction in the risk of death after a heart attack by 26%, recurrent myocardial infarction by 20%, and hospitalization for CHF by 27%.
Currently, the need to use ACE inhibitors starting from the first day in patients with MI is not in doubt. However, there is no consensus: should ACE inhibitors be prescribed to all patients or only those at high risk? Thus, the American Heart Association recommends prescribing ACE inhibitors to all patients in the absence of hypotension, followed by a determination after 6 weeks of the need to continue therapy. According to ESC recommendations (2008), ACE inhibitors should be prescribed only to patients with LV ejection fraction (EF) < 40% or signs of HF, and their long-term use is not mandatory for patients who have had a MI without hypertension, signs of HF and with preserved LV systolic function. VNOK experts emphasize that ACEIs are especially effective in patients with extensive myocardial necrosis, reduced LVEF (<40%), symptoms of HF, and diabetes mellitus. At the same time, they improve the prognosis in patients without a clinically significant decrease in EF. Treatment with ACE inhibitors should be started as early as possible, when hemodynamics are stabilized and in the absence of contraindications, continue indefinitely.
Angiotensin receptor blockers (ARBs)
Despite the high effectiveness of ACE inhibitors in patients with MI, these drugs can cause side effects such as dry cough, angioedema, headache, which makes it impossible for 10–20% of patients to take them, as well as arterial hypotension, which contributes to a further deterioration of coronary perfusion. ACEIs disrupt the degradation of bradykinin and stimulate the synthesis of prostaglandins and nitric oxide, but their effect on the RAAS is very unstable. They disrupt the action of AII on all types of angiotensin receptors: both those that determine negative reactions (AT1) and those that mediate potentially beneficial organoprotective effects (AT2). Another factor limiting the effect of ACE inhibitors is the existence of local “non-ACE-dependent” pathways for the formation of AII. In this regard, the use of drugs that block the RAAS at the receptor level seems more justified. BARs have fewer side effects compared to ACEIs (in particular, they do not have a “first dose” effect), cause less pronounced hyperreninemia, reduce the level of aldosterone in the blood and can cause regression of LV hypertrophy. They increase the fibrinolytic activity of the blood, have a beneficial effect on endothelial dysfunction [16] and slow down the processes of LV remodeling [17].
Comparative studies of ACEI and BAR in CHF have yielded conflicting results. The ELITE study revealed a significant reduction in the risk of death (especially sudden) in patients with CHF taking losartan compared with captopril [18]. However, the ELITE II study, which compared the same drugs, did not confirm the advantages of BAR over ACE inhibitors in terms of their effect on the prognosis of patients with CHF [19]. The feasibility of combination therapy of ACEI and BAR in patients with CHF has been studied in a number of studies. The simultaneous initiation of treatment with these drugs significantly increased the number of side effects without additional impact on morbidity and mortality, however, the addition of a BAR (candesartan or valsartan) to therapy in patients already taking ACE inhibitors led to a significant reduction in mortality and the frequency of hospitalizations due to progression of CHF by 13– 15% [20, 21].
The first data on the use of BAR in patients with MI confirmed the hypothesis of their positive effect on clinical and hemodynamic parameters, comparable to the effects of ACE inhibitors, with fewer side effects [22, 23]. A.N. Parkhomenko et al. (2000) revealed the safety of combined use of irbesartan and captopril starting from the first day of MI, with a more pronounced hemodynamic effect than with the independent use of captopril and a comparable effect on the size of necrosis, as well as the processes of early cardiac remodeling [24]. Similar results were obtained when enalalril and losartan were coadministered [35].
The first large study to examine the efficacy and safety of a BAR (losartan) versus an ACE inhibitor (captopril) in patients with MI with clinical manifestations of AHF was the OPTIMAAL study (n = 5477, mean follow-up 2.7 years). The overall mortality rate in the losartan group was slightly higher (18 vs. 16%), but cardiovascular mortality was significantly more common. There were no significant differences in the ability of the drugs to prevent sudden death or worsening HF. The number of side effects and drug withdrawal rates were lower in the losartan group [26]. It is possible that the results obtained were a consequence of an insufficient dose of losartan (50 mg/day) or an inadequate titration scheme for the drug.
The VALIANT study (n = 14,703) assessed the effectiveness of valsartan compared with captopril and their combination in patients with MI complicated by AHF and/or LV systolic dysfunction. After 36 months of follow-up, there were no significant differences in mortality in all three groups, and there were no differences in cardiovascular mortality, the risk of recurrent myocardial infarction, or the occurrence of heart failure. Side effects were less common with valsartan than with captopril, but with the drug combination the incidence of side effects was significantly higher. The results of the study proved that valsartan can be an alternative to ACEI in patients with MI, but the hypothesis of the advantage of more complete blockade of the RAAS when combining ACEI and BAR was not confirmed [27]. According to European and Russian recommendations, ACEIs and BARs can be used in patients who have had a MI on an alternative basis, depending on tolerability and some other considerations, including economics. The experience of long-term use of BAR after MI is much less, so the prescription of BAR should be resorted to in cases of intolerance to ACE inhibitors with EF ≤ 40% and/or HF and the presence of hypertension.
Aldosterone antagonists
The positive effect of aldosterone antagonists on the long-term period of MI was revealed in the EPHESUS study, which included 6632 patients with MI complicated by the development of AHF or LV dysfunction [28]. In addition to standard therapy, patients in the main group were prescribed a selective aldosterone blocker, eplerenone. After 16 months, there was a significant decrease in overall mortality (14.4% compared to 16.7% in the control group) and the frequency of hospitalizations for cardiovascular reasons. The decrease in mortality was due to a decrease in the incidence of sudden death. The greatest effect from eplerenone therapy was noted when it was prescribed early (on the 3rd–7th day of MI [29].
D. Fraccarollo et al. (2005) in an experiment revealed the advantage of joint administration of eplerenone and the BAR irbesartan in influencing the processes of post-infarction LV remodeling [30].
The use of the non-selective aldosterone antagonist spironolactone in myocardial infarction has only been studied in small studies. According to M. Hayashi et al. (2003), early administration of spironolactone to patients with primary anterior MI helps prevent LV remodeling by suppressing the activity of myocardial collagen synthesis [31]. Long-term combination therapy with spironolactone and BAR (losartan) in patients with MI after successful TLT slowed the progression of CHF and reduced mortality compared with losartan monotherapy [32].
According to the recommendations of the GFOC and ESC, the administration of aldosterone antagonists is indicated for patients who have had an MI, with an EF < 40% in combination with symptoms of HF or suffering from diabetes mellitus. A prerequisite for starting treatment is the level of creatinine in the blood: in men - < 220 µmol/l, in women - < 177 µmol/l, as well as a potassium concentration of no more than 5 mmol/l.
Direct renin inhibitors
The first renin inhibitors (enalkiren, remikiren, zankiren) were synthesized in the mid-1970s, but their clinical use was limited by low bioavailability in the gastrointestinal tract, short half-life and low stability of the components in tablet form [33]. The first success of kirens came with the synthesis of aliskiren, a non-peptide low-molecular renin inhibitor. In 2007, aliskiren was recommended for the treatment of hypertension in the USA and Europe, and a year later information appeared on the effectiveness of its use in patients with CHF [34].
In 2010, the results of two studies on the use of aliskiren in patients with ACS were presented. The ASPIRE study included 820 patients who had had an MI in the previous 2–6 weeks and had evidence of LV dysfunction (EF < 45% and akinesia > 20%). The patients were divided into two groups: in one of them, patients received aliskiren, in the other, placebo against the background of optimal standard therapy, which included statins, beta blockers, antiplatelet agents and ACE inhibitors. There were no significant changes in parameters reflecting the structure and function of the LV in the aliskiren group compared with placebo after 36 weeks of treatment [35]. The AVANT GARDE-TIMI 43 study (n = 1101) examined the need for early blockade of the RAAS to reduce hemodynamic load in ACS patients with preserved LV function. In addition to standard therapy, patients were prescribed valsartan, aliskiren, their combination, or placebo. There were no advantages of blocking the RAAS in reducing brain NUP levels with valsartan, aliskiren, or their combination compared with placebo [36]. Thus, the results of the ASPIRE and AVANT GARDE-TIMI 43 studies cast doubt on the prospects for the use of direct renin inhibitors in patients after MI.
Vasopeptidase inhibitors
Blockade of neutral endopeptidase (NEP) helps to increase the lifespan of NEP by reducing their degradation. Inhibition of vasopeptidases is an attractive approach for the treatment of HF. Several drugs that simultaneously block NEP and ACE are in clinical trials. Simultaneous inhibition of ACE and NEP enhances the natriuretic and vasodilating effects of NUP, suppresses the formation of AII and increases the half-life of other vasodilatory peptides, including bradykinin and adrenomedullin. Preclinical and first clinical studies of the drugs showed their high effectiveness for the treatment of CHF: vascular remodeling and myocardial hypertrophy decreased, natriuretic, diuretic and antiproliferative effects developed [37].
The most studied ACEI/NEP is omapatrilat. The results of early clinical studies demonstrated the high effectiveness of the drug in patients with CHF and hypertension, but later studies showed that omapatrilat has no advantages over the ACE inhibitor enalapril in the treatment of patients with both CHF and hypertension [38].
At the same time, the incidence of angioedema during treatment with omapatrilat was significantly higher, which is a serious obstacle to its implementation in widespread medical practice. In experimental models of MI, omapatrilat was superior to ACE inhibitors, but the use of vasopeptidase inhibitors in clinical settings in patients with MI has not been sufficiently studied.
Endothelin receptor antagonists
Blockade of endothelin receptors may be one of the new ways to treat HF, including in patients who have had an MI. There are non-selective ETA and ETB receptor antagonists (bosentan, enrasentan and tezosentan sodium) and selective ETA receptor antagonists (ambrisentan, atrasentan, darusentan and sitaxentan). The most encouraging results are the use of drugs in this group for the treatment of pulmonary hypertension.
The use of endothelin receptor antagonists in MI has been studied only in experimental studies. A prerequisite for their use in patients with MI can be the study of G. Niccoli et al. (2006), who found that high levels of endothelin-1 are associated with the occurrence of the no-reflow phenomenon during percutaneous myocardial revascularization in patients with primary MI. These data suggest that the use of endothelin-1 antagonists may be effective in the treatment and prevention of the no-reflow phenomenon during emergency and delayed endovascular interventions [39].
Natriuretic peptides
The drug nesiritide is structurally identical to endogenous human brain NUP and is produced by E. coli using recombinant DNA technology. In 2001, nesiritide was approved by the FDA for the treatment of AHF and recommended as first-line therapy in patients with acutely decompensated HF. In 2005, a meta-analysis of several large studies on the use of nesiritide in decompensated heart failure by Sackner-Bernstein et al. showed that the drug may increase the short-term risk of death and worsen renal function, but these data were not subsequently confirmed. However, the role of nesiritide in the treatment of HF still needs to be clarified.
Of no less practical interest is the use of NUP in patients with MI. According to HH Chen et al. (2009), infusion of low doses of nesiritide for 72 hours in patients with anterior MI suppresses aldosterone secretion, protects the structure and function of the LV with an increase in its EF and a decrease in end-diastolic volume (EDV) after a month [40].
RJ Hillock et al. (2008) showed that administration of nesiritide to patients with MI induces increased levels of cardioprotective biomarkers and favorable LV remodeling. In patients who received nesiritide, EDV did not increase and there was a decrease in LV end-systolic volume according to echocardiography; in addition, an increase in the level of NUP and cyclic GMF was noted [41].
M. Kitakaze et al. (2007) found that the addition of human atrial NUP to reperfusion therapy for MI (72-hour infusion of NUP after percutaneous coronary intervention) led to a reduction in the infarct area by 14.7% and a significant increase in LVEF after 6–12 months compared with the group placebo, but arterial hypotension developed significantly more often [42].
Preliminary data show the effectiveness of the use of NUP in patients with MI, but only larger studies will determine their place in the treatment of MI and its complications.
Vasopressin receptor antagonists
Vasopressin receptor antagonists reduce vasoconstriction and promote aquaresis without adversely affecting electrolyte balance. There are non-selective antagonists of V1A/V2 receptors (conivaptan) and selective antagonists of V1A (relcovaptan), V1B (nelivaptan) and V2 receptors (tolvaptan, satavaptan, mozavaptan and lixivaptan). The use of conivaptan and tolvaptan is approved in the USA and Europe for the correction of hyponatremia, including in patients with CHF. The addition of tolvaptan to standard therapy in patients with acutely decompensated HF improves the clinical manifestations of the disease, but does not affect mortality and major cardiovascular complications [43]. Experience with the use of drugs in this group for myocardial infarction is limited to experimental data.
Thus, to date, several methods have been developed for pharmacological correction of the activity of neurohumoral systems in patients with MI. The most promising of the new drugs being studied are natriuretic peptides, the possibilities of clinical use of which require study in large studies.
Pharmacokinetic and pharmacodynamic effects of aliskiren
Aliskiren has favorable physicochemical properties, including high solubility (> 350 mg/ml at pH = 7.4) and hydrophilicity, which significantly improves the bioavailability of the drug [79]. Under experimental conditions, it was found that after taking the first dose, peak plasma concentration is reached after 1–2 hours, bioavailability is within 16.3%, and the half-life is 2.3 hours [79]. In healthy volunteers, the pharmacokinetic properties of the drug were assessed in a dose range from 40 to 1800 mg/day. [4]. It turned out that the plasma concentration of aliskiren increases progressively after taking ranged doses of 40–640 mg/day, reaching a maximum after 3–6 hours. The average half-life is 23.7 hours. Moreover, the stability of the plasma content of aliskiren is observed after 5–8 days of continuous use [45]. In addition, researchers noted the drug’s ability to moderately accumulate when used in high doses, as well as the presence of a direct dependence of the level of bioavailability on food intake [4]. It should be noted that the pharmacokinetic characteristics of aliskiren do not depend on fasting glycemia and plasma concentration of glycosylated hemoglobin [81]. In addition, the drug has a comparable kinetic profile in representatives of different races and ethnic groups [69]. Aliskiren binds moderately to plasma proteins, and the intensity of this interaction does not depend on its plasma concentration [4]. Elimination of the drug occurs unchanged in the bile, excretion in urine is less than 1% [16]. The peculiarities of the drug are low competition with other drugs for binding to blood plasma proteins and the absence of the need for degradation on cytochromes of the P450 system. Aliskiren over a wide range of doses does not have a clinically significant effect on the metabolism of warfarin, lovastatin, atenolol, celecoxib, cimetidine and digoxin [17–19]. In addition, the drug at a daily dose of 300 mg orally does not change the pharmacokinetic profile of other antihypertensive drugs, such as ramipril (10 mg/day), amlodipine (10 mg/day), valsartan (320 mg/day), hydrochlorothiazide (25 mg/day). days) [70].
Aliskiren is a highly selective non-peptide inhibitor of renin synthesis, superior in this regard to other representatives of this class [37, 40, 50]. The drug does not have an additional inhibitory effect on other aspartate peptidases, such as cathepsin D and pepsin, either in experimental or clinical settings [79]. Moreover, aliskiren leads to a significant blockade of renin secretion even in relatively low doses and with limited bioavailability [25, 26].
Early phase 1 and 2 studies showed that the drug provides effective blockade of the RAS and a dose-dependent reduction in systemic blood pressure [45]. Thus, in healthy volunteers, the drug, when administered once compared to placebo, leads to an almost 80% reduction in the initial concentration of angiotensin II, although the plasma renin content decreases more than tenfold. Increasing the observation time from one to eight days while continuing constant administration of aliskiren contributed to the maintenance of deep blockade of the RAS due to a reduction in the plasma pool of angiotensin II by 75% of the initial level. At a dose of 160 mg/day, aliskiren has the same depressant effect on the plasma concentration of angiotensin II as the ACE inhibitor enalapril at a dose of 20 mg/day. In addition, at a dose of more than 80 mg/day, the drug promotes a significant regression of plasma aldosterone levels (Nussberger et al., 2002).
In a cohort of patients with hypertension, over four weeks of therapy, aliskiren at a dose of 75 mg/day led to a reduction in plasma renin activity (PAR) by 34 ± 7% of the initial level; after increasing the dose to 150 mg/day, the drug contributed to a decrease in PAR by 27 ± 6% to the outcome of the eighth week of continuous use [78]. It should be noted that the initial significant decrease in plasma renin activity is accompanied by a gradual increase that does not reach the initial level. It is important that this phenomenon is not accompanied by a loss of the antihypertensive effect of the drug [45, 78]. Nevertheless, the possibility of the phenomenon of “escaping” renin secretion from the influence of aliskiren has led to the need to continue research in the direction of assessing the prospects for the effectiveness of the combination of PIR and ARA, which are also capable of reducing plasma renin activity. Thus, in a small pilot crossover study, it was found that aliskiren at a dose of 300 mg/day was superior to valsartan at a dose of 160 mg/day in terms of reducing plasma renin activity. However, the combination of aliskiren and valsartan in half daily doses turned out to be preferable compared to the isolated use of each drug in terms of its ability to block RAS activity. This was reflected in a deeper decrease not only in PAR, but also in the levels of angiotensin II and angiotensin II. The researchers came to the conclusion that the effect of both drugs on the activity of the RAS is synergistic [3]. Similar data were obtained by O'Brien et al. (2007) when using aliskiren (150 mg/day) in combination with hydrochlorothiazide, ramipril or irbesartan in patients with mild and moderate hypertension [46]. It turned out that aliskiren contributed to a significant reduction in PAR by 65% (p < 0.0001) from the initial level, while ramipril and irbesartan in monotherapy led to a 90% and 175% reduction in PAR, respectively. The addition of aliskiren to antihypertensive drugs did not result in an additional reduction in PAR, but led to more effective control of office BP and the 24-hour BP profile [46].
Thus, aliskiren is capable of quite serious blockade of the RAS, which is accompanied by the expected clinical effects in the form of a reduction in vascular tone and a decrease in systemic blood pressure. However, the drug is not without fundamentally negative qualities, primarily associated with the implementation of the phenomenon of PAR “escape,” which is, in principle, characteristic of all drugs that mediate their pharmacodynamic effect through chronic blockade of the RAS [31]. It has been established that theoretical concerns regarding a decrease in the effectiveness of aliskiren due to restoration of renin secretion or the presence of a withdrawal syndrome after sudden cessation of treatment are not confirmed by clinical observations [28, 55].
ON THE APPROACHES TO RENIN: 110 YEARS OF SEARCH
There is perhaps no more common chronic disease today than hypertension (high blood pressure). Even its slow and seemingly imperceptible course ultimately leads to fatal consequences - heart attacks, strokes, heart failure, kidney damage. Back in the century before last, scientists discovered that the kidneys produce a protein called renin, which causes an increase in blood pressure in the vessels. But only 110 years later, through the joint efforts of biochemists and pharmacologists, it was possible to find an effective remedy that can resist the dangerous effects of a long-known substance.
Science and life // Illustrations
Rice. 1. Liver cells constantly release the long peptide angiotensinogen into the bloodstream.
Rice. 2. Cardiovascular continuum: the path from hypertension to damage to the heart, blood vessels, kidneys and other organs.
Rice. 3. Direct renin inhibitor (PIR) is built into the active center of renin and prevents it from breaking down angiotensinogen.
‹
›
In the early 1990s, the number of cardiovascular patients began to increase in Russia. And still in our country the mortality rate among the working-age population exceeds European indicators. Representatives of the male half of the population turned out to be especially unstable to social cataclysms. According to the World Health Organization, the life expectancy of men in our country is only 59 years. Women turned out to be more resilient - they live on average 72 years. Every second citizen of our country dies from cardiovascular diseases and their consequences - heart attacks, strokes, heart failure, etc.
One of the main causes of cardiovascular diseases is atherosclerotic vascular damage. With atherosclerosis, the inner lining of the vessel thickens, so-called plaques form, which narrow or completely block the lumen of the artery, which impairs the blood supply to vital organs. The main cause of atherosclerotic vascular damage is a violation of fat metabolism, mainly an increase in cholesterol levels.
Another, no less important and most common cause of cardiovascular disease is hypertension, which is manifested by a sustained increase in blood pressure. Increased blood pressure also leads to vascular damage. Namely, the lumen of the vessel narrows, its wall thickens (hypertrophy of the muscle layer develops), and the integrity of the internal lining of the vessel—the endothelium—is compromised. Such changes are called vascular remodeling. All this leads to the fact that a vessel affected by atherosclerosis loses its elasticity and stops pulsating under the influence of blood flow. If healthy vessels can be compared to flexible rubber tubes that transmit a pulse wave and dampen the turbulence of the blood flow, then pathological vessels are similar to a metal pipeline. Vascular remodeling contributes to the progression of atherosclerosis.
Hypertension as a cause of heart attacks and strokes
Hypertension often goes unnoticed. Patients do not know that they are sick, do not change their lifestyle, do not see a doctor or take medications. Meanwhile, hypertension, due to its destructive effect on the body, can be called a “silent killer”. If the disease develops quickly, it leads to the progression of atherosclerosis and ultimately to heart attack, stroke, and gangrene of the lower extremities. If the disease proceeds for a long time and the body manages to adapt to the blockage of blood vessels, damage develops to the heart muscle (first hypertrophy, and then atrophy of the myocardium, which leads to chronic heart failure), kidneys (albuminuria - loss of protein in the urine, impaired renal function and ultimately - renal failure) and metabolic disorders (glucose intolerance, and then diabetes).
The causes of hypertension are not fully understood, although research in this direction has been conducted for more than a century. How does hypertension occur and why does it cause such deadly complications? Biochemistry provides the answer to these questions.
Molecules that increase blood pressure
The role of biochemical disorders in the development of hypertension has been known for a long time. In 1897, Robert Tigerstedt, a professor of physiology at Karolinska University in Stockholm, a Finn by origin, reported his discovery at an international conference in Moscow. Together with his assistant Per Gustav Bergman, he discovered that intravenous administration of kidney extract caused an increase in blood pressure in rabbits. Scientists named the substance that increases blood pressure renin. Tigerstedt's report did not create a sensation; moreover, the study was considered small, insignificant, done for the sake of another publication. The disappointed professor stopped his research and returned to Helsinki in 1900. Bergman began practicing medicine, and the scientific world forgot about the pioneering work of Scandinavian physiologists for 40 years.
In 1934, a Canadian scientist working in California, Harry Goldblatt, caused symptoms of arterial hypertension in dogs by clamping the renal artery and proceeded to isolate a protein substance, renin, from the kidney tissue. This marked the beginning of discoveries in the field of the mechanism of blood pressure regulation. True, Goldblatt managed to obtain a pure renin preparation only 30 years later.
Literally a year after Goldblatt's first publication, in 1935, two research groups at once - from Buenos Aires under the leadership of Eduardo Mendez and an American one under the leadership of Irving Page - independently of each other, also using the technique of clamping the renal artery, isolated another substance that increases arterial blood pressure. pressure. Unlike the large protein molecule renin, it was a small peptide consisting of only eight amino acids. American researchers called it hypertensin, and Argentine researchers called it angiotonin. In 1958, during an informal meeting over a glass of martini, scientists compared the results of their research, realized that they were dealing with the same compound and came to a compromise agreement on the chimeric name of the peptide they discovered - angiotensin.
So, the main connections that increase blood pressure were discovered; only the connecting links in the mechanism of development of hypertension were missing. And they appeared. At the end of the 50s of the twentieth century, the concept of the functioning of the renin-angiotensin system (RAS) was formed.
The classic idea of how the RAS functions is shown in Fig. 1.
It is angiotensin II, acting on certain receptors, that leads to an increase in blood pressure, and with prolonged activation of the RAS, to dramatic consequences in the form of damage to the heart, blood vessels, kidneys, and ultimately to death (Fig. 2).
Several types of angiotensin II receptors have been discovered, the most studied of which are receptors of the 1st and 2nd types. When angiotensin II interacts with type 1 receptors, the body responds with vasospasm and increased aldosterone production. Aldosterone is a hormone of the adrenal cortex that is responsible for fluid retention in the body, which also contributes to an increase in blood pressure. So type 1 receptors are responsible for the “harmful” effect of angiotensin II, that is, for increasing blood pressure. The interaction of angiotensin II with type 2 receptors, on the contrary, leads to a beneficial effect in the form of vasodilation.
As it turned out, the destructive effect of angiotensin II is not limited to an increase in blood pressure. Recent studies show that binding of angiotensin II to type 1 receptors contributes to the development of atherosclerosis. It turned out that angiotensin II causes inflammatory processes in the walls of blood vessels, promotes the formation of reactive oxygen species and, as a result, disrupts the structure and function of the endothelium - the cells lining the walls of blood vessels. Dysfunction of the endothelium leads to the development of atherosclerosis and remodeling of vessel walls.
So, the renin-angiotensin system (RAS) plays a key role in both increased blood pressure and the development of atherosclerosis. Scientists have found that genes responsible for the functioning of proteins involved in ASD determine a person's susceptibility to hypertension and cardiovascular diseases. If certain genes are active, the RAS is also hyperactivated, and the likelihood of developing hypertension and cardiovascular diseases increases several times.
Search for drugs for hypertension. Three targets in a molecular chain
As soon as the idea of the renin-angiotensin system (RAS) was formed, three molecular targets were immediately identified that could be used to prevent the development of hypertension. Therefore, the strategy for searching for new drugs developed in three main directions (see Fig. 1): the search for renin inhibitors; search for angiotensin-converting enzyme (ACE) inhibitors; search for angiotensin II type 1 receptor blockers (ARBs).
The most attractive target for pharmacologists was and remains the enzyme renin, since it is the key molecule of the RAS. If there is no renin, angiotensin II is not produced. However, the first inhibitors (substances that block activity) of renin, developed back in the 60s of the last century, could not be put into practice due to unsatisfactory pharmacological properties and the high cost of synthesis. They were poorly absorbed from the gastrointestinal tract and had to be administered intravenously.
After the failure with renin, pharmacologists began searching for another molecular target. Scientists were helped to find it by the poisonous snake Bothrops gararaca, the bite of which leads to a long-term and sometimes fatal drop in blood pressure. In 1960, the Brazilian Sergio Ferreiro began searching for a substance contained in the poison that causes “vascular paralysis.” In 1968, it was discovered that the desired substance is an inhibitor of a certain enzyme that converts angiotensin I into angiotensin II. This is how angiotensin-converting enzyme (ACE) was discovered. In 1975, captopril was introduced, the first synthetic ACE inhibitor that could be taken in tablet form and whose effectiveness was not surpassed by other ACE inhibitors. This was a breakthrough and a real success in the treatment of hypertension. Now the number of ACE inhibitors is very large, there are more than 30 of them.
Along with successes, data appeared on the side effects of captopril and other ACE inhibitors, in particular the appearance of rash, itching, and painful dry cough. In addition, even at maximum doses, ACE inhibitors cannot completely neutralize the harmful effects of angiotensin II. In addition, the formation of angiotensin II during treatment with ACE inhibitors is very quickly restored due to alternative mechanisms. This is the so-called escape effect, which causes doctors to increase the dose or change the drug.
In Europe and the USA, over the past 10 years, ACE inhibitors have given way to a new class of drugs - angiotensin receptor blockers (ARBs). Modern ARBs completely turn off “harmful” type 1 receptors without affecting “useful” type 2 receptors. These drugs, the first of which was losartan, have virtually no side effects characteristic of ACE inhibitors, in particular they do not cause a dry cough. ARBs are in no way inferior to ACE inhibitors in lowering blood pressure and more. Recent studies show that ACE inhibitors and angiotensin receptor blockers (ARBs) prevent damage to the heart and blood vessels and even help improve the condition of blood vessels and myocardium affected by hypertension.
It is curious that if captopril is still not inferior in effectiveness to newer ACE inhibitors, then ARBs are being improved all the time. Newer ARBs are more specific to type 1 receptors and remain active in the body longer.
The final assault
Despite the success of ACE inhibitors and ARBs, pharmacologists have not given up hope of “overcoming” the substance that plays a key role in hypertension, renin. A very attractive goal is to turn off the molecule that “triggers” the biochemical cascade of the RAS.
A more complete blockade of the angiotensin II synthesis system was expected from renin inhibitors. The enzyme renin catalyzes the process of conversion of angiotensinogen, that is, in the biochemical cascade it interacts with only one molecule (Fig. 3). This means that renin inhibitors should not have significant side effects, unlike ACE inhibitors, which affect not only ACE, but also other regulatory systems.
Many years of searching for renin inhibitors resulted in the synthesis of several molecules, one of which, aliskiren, already appeared in the arsenal of American doctors in 2007. Direct renin inhibitors (DRIs) have many advantages. They are easily tolerated by patients, are slowly eliminated from the body, they reduce blood pressure well (better than ACE inhibitors), and do not cause withdrawal effects when stopping taking them.
So, our story began with Renin, and it will end with him. Advances in science have finally given scientists the opportunity to approach a protein discovered 110 years ago at a completely new molecular level. But perhaps the new drug is just the beginning. It turned out that renin is not only an enzyme, but also a hormone that interacts with special receptors discovered in 2002. It is likely that renin inhibitors may not only block its enzymatic activity, but also interfere with the binding of renin to renin receptors. This possibility is currently being actively studied. The next step in the search for new drugs for the treatment of hypertension may be the synthesis of renin receptor blockers or even therapy at the gene level. The development of inhibitors of aldosterone synthesis enzymes and other enzymes—endopeptidases—is also promising. But this is a topic for another article.
In any case, in the near future, patients will have access to drugs that are far superior to anything known today and that can reverse the terrifying mortality statistics from cardiovascular diseases. All this is thanks to scientific research and the introduction of scientists’ developments into medical practice.
***
Based on the non-commercial name of a drug for hypertension, one can draw a conclusion about its mechanism of action. Angiotensin-converting enzyme (ACE) inhibitors have the ending -pril in their names (enalapril, lisinopril, ramipril). Angiotensin receptor blockers (ARBs) - ending with sartan (valsartan, irbesartan, telmisartan). Direct renin inhibitors (DRIs) can be distinguished by their kyren endings (aliskiren, remikiren, enalkiren).
A non-commercial name should not be confused with a trademark. There are usually no rules or patterns in the brand names of original drugs.
Glossary for the article
Blockers are substances that block the interaction of physiologically active substances with receptors.
Inhibitors are substances that block enzyme activity.
Receptors are protein molecules on the surface of the cell membrane. The interaction of other molecules with them leads to the launch of a chain of reactions inside the cell.
Enzymes are protein molecules that catalyze processes in a living cell.
Organoprotective qualities of aliskiren
It has been established that chronic blockade of the RAS in patients with hypertension improves clinical outcomes not only due to the reduction of blood pressure, but also, possibly, due to effective organ protection [12, 13, 34]. However, the contribution of the intrinsic qualities of antihypertensive drugs to the reduction of global cardiovascular risk is widely debated [77]. It is believed that it is the control of blood pressure that is the main determinant in the implementation of the organoprotective effects of antihypertensive therapy [12, 60, 73]. However, PIRs may potentially have beneficial effects on end organs and clinical outcomes [28]. It is assumed that aliskiren may have an organoprotective effect through inhibition of specific renin receptors [44], present in the mesangial tissue of the kidneys, in the subendothelium of the renal and coronary arteries [4]. In addition, there is evidence of a beneficial effect of aliskiren on the activity of local renal RAS [38].
The experiment proved the ability of aliskiren to induce vasodilation of the renal arteries and promote an increase in minute diuresis [32], lead to the reversal of albuminuria, and also contribute to the reduction of LV hypertrophy [48]. At the same time, the reno- and cardioprotective qualities of aliskiren were comparable to those of valsartan [48].
In clinical studies, aliskiren demonstrated a positive effect in reducing albuminuria, preventing a decrease in glomerular filtration rate and increasing plasma creatinine [66]. Moreover, the nephroprotective activity of the drug was not inferior to the ARA losartan. In addition, aliskiren is able to reduce the severity of proinflammatory and neurohumoral activation not only in experiments [42], but also in clinical settings [59]. The possibility of reversing LV hypertrophy with long-term administration of aliskiren and potentiation of this effect with the addition of losartan have been shown [58].
Tolerability and safety of aliskiren in monotherapy and in combination administration
Aliskiren showed high safety both in healthy volunteers during phase 1 trials and in patients with hypertension. The incidence of adverse side effects or adverse reactions leading to patient refusal to continue the study was comparable to that in the placebo groups. The most frequently reported side effects were fatigue, headache, dizziness and diarrhea [29, 62, 76]. It should be noted that the incidence of side effects depends on the dose of the drug [62, 76]. It is important that aliskiren does not affect the metabolism of endogenous bradykinin and substance P, so the drug does not lead to the manifestation of cough and angioedema as often as ACE inhibitors. In general, the tolerability of aliskiren is comparable to that of ARBs and placebo [15].
Aliskiren is not only well tolerated by patients with impaired liver function, but also has a pharmacokinetic profile independent of the severity of liver failure [71]. There is data on the safety of aliskiren in patients with renal failure, diabetes mellitus, obesity, metabolic syndrome and heart failure [4, 33], as well as in older age groups [68]. However, there is a potential danger of deterioration in renal function when using aliskiren in monotherapy or when combining it with ARA in patients with renal artery stenosis, during parenteral anesthesia, as well as in a cohort of people receiving COX-2 inhibitors [4].
In conclusion, it should be noted that the new class of antihypertensive drugs certainly deserves attention. However, regarding the clinical efficacy of PIR and aliskiren in particular, additional research is required to increase the body of evidence regarding possible beneficial effects on target organs. The amount of existing data regarding the prospects for the use of PIR in the treatment of not only hypertension, but also HF and diabetes mellitus is currently limited. However, high safety, good tolerability, favorable therapeutic profile, and the possibility of wide combination with various drugs allow us to hope that PIR will take its rightful place among antihypertensive drugs.
Direct renin inhibitor - aliskiren in the treatment of arterial hypertension
The renin-angiotensin-aldosterone system plays a key role in regulating blood pressure and water-electrolyte balance. A direct renin inhibitor, aliskiren, reduces plasma renin activity and has cardio- and nephroprotective effects. The antihypertensive effect does not depend on gender, race, age, or body mass index. The antihypertensive effect of aliskiren and angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and calcium antagonists is comparable. Aliskiren is effective in patients with obesity, diabetes mellitus, impaired renal function and metabolic syndrome.
Direct renin inhibitors — aliskiren in the treatment of arterial hypertension
The Renin-angiotensin-aldosterone system plays a key role in the regulation of blood pressure and fluid and electrolyte balance. Direct renin inhibitor - aliskiren, decreasing plasma renin activity, providing cardio- and nephroprotective effects. Antihypertensive effect is independent of gender, race, age, body mass index. Antihypertensive effect of aliskiren and the angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, calcium antagonists is comparable. Aliskiren is effective in patients with obesity, diabetes, renal dysfunction and metabolic syndrome.
In the course of studying the renin-angiotensin-aldosterone system (RAAS), approaches have been developed to regulate its pharmacological activity. The first component of the RAAS, renin, was identified 110 years ago. Subsequently, its importance in the regulation of RAAS activity in pathological conditions was demonstrated, which became the basis for the development of direct renin inhibitors (DRIs). The RAAS plays a key role in regulating blood pressure (BP) and water and electrolyte balance. Increased activity of the RAAS plays an important role in the formation and progression of arterial hypertension (AH), chronic heart failure (CHF), chronic kidney disease, and systemic atherosclerosis. The RAAS is directly involved in the processes of tissue growth and differentiation, modulation of inflammation and apoptosis, as well as potentiation of the synthesis and secretion of a number of neurohumoral substances. The main effects of the RAAS are realized through angiotensin II (ATII) through stimulation of specific receptors. Activation of angiotensin receptor subtype 1 (AT1) leads to vasoconstriction and stimulates the release of vasopressin, aldosterone, endothelin, and norepinephrine. The physiological role of other angiotensin receptor subtypes (AT3, AT4 and ATx) continues to be studied. ATII promotes the accumulation of collagen matrix, the production of cytokines, adhesive molecules, activation of the intracellular signaling system, increased expression of fetal phenotype genes, plays a major role in myocardial remodeling and left ventricular (LV) hypertrophy, ATII is involved in the processes of arterial remodeling, intensification of oxidative stress and apoptosis, promotes the formation and progression of hypertension, CHF, atherosclerotic vascular damage, diabetic and non-diabetic nephropathy, angiopathy in diabetes mellitus (DM), eclampsia in pregnant women, Alzheimer's disease. The progression of cardiovascular diseases does not depend on the vasopressor effect of ATII [1].
Renin secretion is the first step in increasing the synthesis of ATI, ATII and other products of the RAAS cascade. The implementation of subsequent effects of the RAAS is modulated by the influence of renin on specific receptors, inducing an increase in ATII [2].
Until recently, the following RAAS inhibitors existed: angiotensin-converting enzyme inhibitors (ACEIs) and ATII receptor blockers (ARBs). The mechanism of action of ACE inhibitors is as follows: the activity of ACE is suppressed, which leads to a decrease in the effects of ATII and a slowdown in the degradation of vasopressors (bradykinin and prostaglandin E2). ARBs competitively inhibit ATII receptors and reduce the effects of ATII. Receptors for renin and prorenin are located on the cell surface. Activation of the cellular signaling pathway by renin leads to fibrosis and cellular hypertrophy. In recent years, control of the activity of the RAAS has been carried out by limiting the production of ATII, blocking ATII and aldosterone receptors, by limiting the secretion of renin, mainly through the use of β-blockers. Numerous studies have shown that adequate reduction of RAAS activity with the help of ACE inhibitors, ARBs or aldosterone is more postulated than actually achieved, since the phenomenon of “escape” of the antihypertensive and organoprotective effects of RAAS blockers with their long-term use develops. To overcome this phenomenon, combinations of ACEI + ARB, ACEI + β-blocker, ACEI + spironolactone are used. The emergence of PIR is considered as a way to achieve more complete control of RAAS activity and overcome the “escape” phenomenon [3].
The first PIRs were synthesized in the 70s of the twentieth century, but the first drug suitable for oral administration was aliskiren (A). A., by binding to the active part of the target molecule, prevents its interaction with angiotensinogen. By reducing plasma renin activity (PRA), A. has cardio- and nephroprotective effects. RAAS inhibitors stimulate ARP, resulting in the following effects: vasoconstriction in the glomerulus, inflammation, fibrosis (kidneys); hypertrophy, fibrosis, vasoconstriction (heart); hyperplasia, hypertrophy, inflammation, lipid oxidation, fibrosis (vascular); vasoconstriction (brain). A. acts at the point of activation of the RAAS and reduces ARP. Unlike ACE inhibitors and ARBs, A reduces the level of ATI, AII and ARP. Renin has enzymatic as well as receptor-mediated activity [4].
Pharmacokinetics A. Clinical studies have shown that the tolerability of A is comparable to placebo. The duration of action of this drug exceeds 24 hours, and renal vasodilation may persist for up to 48 hours. The half-life of A is approximately 40 hours, which allows for once daily dosing. The recommended starting dose A is 150 mg with a further increase to 300 mg. The pharmacokinetic characteristics of A do not depend on fasting glycemia and the concentration of glycosylated hemoglobin in the blood plasma. Elimination of the drug occurs unchanged in the bile, excretion in urine is <1%. Phase 1 and 2 studies have shown that the drug promotes effective blockade of the RAAS and dose-dependent prevention of increases in blood pressure [5]. The full antihypertensive effect occurs after 2 weeks and does not depend on gender, race, age, or body mass index. And it has a minimal risk of drug interactions and does not require dose adjustment in chronic renal failure (CRF) or liver damage. The addition of A to lovastatin, atenolol, warfarin, furosemide, digoxin, celecoxib, hydrochlorothiazide (HCTZ), ramipril, valsartan, metformin and amlodipine did not lead to a clinically significant increase in exposure to A. Its combined use with atorvastatin resulted in a 50% increase in Cmax (maximum concentration drug) and AUC (area under the concentration-time curve) after multiple doses. Co-administration of ketoconazole 200 mg twice daily with A resulted in an 80% increase in plasma A levels. When A was co-administered with furosemide, the AUC and Cmax of furosemide decreased by 30 and 50%, respectively [6]. No dose adjustment is required in patients with chronic renal failure. In patients with chronic renal failure, there was a moderate (~2-fold) increase in A exposure, but it did not correlate with the severity of kidney damage and creatinine clearance. Clearance A was 60–70% in healthy people. Renal clearance of A decreased with increasing severity of renal damage. Because renal impairment has only a modest effect on A exposure, dosage adjustment of A is likely not required in patients with hypertension and renal impairment. No dose adjustment is required in patients with liver damage. There was no significant correlation between A exposure and the severity of liver damage. And it is capable of blocking the RAAS, which leads to a decrease in vascular tone and systemic blood pressure. However, the drug is not without negative qualities associated with the “escape” phenomenon, which is typical for all drugs that block the activity of the RAAS. A decrease in the effectiveness of A due to restoration of renin secretion or the presence of withdrawal syndrome is not confirmed by clinical observations [7].
Antihypertensive effectiveness A. ARP is an indicator necessary not only for diagnosing rare secondary forms of hypertension (renovascular). The clinical and prognostic significance of ARP is as follows: the indicator increases with hypertension in combination with other risk factors (male gender, smoking, type 2 diabetes, obesity, metabolic syndrome) and in the presence of target organ damage (TOD) ( persistent decrease in glomerular filtration rate); the increase in renin ARP can be iatrogenic, provoked by ACE inhibitors and/or diuretics (loop, thiazide), causing renal sodium loss: in this case, further activation of the RAAS is observed, which leads to loss of control over blood pressure and progression of CHF; an increase in ARP always predisposes to worsening POM and potentially fatal cardiovascular complications (CVD) and renal complications; Increased ARP is an independent factor for the pharmacological effects of PIR, which makes it possible to achieve a decrease in blood pressure and inhibit the progression of POM. A. can claim to be an effective antihypertensive drug in monotherapy and in combination with other drugs. Indications for the use of PIR are: hyperrenin variants of hypertension, normorenin hypertension, in which prorenin and indirect activation of prorenin receptors leads to tissue destruction. PIR is indicated not only for renovascular hypertension and CHF, but also for increased plasma concentrations of prorenin (hypertension with hyperactivation of the sympathetic nervous system, metabolic syndrome, type 2 diabetes, menopause).
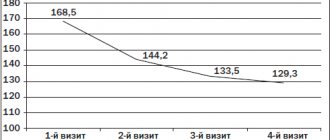
Monotherapy A. provides a dose-dependent reduction in diastolic blood pressure (DBP) and systolic blood pressure (SBP) in patients with mild and moderate hypertension [8]. An assessment of the effectiveness and safety of A. in 672 patients with stage I-II hypertension in an 8-week placebo-controlled study revealed a dose-dependent decrease in SBP and DBP. The antihypertensive effect of A persisted for two weeks after its discontinuation; A was well tolerated; the incidence of adverse events did not differ from placebo. A - trade name rasilez (P) - at a dose of 150 mg reduces SBP by 13 mm Hg. Art., and DBP by 10.3 mm Hg. Art., and at a dose of 300 mg reduces SBP from 15 to 22 mm Hg. Art. (depending on the level of hypertension), and DBP - by 11 mm Hg. Art. And provides blood pressure control in the early morning. After canceling A, the “rebound” phenomenon does not occur [8]. Pooled analysis of clinical studies including 8481 patients [9]. receiving monotherapy A or placebo, showed that a single dose of A at a dose of 150 or 300 mg/day caused a decrease in SBP by 12.5 and 15.2 mm Hg. Art. respectively, compared with a decrease of 5.9 mm Hg. Art. in patients receiving placebo (p<0.0001). DBP decreased by 10.1 (at a dose of 150 mg) and 11.8 mmHg. Art. (at a dose of 300 mg) respectively (in the placebo group - by 6.2 mm Hg, p <0.0001). There were no differences in the antihypertensive effect of A in men and women, as well as in persons over and under 65 years of age. When using ACE inhibitors, the concentrations of prorenin and ARP increase (the effectiveness of ACE inhibitors decreases). With an increase in the dose of ACE inhibitors, ARP and plasma concentration of ATI significantly increase [10]. Study A compared with ACE inhibitors in patients with mild to moderate hypertension found the following: A significantly reduced DBP and SBP more than ramipril after 12 weeks of treatment (monotherapy). A ± hydrochlorothiazide (HCTZ) significantly reduced DBP and SBP more than ramipril ± HCTZ after 26 weeks of treatment. And it significantly lowers DBP and SBP than ramipril after 12 weeks of treatment (monotherapy) in patients with stage II hypertension. Therapy A provides significantly better blood pressure control compared to ramipril. SBP and DBP return to baseline levels more quickly after discontinuation of ramipril than after discontinuation of A. Comparison of the hypotensive effectiveness of A, irbesartan and ramipril after a missed dose showed the following: after a missed dose, the achieved reduction in blood pressure was significantly greater in group A than in the ramipril group [eleven]. A significantly greater percentage of blood pressure reduction was maintained after a missed dose of A compared with irbesartan or ramipril. The return to initial blood pressure occurs more smoothly after discontinuation of A. than ramipril. A. and enalapril almost equally reduce the plasma concentration of ATP, but unlike A, taking enalapril led to a more than 15-fold increase in ATP. Under conditions of a low-salt diet, A-induced organ (in particular, renal) vasodilation can persist for up to 48 hours. Drugs that stimulate natriuresis (thiazide and loop diuretics) can provoke an increase in ARP. Prescribing A. in this situation is one of the most effective approaches to eliminating the reactive increase in ARP when combined with an ACE inhibitor and a thiazide diuretic.
In 2009, the results of a multicenter controlled clinical trial were published, in which the effectiveness of A and HCTZ (initial antihypertensive therapy) was compared in 1124 patients with hypertension; if necessary, amlodipine was added to these drugs [12]. By the end of the monotherapy period (week 12), it became clear that A leads to a more pronounced decrease in blood pressure than HCTZ (-17.4/-12.2 mmHg versus -14.7/-10.3 mmHg Hg, p<0.001). These results are important because most patients with hypertension initially require combination antihypertensive therapy. Optimization of combination antihypertensive therapy is important in patients with obesity. Moreover, A has additional advantages [13]. Patients with obesity, in whom the full (25 mg/day) dose of HCTZ did not lead to a decrease in blood pressure, were randomized into groups that received amlodipine + HCTZ (10/25 mg/day), irbesartan + HCTZ (300/25 mg/day ) and A. + HCTZ (300/25 mg/day). As Art. The antihypertensive effectiveness of treatment regimens (ARB + HCTZ, calcium antagonist + HCTZ) decreases. In a group with Ozh. III Art. (BMI≥40 kg/m2) only 50% managed to achieve target blood pressure with irbesartan + HCTZ, 43.8% with amlodipine + HCTZ, and only 16.7% with HCTZ. With less severe (grades I–II) obesity, target blood pressure was not achieved in more than 40% of patients receiving ARB + HCTZ or amlodipine + HCTZ, and in more than 60% of patients receiving only HCTZ. In the group of patients with obesity. Stages I-II, receiving A. + HCTZ, the target blood pressure was achieved in 56.7% of patients, and with Ozh. III Art. - 68.8%. Hypertension combined with obesity is often associated with an increase in the activity of the RAAS and is difficult to correct, so in such cases A. may be prescribed.
A.’s ability to lower blood pressure and reduce albuminuria has been established [14]. In the AVOID study [15], the effect of a combination of maximum doses of losartan and A on albuminuria (based on urine albumin/creatinine ratio) was assessed in 599 patients with diabetic nephropathy with hypertension. The addition of A (300 mg/day) to losartan (100 mg/day) was accompanied by a significant decrease in the urine albumin/creatinine ratio by 20% in the group as a whole (100%), and in 24.7% by 50% or more. In the losartan + placebo group, a decrease in urine albumin/creatinine ratio by 50% or more was achieved only in 12.5% of patients (p <0.001). PIRs can reduce albuminuria both in monotherapy and in combination with ARBs, allowing to achieve optimal st. blockade of the RAAS, which ensures the elimination of generalized and local renal endothelial dysfunction.
And with combination therapy of hypertension. In patients with mild and moderate hypertension without and with obesity. A. + HCTZ provide a significant reduction in DBP and SBP. More patients achieve BP control with the A + HCTZ combination than with other HCTZ combinations [16]. In patients with hypertension and diabetes, A + ramipril significantly lowers blood pressure than both components of monotherapy. A provides significantly better blood pressure control than ramipril [17]. In patients with mild and moderate hypertension, A + valsartan significantly reduces blood pressure better than both components of monotherapy [18]. A significantly reduces blood pressure when combined with amlodipine at a dose of 5 mg/day. A increases the level of blood pressure control compared to amlodipine at a dose of 5 mg/day [19]. A. ± HCTZ are effective in long-term therapy of hypertension. A + valsartan ± HCTZ provide long-term antihypertensive efficacy (interim analysis of 6 months of therapy) [20].
In 2009, the design of the ALTITUDE (Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints) study was published (part of the ASPIRE HIGHER program), which studied the effect of double blockade of the RAAS using a combination of A and standard therapy (ACEIs or ARBs) in patients with diabetes. Type 2 with a high risk of cardiovascular complications and renal complications, partly due to an increase in ARP. The primary objective of this study is to evaluate the effectiveness of adding A to standard therapy on the effect on the composite endpoint (cardiovascular death and complications: successful resuscitation, non-fatal MI, non-fatal stroke, unplanned hospitalization for CHF; development of end-stage renal failure, doubling of serum creatinine, death from causes related to kidney damage) [21]. This study should last about 4 years, and its results are intended to justify the use of a combination of A. with an ACE inhibitor or ARB to inhibit the progression of cardiorenal syndrome in type 2 diabetes. The greatest effectiveness of A can be expected in those variants of hypertension in which there is a tendency to increase ARP (forming essential hypertension, obesity, metabolic syndrome, type 2 diabetes, chronic renal failure). The vast majority of patients with hypertension require combination antihypertensive therapy already at the first stage of treatment and, as shown in one of the recently published clinical studies [22], in combinations A retains its activity regardless of the initial antihypertensive therapy. The increase in ARP in patients with hypertension is considered as a diagnostic marker and as an independent risk factor for potentially fatal cardiovascular events. Pharmacological modulation of ARP is one of the most promising approaches to managing the risk of cardiovascular events in patients with hypertension combined with kidney damage, metabolic syndrome and obesity. [23]. The AVOID (Aliskiren in the eValuation of prOteinuria In Diabetes) study (part of the ASPIRE HIGHER program) was designed to evaluate the potential of a specific antihypertensive drug in protecting end organs in various situations characterized by a very high risk of potentially fatal complications (LV hypertrophy, type 2 diabetes , CHF). Interim results suggest that direct renin blockade with A is one of the most accessible strategies to improve long-term prognosis. In the ALLAY study (The Aliskiren Left Ventricular Assessment of Hypertrophy), A caused a decrease in the LV myocardial mass index, reflecting regression of its hypertrophy, in patients with hypertension and excess body weight. The combination of A and losartan provided a further reduction in LV myocardial mass index by an additional 20% compared with losartan monotherapy, but this difference did not reach a statistically significant value [24]. According to the results of the ALOFT study (ALiskiren Observation of heart Failure Treatment study), the addition of A to the standard treatment regimen for CHF with signs of an unfavorable prognosis (persistent increase in plasma concentration of natriuretic peptide) and hypertension made it possible to further improve the ratio of the magnitude of mitral regurgitation to the area of the mitral orifice and transmitral blood flow . Thanks to A, it was possible to achieve a decrease in the concentration of markers of maladaptive neurohumoral activation (plasma levels of brain natriuretic peptide and its N-aminoterminal precursor (NT-pro BNP), urinary aldosterone concentration, ARP) [25]. Prospects for the use of A for the purpose of inhibiting the development of kidney damage are determined by its high safety, obviously significantly superior to other RAAS blockers (ACE inhibitors, ARBs and aldosterone antagonists) due to the lower risk of increasing creatininemia and kalemia. Excreted primarily in bile rather than urine [5], A retains its antihypertensive effect, but does not lead to deterioration of renal function in patients with a persistent decrease in glomerular filtration rate. It is in nephrology that aggressive blockade of the RAAS using simultaneously used several classes of drugs may be effective in preventing terminal chronic renal failure. A reduces albuminuria (significantly superior to monotherapy with each drug) and the likelihood of irreversible deterioration of renal function in the group of patients (with proteinuria >1 g/day), as shown in the COOPERATE study (COcombination treatment of angiOtensin-II recePtor blockEr and angiotensin-conveRting-enzyme inhibitor in non-diAbeTic rEnal disease) [26]. The ONTARGET study (OngoiNg Telmisartan Alone and in combination with Ramipril Global Endpoint Trial) showed that the combination of ACEIs and ARBs is associated with a greater likelihood of arterial hypotension and hyperkalemia, and, compared with monotherapy with these drugs, is associated with an increase in the rate of initiation of program hemodialysis and a doubling of creatininemia in high-risk patients with POM [27]. The following clinical situations can be objects for demonstrating the nephroprotective properties of A.: hypertension/metabolic syndrome or type 2 diabetes with albuminuria; Hypertension associated with a persistent decrease in glomerular filtration rate; Hypertension in chronic kidney diseases with and without proteinuria (including nephrotic) (for example, tubulointerstitial nephropathies); renovascular hypertension of various origins; patients who, for various reasons, have experienced an increase in creatininemia or hyperkalemia when using ACE inhibitors or ARBs; terminal chronic renal failure, including those treated with program hemodialysis or continuous ambulatory peritoneal dialysis; kidney transplant recipients [28, 29].
A new class of antihypertensive drugs (ADMs) requires additional research to increase the body of evidence regarding slowing the progression of POM.
And, obviously, it is indicated for most categories of patients suffering from hypertension, and this circumstance is reflected in the Russian recommendations on hypertension for the diagnosis and treatment of hypertension (fourth revision, 2010) as an additional class of antihypertensive drugs for combination therapy [30]. With the development of kidney damage, A may be effective in preventing terminal chronic renal failure and improving the prognosis of these patients.
ON THE. Andreichev, Z.M. Galeeva
Kazan State Medical University
Andreichev Nail Aleksandrovich - Candidate of Medical Sciences, Associate Professor of the Department of Faculty Therapy and Cardiology
Literature:
1. Bauer JH, Reams GP The angiotensin II type 1 receptor antagonists. A new class of antihypertensive drugs // Arch. Intern. Med. - 1995. - Vol. 155 (13). - R. 1361-1368.
2. Kim S., Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases // Pharmacol. Rev. - 2000. - Vol. 52(1). - R. 11-34.
3. Brown MJ Aliskiren // Circulation. - 2008. - Vol. 118(7). - R. 773-784.
4. Nguyen G., Delarue F., Burckle C. et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to rennin // Clin. Invest. - 2002. - Vol. 109(11). - R. 1417-1427.
5. Nussberger J., Wuerzner G., Jensen C. et al. Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): comparison with enalapril // Hypertension. - 2002. - Vol. 39(1). - R. 1-8.
6. Zhao C, Vaidyanathan S, Dieterich HA et al. Assessment of the pharmacokinetic interaction between the oral direct renin inhibitor aliskiren and furosemide: a study in healthy volunteers // Clin. Pharmacol. Ther. - 2007. - Vol. 81 (Suppl 1). - S. 110 (PIII-78).
7. Gradman AH, Kad R. Renin inhibition in hypertension // Am. Coll. Cardiol. - 2008. - Vol. 51(5). - S. 519-528.
8. Oh B.-H., Mitchell J., Herron JR et al. Aliskiren, an oral renin inhibitor, provides dose-dependent efficacy and sustained 24-hour blood pressure control in patients with hypertension // Am. Coll. Cardiol. - 2007. - Vol. 49. - S. 1157-1163.
9. Fisher ND, Jan Danser AH, Nussberger J. et al. Renal and hormonal responses to direct renin inhibition with aliskiren in healthy human // Circulation. - 2008. - Vol. 117 (25). - S. 3199-3205.
10. Dahlo FB, Anderson DR, Arora V. et al. Aliskiren, a direct renin inhibitor, provides antihypertensive efficacy and excellent tolerability independent of age or gender in patients with hypertension (abstr) // Clin. Hypertension. - 2007. - Vol. 9 (Suppl. A). - S. A157.
11. Andersen K., Weinberger MH, Egan B. et al. Comparative efficacy and safety of aliskiren, an oral direct renin inhibitor, and ramipril in hypertension: a 6-month, randomized, double-blind trial // Hypertension. - 2008. - Vol. 26. - S. 589-599.
12. Schmieder RE, Philipp T, Guerediaga J et al. Long-Term Antihypertensive Efficacy and Safety of the Oral Direct Renin Inhibitor Aliskiren. A 12-Month Randomized, Double-Blind Comparator Trial With Hydrochlorothiazide // Circulation. - 2009. - Vol. 119. - S. 417-425.
13. Prescott MF, Boye SW, Le Breton S. et al. Antihypertensive efficacy of the direct renin inhibitor aliskiren when added to hydrochlorothiazide treatment in patients with extreme obesity and hypertension // Am. Coll. Cardiol. - 2007. - Vol. 49(9, Suppl. A). - S. 370A. — (P. 1014-169).
14. Persson F., Rossing P., Schjoedl KJ et al. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes // Kidney Int. - 2008. - Vol. 73 (12). - S. 1419-1425.
15. Parving HH, Persson F, Lewis JB et al. AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy // N. Engl. J. Med. - 2008. - Vol. 358(23). - S. 2433-2446.
16. Jordan J, Engli S, Boye SW et al. Direct renin inhibition with aliskiren in obese patients with arterial hypertension // Hypertension. - 2007. - Vol. 49. - S. 1-9.
17. Uresin Y., Taylor A., Kilo C. et al. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension // Renin. Angiotensin Aldosterone Syst. - 2007. - Vol. 8. - S. 190-198.
18. Oparil S., Yarrows S., Patel S. et al. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomized, double-blind trial // Lancet. - 2007. - Vol. 370. - S. 221-229.
19. Drummond W, Munger MA, Essop MR et al. Antihypertensive efficacy of the oral direct renin inhibitor aliskiren as add-on therapy in patients not responding to amlodipine monotherap // Clin. Hypertension. - 2007. - Vol. 9. - S. 742-750.
20. Chrysant SG, Murray AV, Hoppe UC et al. Long-term safety, tolerability and efficacy of aliskiren in combination with valsartan in patients with hypertension: a 6-month interim analysis // Curr. Med. Res. Opin. - 2008. - Vol. 24 (4). — S. 1039-1047.
21. Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE): rationale and study design // Nephrol Dial Transplant. - 2009. - Vol. 24 (5). — S. 1663-1671.
22. Stanton AV, Dicker P., O'Brien ET Aliskiren monotherapy results in the greatest and the least blood pressure lowering in patients with high- and low-baseline PRA levels, respectively // Am. J. Hypertension. - 2009. - Vol. 22. - S. 954-957.
23. Mukhin N. A., Fomin V. V. Plasma renin activity is a risk factor and an independent target of antihypertensive therapy: the role of aliskiren // Consilium Medicum. - T. 11. - 2009. - No. 10. - P. 3-6.
24. Solomon SD, Appelbaum E, Manning WJ et al. Effect of the Direct Renin Inhibitor Aliskiren, Either Alone or in Combination With Losartan, Compared to Losartan, on Left Ventricular Mass in Patients With Hypertension and Left Ventricular Hypertrophy // The Aliskiren Left Ventricular Assessment of Hypertrophy (ALLAY) Trial. Presented at the American College of Cardiology. 57th Annual Scientific Session, March 31, 2008.
25.Mc. Murray JV, Pitt B, Latini R et al. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure // Circulation-Heart Failure. - 2008. - Vol. 1. - S. 17-24.
26. Nakao N., Yoshimura A., Morita H. et al. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomized controlled trial // Lancet. - 2003. - Vol. 361 (9352). — S. 117-124.
27. Mann IF, Schmieder RE, McQueen M. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomized, double-blind, controlled trial // Lancet. - 2008. - Vol. 372 (9638). - S. 547-553.
28. Chazova I.E., Fomin V.V., Paltseva E.M. Direct renin inhibitor aliskiren - new possibilities for protecting the kidneys in arterial hypertension // Clinical Nephrology. - No. 1. - 2009. - P. 44-49.
29. Chazova I.E., Fomin V.V. Direct renin inhibitor aliskiren: possibilities for correction of cardiorenal syndrome // Systemic hypertension. - 2009. - No. 4. - P. 53-58.
30. Diagnosis and treatment of arterial hypertension: Russian recommendations // Systemic hypertension. - 2010. - No. 3. - P. 5-26.