Composition and release form
The medicine is flat round white tablets. There is a dividing line on the front side. The corner is a little skewed. This chamfer makes the tablet easy to swallow. The letter D is engraved on the reverse side. The drug is sold in bottles. Each bottle contains 50 tablets.
The main active ingredient of the drug is digoxin. Auxiliary elements have been added to it. 1 tablet contains 0.25 mg of digoxin. It is obtained from raw materials of foxglove (Digitalis lanata). This perennial plant contains many cardiac glycosides that are used in medicine.
Digoxin is also available as a solution for injections. The amount of digoxin in it is 0.25 mg. It is supplemented with injection water, ethyl alcohol, glycerin and citric acid.
This medicine is a cardiac glycoside that normalizes blood pressure, rhythm and heart sounds. Digoxin has a beneficial effect on the heart muscle and belongs to the group of cardiotonic drugs.
How it affects the body
Digoxin inhibits and blocks the enzyme sodium-potassium adenosine triphosphatase. The task of Na+/K+ ATPase is to remove sodium ions from myocardial cells. When the activity of this enzyme is inhibited, the amount of sodium (Na+) in the muscle cells of the heart increases dramatically. Thanks to this, channels open in the cells through which calcium (Ca2+) enters the cardiomyocytes. Due to excess sodium, calcium accumulates very quickly in the membranes of heart muscle cells. High levels of calcium in myocardial cells inhibit the effects of the troponin complex, which prevents the mutual influence of two important proteins - myosin and actin.
Due to this complex mechanism, the myocardium begins to contract stronger and faster. Thanks to this, blood circulation improves: stroke and minute volume of blood flow increases. Systolic and diastolic volumes of the heart are reduced. The myocardium decreases in volume, but its tone increases, so the heart muscle begins to need less oxygen. Systolic output increases.
Digoxin reduces the heart rate during atrial fibrillation and has a beneficial effect on hemodynamics both inside the heart and throughout the body.
Positive effect of Digoxin:
- it becomes easier for the patient to breathe in a lying position;
- the pressure in the vena cava, pulmonary artery and right atrium decreases;
- heart rate normalizes;
- shortness of breath decreases in chronic heart failure;
- the left ventricle empties better due to faster and stronger muscle contraction;
- the load on all right parts of the heart (atrium and ventricle) is reduced;
- the total peripheral vascular resistance (TPVR) decreases.
One of the most important properties of digoxin is its ability to change the strength of heart contractions. This effect is called the inotropic effect.
Indications
The drug "Digoxin" in tablets or injections is prescribed for a variety of diseases:
- chronic heart failure (CHF) class II (with obvious symptoms);
- CHF classes III and IV;
- atrial fibrillation with a heart rate of more than 90–100 per minute.
Most often, Digoxin is included in the complex therapy of heart and vascular diseases. This applies to the treatment of class II CHF. The medicine has a positive effect in both acute and chronic forms of atrial fibrillation and flutter.
Dosage and instructions for use
Digoxin is prescribed only in the hospital for inpatient treatment, so that doctors have the opportunity to monitor the patient’s condition. The dose is selected individually for each patient, taking into account his muscle mass.
Types of therapy:
- rapid digitalization - the patient receives about 0.75–1.5 mg;
- gradual digitalization - the patient is prescribed 1-3 tablets per day (0.25-0.75 mg per day) for 5-7 days;
- therapeutic support - 0.5–1 tablet per day (0.125–0.25 mg of substance).
For older people, reduced doses are chosen. Patients should not change the dosage themselves.
For atrial fibrillation, a higher dose is prescribed than for CHF. It decreases slightly if the patient has serious kidney disease, hypothyroidism, low calcium levels, or if the muscles are underweight.
Pharmacological properties of the drug Digoxin
A mid-acting, highly lipophilic cardiac glycoside obtained from the leaves of foxglove woolly. It has a positive inotropic effect through the formation of a complex with adenosine triphosphatase and disruption of the transport of sodium and potassium ions through the membranes of cardiomyocytes. As a result, the transmembrane transport of calcium ions increases and their release inside cardiomyocytes increases and, as a result, the activity of myofibrils increases. Slows down AV conduction, lengthens the effective refractory period and reduces heart rate mainly by increasing the tone of the parasympathetic and decreasing the tone of the sympathetic part of the autonomic nervous system. Quickly and almost completely absorbed from the digestive tract when taken orally; The therapeutic concentration in the blood serum is achieved after 1 hour, the maximum concentration is achieved 1.5 hours after administration. The onset of action is after 5–30 minutes when administered intravenously and after 30 minutes–2 hours when taken orally. The maximum effect when taken orally is achieved after 2–6 hours, when administered intravenously - after 1–4 hours. Bioavailability, depending on the dosage form used, is 60–85%, but this figure varies widely depending on age and condition the patient, the nature of the food consumed. Concomitant use with food reduces the rate, but not the extent of absorption. Therapeutic concentration in the blood is 0.5–2 ng/ml. Plasma protein binding is low - 20–25%. The half-life averages 58 hours and depends on the age and condition of the patient (in young people - 36 hours, in elderly people - 68 hours, with anuria it increases to several days). Duration of action is about 6 days. Slightly biotransformed in the liver; 50–70% of digoxin is excreted unchanged in the urine. In some patients, digoxin is converted in the large intestine into inactive products under the influence of intestinal microflora. Digoxin passes into breast milk in quantities that do not have a negative effect on the baby (the ratio of the concentration of digoxin in breast milk and maternal blood plasma is 0.6–0.9%).
Side effects
When using Digoxin, some patients may experience side effects:
- headache;
- apathy;
- paroxysmal (acute) ventricular tachycardia;
- ventricular extrasystole;
- AV block;
- poor appetite;
- blood from the nose;
- SA blockade;
- digestive disorders;
- intestinal necrosis;
- skin rash;
- atrial flutter and fibrillation;
- visual disturbances - “floaters” before the eyes, objects appear yellow-green, too big or too small;
- AVNRT - AV nodal tachycardia;
- disturbances of consciousness, up to psychosis;
- drowsiness;
- ST segment depression;
- sensitivity disorders;
- radiculitis;
- neuritis;
- atrial tachycardia in combination with blockade.
To reduce side effects, your doctor may prescribe antidotes and additional medications.
Special instructions for the use of Digoxin
In patients with impaired renal function, elderly and debilitated patients, as well as in patients with an implanted pacemaker, careful dosage selection is necessary, since toxic effects may occur when using doses that are usually well tolerated by other patients. In patients with hypokalemia, hypomagnesemia, hypercalcemia, myxedema, cor pulmonale, digitalization should be carried out carefully and the use of digoxin in high single doses should be avoided. It is necessary to correct the electrolyte balance. Hypokalemia and hypomagnesemia increase the toxicity of digitalis glycosides. When using digoxin orally, you should limit your intake of hard-to-digest foods and foods containing pectins.
Contraindications
There are many contraindications for which Digoxin cannot be taken.
- congenital heart defects - stenosis, Wolff-Parkinson-White syndrome (WPW);
- ventricular fibrillation;
- poisoning with cardiac glycosides;
- II degree of AV heart block;
- ventricular tachycardia;
- tamponade;
- intermittent complete block of the bundle branches;
- allergy to medication components;
- transient complete AV block;
- lactation period;
- age under 3 years;
- obstructive hypertrophic cardiomyopathy (without CHF and atrial fibrillation);
- disturbances in the absorption of lactase, glucose and galactose.
Digoxin should be taken with great caution for a number of diseases and conditions:
- mitral valve stenosis;
- I degree of AV block;
- unstable forms of angina;
- sick sinus syndrome;
- acute stage of myocardial infarction;
- renal or liver failure;
- manifestations in the past of MAS syndrome (Morgagni-Adams-Stokes);
- obesity;
- risk of unstable impulse conduction through the AV node;
- oxygen starvation;
- cardiac asthma (especially in combination with mitral valve stenosis);
- pericarditis;
- arteriovenous fistula (shunt);
- hypothyroidism;
- amyloid cardiac dystrophy;
- any type of restrictive cardiomyopathy;
- dilatation of the heart chambers;
- inflammatory myocardial disease;
- excess alkaline substances in the body (alkalosis;
- extrasystole;
- electrolyte imbalance (increased calcium and sodium, decreased magnesium and potassium);
- elderly age of the patient;
- slow heart rate (bradycardia);
- severe or chronic lung diseases;
- carotid sinus syndrome;
- "pulmonary" heart.
The doctor carefully examines the patient’s health condition and prescribes the appropriate medicine and its exact dosage.
Digoxin overdose, symptoms and treatment
Overdose symptoms develop gradually over several hours. The most dangerous are cardiac arrhythmias (the possibility of death with the development of ventricular arrhythmias or heart block with asystole). Deaths have been described after taking digoxin at a dose of about 20 mg. In case of oral overdose, gastric lavage and administration of activated carbon, colestipol or cholestyramine are indicated. In case of hypokalemia in the absence of complete heart block, it is advisable to administer potassium salts. To correct arrhythmias caused by digoxin overdose, lidocaine, procainamide, propranolol and phenytoin are prescribed. In case of complete heart block, pacing is performed. In case of a life-threatening overdose of digoxin, intravenous administration of fragments of sheep antibodies that bind digoxin (Digoxin immune Fab, Digitalis-Antidote BM) through a membrane filter. 40 mg of antidote binds approximately 0.6 mg of digoxin. Dialysis and exchange blood transfusion for poisoning with digitalis glycosides are ineffective.
List of pharmacies where you can buy Digoxin:
- Moscow
- Saint Petersburg
Can I take it during pregnancy?
Pregnant women can take Digoxin only as prescribed by a doctor. This medicine does not harm the baby, but can penetrate the placenta into the fetus. A pregnant woman may need an increased dosage of the drug. Digoxin can lead to the birth of a low birth weight baby. Excess of substances contained in digitalis greatly harms the unborn child. During breastfeeding, you should also avoid taking Digoxin, because it enters the baby’s body through breast milk.
Overdose
An excessive dose of Digoxin is extremely dangerous and can lead to the death of the patient. Overdose happens quite often. If the level of digoxin in the blood plasma reaches 2 ng/ml, this concentration is toxic and life-threatening. The lethal dose is 10 mg.
Main symptoms:
- any heart rhythm disturbances;
- sweating;
- the ECG shows trough-shaped depression of the ST segment (a sign of the presence of digitalis in the body, and not just an overdose);
- hallucinations;
- dizziness and pain;
- distorted color perception;
- digestive disorders;
- drooling, poor appetite;
- weakness;
- sensitivity disorders;
- depressed mental state;
- disorientation;
- AV block;
- various cardiac conduction disorders;
- on the ECG the PQ interval lengthens.
In children, signs of gastrointestinal disorders are more common. Cardiac conduction disturbances and some types of arrhythmias are also observed.
The doctor immediately prescribes an antidote and other therapy that will eliminate the symptoms and improve the patient’s condition. An electrical pacemaker will be needed if the patient has Morgagni-Adams-Stokes attacks and complete transverse heart block. A pacemaker is installed temporarily until the serious condition disappears.
Digoxin
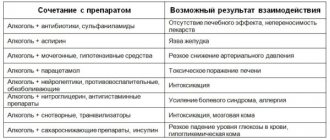
When digoxin is co-administered with drugs that cause electrolyte imbalance, in particular hypokalemia (for example, diuretics, glucocorticosteroids, insulin, beta-agonists, amphotericin B), the risk of arrhythmia and the development of other toxic effects of digoxin increases.
Hypercalcemia can also lead to the development of toxic effects of digoxin, so intravenous administration of calcium salts should be avoided in patients taking digoxin. In these cases, the dose of digoxin must be reduced. Some drugs may increase serum digoxin concentrations, such as quinidine, calcium channel blockers (especially verapamil), amiodarone, spironolactone, and triamterene. Absorption of digoxin in the intestine can be reduced by the action of cholestyramine, colestipol, aluminum-containing antacids, neomycin, and tetracyclines. There is evidence that the concomitant use of spironolactone not only changes the concentration of digoxin in the blood serum, but also may affect the results of the method for determining the concentration of digoxin, therefore special care is required when assessing the results obtained.
Reduced bioavailability: activated carbon, JIC astringents, kaolin, sulfasalazine (binding in the lumen of the gastrointestinal tract); metoclopramide, proserin (increased gastrointestinal motility). Increased bioavailability: broad-spectrum antibiotics that suppress intestinal microflora (reduce destruction in the gastrointestinal tract).
Beta-blockers and verapamil increase the severity of the negative chronotropic effect and reduce the strength of the inotropic effect.
Inducers of microsomal oxidation (barbiturates, phenylbutazone, phenytoin, rifampicin, antiepileptics, oral contraceptives) can stimulate digitoxin metabolism (if they are discontinued, digitalis intoxication is possible). When used simultaneously with digoxin, the following drugs may interact, as a result of which the therapeutic effect
or there is a side or toxic effect of digoxin: mineralocorticosteroids, glucocorticosteroids with significant mineralocorticosteroid effects; amphotericin B for injection; carbonic anhydrase inhibitors; adrenocorticotropic hormone (ACTH); diuretics that promote the release of water and potassium (bumetadine, ethacrynic acid, furosemide, indapamide, mannitol and thiazide derivatives); sodium phosphate.
Hypokalemia caused by these drugs increases the risk of toxic effects of digoxin, therefore, when used simultaneously with digoxin, constant monitoring of potassium concentration in the blood is required.
— St. John's wort preparations (their interaction with digoxin induces P-glycoprotein and cytochrome P450, i.e. it reduces bioavailability, increases metabolism and significantly reduces the concentration of digoxin in plasma).
— Amiodarone (increases the concentration of digoxin in the blood plasma to a toxic level). The interaction of amiodarone and digoxin inhibits the activity of the sinus and atrioventricular nodes of the heart and the conduction of nerve impulses through the conduction system of the heart. Therefore, after prescribing amiodarone, digoxin is canceled or its dose is reduced by half;
- Preparations of aluminum and magnesium salts and other antacids can reduce the absorption of digoxin and reduce its concentration in the blood;
- Simultaneous use with digoxin: antiarrhythmic drugs, calcium salts, pancuronium, rauwolfia alkaloids, succinylcholine and sympathomimetics can provoke the development of heart rhythm disturbances, therefore in these cases it is necessary to monitor the patient’s cardiac activity and ECG;
- Kaolin, pectin and other adsorbents, cholestyramine, colestipol, laxatives, neomycin and sulfosalazine reduce the absorption of digoxin and thereby reduce its therapeutic effect;
- Blockers of “slow” calcium channels, captopril - increase the concentration of digoxin in the blood plasma, therefore, when using them together, it is necessary to reduce the dose of digoxin so that the toxic effect of the drug does not manifest itself;
— Edrophonium (an anticholinesterase drug) — increases the tone of the parasympathetic nervous system, so its interaction with digoxin can cause severe bradycardia;
- Erythromycin - its action improves the absorption of digoxin in the intestine;
- Heparin - digoxin reduces the anticoagulant effect of heparin, so its dose has to be increased;
— Indomethacin reduces the release of digoxin, so the risk of toxic effects of the drug increases;
— Magnesium sulfate solution for injection is used to reduce the toxic effects of cardiac glycosides;
- Phenylbutazone - reduces the concentration of digoxin in the blood serum;
— Potassium salt preparations, they should not be taken if conduction disturbances on the ECG appear under the influence of digoxin. However, potassium salts are often prescribed along with digitalis preparations to prevent cardiac arrhythmias;
- Quinidine and quinine - these drugs can sharply increase the concentration of digoxin;
- Spironolactone - reduces the rate of release of digoxin, so it is necessary to adjust the dosage of the drug when used together;
— Thallium chloride (TL 201) — when studying myocardial perfusion with thalium drugs, digoxin reduces the degree of thalium accumulation in areas of damage to the heart muscle and distorts the study data;
- Thyroid hormones - when prescribed, metabolism increases, so the dose of digoxin must be increased.
Interaction with other drugs
Digoxin affects some processes in tissues and organs. To ensure that the drugs work well together, your doctor may prescribe a blood test that will determine the concentration of digoxin.
You cannot combine Digoxin with calcium injections, as well as with tanning agents, acids and alkalis, and salts of heavy metals.
When combined with medications that disrupt the electrolyte balance, Digoxin will exhibit its negative properties. Before prescribing this drug, the doctor administers drugs that normalize the potassium level in the blood.
The risk of poisoning from digitalis components increases with the use of beta-blockers, many antibiotics, NSAID painkillers, insulin, calcium channel blockers, diuretics, anxiolytics, some antifungal medications, and proton pump inhibitors.
Ventricular arrhythmias occur with the simultaneous use of sympathomimetics and digoxin, as well as due to its combination with propranolol, reserpine and phenytoin. The rhythm is greatly reduced when digoxin and ivabradine are combined.
The effectiveness of Digoxin is reduced in combination with metoclopramide, barbiturates, phenylbutazone, as well as with drugs that reduce the acidity of gastric juice.
In accordance with current recommendations, digoxin can be prescribed to patients with CHF with reduced LVEF and sinus rhythm, who, despite optimal drug therapy (ACE inhibitors, beta blockers, mineralocorticoid receptor antagonists), remain symptomatic. The goal of such therapy is to reduce the frequency of re-hospitalization due to decompensation of CHF. This recommendation is primarily based on the results of the RADIANCE and PROVED studies, which demonstrated an increase in the incidence of hospitalization in patients with CHF when digoxin was discontinued. An important feature of these studies was the fact that patients did not receive optimal drug therapy according to current guidelines.
The JACC journal published the results of an analysis of data from the OPTIMIZE-HF registry. The analysis included hospitalized patients with CHF with reduced LVEF receiving optimal drug therapy, as well as digoxin. The prognosis of patients in whom digoxin was discontinued was subsequently assessed compared with patients in whom therapy was continued.
A total of 3499 patients were included in the analysis (mean age 76 years, LVEF 28%). Digoxin was discontinued during hospitalization in 721 patients, in most cases due to deterioration of renal function. Almost every such patient x was matched with a control pair (the pairs were balanced according to 50 main characteristics); further comparisons were made in 698 such pairs. The follow-up period was 4 years.
After one month of follow-up, a significantly higher overall mortality rate was recorded in the digoxin withdrawal group (OR 1.80; 95% CI 1.26 - 2.57; p = 0.001). Subsequently, withdrawal of digoxin did not have a significant effect on mortality.
After 6, 12, and 48 months of follow-up, digoxin discontinuation was significantly associated with an increased likelihood of readmission, including due to decompensated CHF. After 4 years of follow-up, the OR for the risk of readmission due to decompensated CHF in the digoxin withdrawal group was 1.21 (95% CI 1.05 - 1.39; p = 0.007).
The authors consider activation of the sympathetic nervous system as a putative mechanism for increased mortality after digoxin discontinuation. This is indirectly confirmed by the fact that, according to subgroup analysis, the negative effect of digoxin withdrawal was observed only in those patients who did not receive beta blockers at discharge (35%). Stopping ACEIs had no effect on the negative effects of digoxin withdrawal.
Of course, this kind of analysis does not allow us to fully reliably assess the advisability of prescribing digoxin de novo. It is unlikely that such a randomized trial will be performed. However, the DIGIT-HF randomized placebo-controlled trial is currently being conducted to evaluate the prognostic effect of another glycoside, digitoxin, the excretion of which is independent of renal function.
Based on materials:
1) Digoxin Discontinuation and Outcomes in Patients With Heart Failure With Reduced Ejection Fraction. Awais Malik, Ravi Masson, Steven Singh, et al. Journal of the American College of Cardiology Aug 2021, 74 (5) 617-627; DOI: 10.1016/j.jacc.2019.05.064
https://www.onlinejacc.org/content/74/5/617
2) Bavendiek U, Berliner D, Dávila LA, et al. Rationale and design of the DIGIT-HF trial (DIGitoxin to Improve outcomes in patients with advanced chronic Heart Failure): a randomized, double-blind, placebo-controlled study. Eur J Heart Fail. 2019;21(5):676–684. doi:10.1002/ejhf.1452
Text: Ph.D. Shakhmatova O.O.