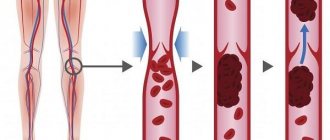
Deep vein thrombosis (DVT) - what is it?
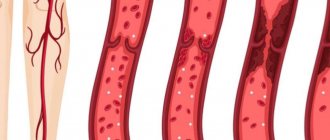
Deep vein thrombosis is a disease in which blood clots (thrombi) form in the lumen of the deep veins. The lower extremities are most often affected.
The mechanism of development of deep vein thrombosis
As the disease progresses, the health of the deep vessels is at risk. If treatment is not prescribed in time, there can be serious consequences.
Do people die from a blood clot: mortality statistics
Thrombus
The formation of a blood clot can occur in different parts of the body and it is very difficult to predict where exactly the blood clot is located. At a critical moment, it breaks away and begins to move, which is instantly fatal. People die from blood clots if treatment is not started in a timely manner and the person does not seek the help of doctors for a long time.
- Mortality statistics are such that more people die from thrombosis than from breast or prostate cancer.
- More than 10 million cases of thromboembolism occur worldwide each year.
Smoking, alcohol, and stress can develop this disease. If you want to be healthy, you should pay more attention to physical education, do morning exercises and worry less. It is also necessary to eat properly and drink clean water. This will guarantee your good health.
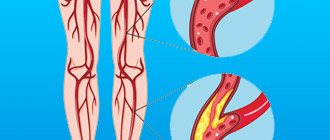
Deep vein thrombosis with varicose veins
Very often, deep vein thrombosis becomes a complication of varicose veins.
Deep vein thrombosis is often a complication of varicose veins
The severity of the disease will depend on the location of the blood clot and its size. If there is no complete blockage of the vessel, there may be no symptoms of the disease.
Deep vein thrombosis - causes of DVT development
Deep vein thrombosis most often occurs when several factors combine:
- in case of blood clotting disorders;
- when blood flow slows;
- in case of damage to the vascular walls.
There are risk factors that provoke the occurrence of thrombosis, these are:
- elderly age;
- smoking;
- overweight;
- the use of certain medications, including oral contraceptives;
- pregnancy and childbirth;
- sedentary lifestyle;
- some operations;
- injuries that damage blood vessels.
Mechanical sense of platelet
N. L. Reznik “Chemistry and Life” No. 12, 2014
Activated platelet does not look like a platelet
Platelet on the embrasure
One milliliter of human blood contains from 150 to 300 thousand platelets, sometimes called platelets - they are flat and nuclear-free. The length of the platelet in its longest part is 1–4 µm, thickness 0.5–0.75 µm. This volume contains mitochondria, glycogen, granules with biologically active substances and calcium ions, enzymes necessary for the life of the cell itself, as well as actin and myosin molecules that make up the cytoskeleton. Platelets are formed in the bone marrow, circulate in the blood for 5–11 days and find their end in the liver, lungs or spleen.
What are platelets for? Mainly to form a blood clot (Fig. 1). Thrombosis for them consists of three stages: adhesion, activation and aggregation.
Rice.
1. The main stages of thrombus formation.
At the top
- platelet adhesion and aggregation.
Below -
the coagulation cascade and the interaction of blood cells with fibrin fibers
The formation of a blood clot in a healthy body begins with damage to the vessel. In this case, the inner membrane, the endothelium, is destroyed, and the platelets come into contact with the exposed collagen layer of the vascular wall: the platelet seems to roll along it. And on its surface there are receptors that interact with collagen and the protein that coats collagen, von Willebrand factor (VWF). Interaction with VWF allows platelets to stay on collagen, otherwise they would be washed away by the blood flow. Once a cell has attached to the collagen, it literally spreads out onto it and becomes activated. At the same time, its shape changes, and it no longer resembles a plate (see the picture at the beginning of the article). An activated platelet secretes various platelet factors - serotonin, ADP and many others - which narrow the lumen of the vessel, attract further platelets to the damaged area, stimulate their activation and aggregation. As a result, each activated platelet becomes associated with the surface of the vessel and/or with other platelets. Together they plug the narrowed gap.
At the same time, a cascade of coagulation reactions occurs at the site of blood vessel damage and platelet aggregation. It is also triggered by exposed connective tissue and tissue factor, which is released by damaged endothelial cells. This cascade involves proteins (coagulation factors) present in the blood plasma in an inactive form. When activated, each factor serves to activate the next. Platelets are also involved in this process; molecules on their surface are necessary for many coagulation reactions. As a result of a series of reactions, the protease thrombin is formed from the inactive protein prothrombin and converts the soluble protein fibrinogen into fibrin. Fibrin formation is the goal of the coagulation cascade. Fibrin monomers polymerize, forming insoluble filaments that fold into a network of complex structures. The fibrin network holds platelets and strengthens the clot. Red blood cells also become entangled in it, which is why the blood clot is red.
But we got ahead of ourselves a little. Let's go back to the moment when platelets just started aggregating. To make the connection between them strong, two proteins are needed: integrin and the already familiar fibrinogen (Fig. 2).
Rice. 2.
Platelet aggregation. They attach to the collagen of the vascular wall, clinging to von Willebrand factor, and fibrinogen molecules connect cells to each other
Integrins are located on the surface of the platelet and ensure the interaction of cells with the environment, that is, the transmission of external signals into the cell and information from its depths to the outside. In fact, integrins are receptors that bind to various signaling molecules. The most abundant integrin in platelets, αIIbβ3, mediates their association with fibrinogen. By combining with integrin, fibrinogen monomers crosslink platelets into a dense clot that can only be destroyed by the enzyme plasmin. In addition, fibrinogen, interacting with integrin, acts as an activation signal.
The fibrinogen adhesive provides not only the strength of the blood clot, but also its compressibility. A few hours after the clot forms, the platelet's actin and myosin cables contract and the cell shrinks. And since platelets are connected to each other, as well as to extracellular fibrin, the entire thrombus is compressed and tightens the edges of the wound. Then the damaged area is restored, and the blood clot dissolves.
The platelet shrinks to the touch
The coagulation cascade and thrombus formation are described here in general terms. Anyone who wants to know the details will find them in the textbook; the biochemical signals that control the behavior of platelets are well studied. However, mechanical forces also act on platelets: blood flow, contacts with polymerized fibrin and thrombus neighbors. And since the structure and mechanical properties of a blood clot are very heterogeneous, the ability to sense them would be useful for blood platelets.
The hypothesis of platelet mechanosensitivity was tested by specialists from Emory University and the Georgia Institute of Technology, Atlanta, USA, under the leadership of Wilbur Lamb, MD ( Proceedings of the National Academy of Sciences
, 2014, 111, 14430–14435; DOI:10.1073/pnas.1322917111). The researchers prepared polyacrylamide gels of varying stiffness (from 0.25 to 100 kPa), and placed fibrinogen on top of them in the same concentration. Thus, the density of molecules that bind and activate platelets remained constant in all cases, only the rigidity of the biochemically inert polyacrylamide substrate changed.
Pieces of the gel were incubated for two hours in a suspension of platelets obtained from healthy people, and then the number of cells attached to the substrate was determined (Fig. 3). The number of platelets anchored on the surface of the gel increased with increasing its rigidity and at 50 kPa reached a maximum of 10 thousand cells per square millimeter. With a further increase in rigidity, the number of attached cells did not change. The dependence of adhesion on gel stiffness remains the same in liquid flow.
Rice. 3.
An experiment proving the mechanosensitivity of platelets. They sit more actively on the hard gel and spread out more strongly on it
As we remember, having attached to the surface, the platelet presses against it. The average area of the spread cell also increases with increasing gel rigidity and is maximum at 50 kPa - about 23 square micrometers.
Apparently, platelets react to the hardness of the surface they come into contact with. But it remained unclear how they did it. Typically, the mechanical sense of cells is provided by various elements associated with the nucleus. However, platelets do not have a nucleus and the cytoskeleton is much simpler than that of other cells, therefore they cannot sense anything.
It is logical to assume that if platelets have a mechanical sense, it is provided by the same factors that take part in adhesion and activation. The scientists used inhibitors that selectively blocked the action of these factors and observed whether they affected the dependence of platelet adhesion and cell spreading on gel stiffness. After complex experiments, researchers proposed a mechanism for platelet mechanosensitivity (Figure 4).
Rice. 4.
Only a rigid substrate sufficiently stimulates integrin, causing platelet adhesion, activation and aggregation
It is provided by integrin αIIbβ3, which interacts with fibrinogen. When a platelet comes into contact with the substrate, it touches it with integrin, which can be likened to the landing gear of an airplane. When landing on a soft substrate, the cell experiences little resistance, and the integrin enters a low-affinity state, that is, it performs receptor functions rather sluggishly and causes relatively low platelet activation and adhesion. Hitting a harder substrate turns on the integrin at full capacity. The protein transmits a signal received from outside into the cell, where it is received by the Rac1 factor. It has many functions: it controls the secretion of platelet factors and stimulates the Rap1b protein, which also activates integrin, but not from the outside, but from the inside. Rac1 also regulates the formation of platelet actinomyosin cords, and when they contract, they ensure the cell spreads out on the substrate. Spreading, by the way, is an important stage of activation: only in this state do platelets expose phosphatidylserine, which is involved in the coagulation cascade, on the surface of the membrane. In addition, it is the reduction of actomyosin that ensures a change in the shape of activated platelets.
So, the “integrin - Rac1 - Rap1b” ligament forms a mechanosensitive device, which turns on during a hard landing, causing further activation of platelets, their irreversible aggregation and the formation of blood clots.
One should not, however, go to the extreme of believing that platelet adhesion and activation is influenced solely by the rigidity of the substrate. The concentration of fibrinogen on it also matters. Researchers have shown that platelets adhere better to surfaces with lower concentrations of fibrinogen. They explain this by saying that fibrinogen molecules, freely distributed throughout the glass, expose more binding sites to the integrin. And if there is too much protein on the same glass, its molecules are oriented vertically and open only part of the binding sites. Consequently, platelet aggregation is most effective when there are more integrin binding sites on the substrate and the substrate itself is stiffer.
Studies of the mechanosensitivity of platelets are also interesting because they allow us to predict the rate of their adhesion on surfaces of different rigidities. The fact is that fibrinogen settles not only on the collagen of the damaged vascular wall, but also on the atherosclerotic plaque, as well as on artificial surfaces, including medical equipment: artificial vessels, implants and catheters. And αIIbβ3 integrin binds to fibrinogen even when the platelet is not activated. Therefore, blood clots form on collapsing plaques and biomaterials, creating a lot of problems for patients and doctors. By slightly changing the rigidity of the material, it is theoretically possible to weaken the intensity of platelet adhesion and aggregation. But this is a matter for the future.
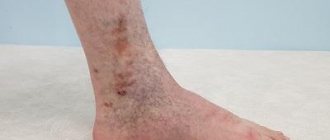
What symptoms develop with deep vein thrombosis?
As a rule, symptoms do not appear immediately, only if the blood clot enlarges. If a clot ruptures, shortness of breath, chest pain, and hemoptysis may occur.
The development of the disease can be recognized by the following symptoms:
- swelling of the legs;
- bluish skin tone;
- pain when moving.
The main symptom of deep vein thrombosis is leg pain!
If you have these signs, you most likely have deep vein thrombosis. Stages or variants of the course determine the method of treatment.
How is pulmonary embolism diagnosed?
As a result of pulmonary embolism, cardiac function, pulmonary blood flow and gas exchange are disrupted. This condition is accompanied by characteristic symptoms, primarily a rapid heartbeat. There may also be a stabbing pain in the chest that gets worse while breathing. Patients may experience severe shortness of breath, and the respiratory rate may increase to 30-40 per minute. In addition, cyanosis or pallor of the skin, decreased blood pressure, cough, and hemoptysis may be observed.
“In the case of pulmonary embolism, difficulty breathing and chest pain may occur. In people with a disease of the vascular-cardiac system, for example, atrial fibrillation, pulmonary embolism can occur quite often,” says Shpachenko.
If thromboembolism is suspected, urgent hospitalization is required. To confirm the diagnosis, the hospital will order electrocardiography, chest x-ray and echocardiography. A radionuclide scan of the lungs, probing of the right side of the heart, and computed tomography with contrast of the pulmonary arteries can also be performed.
Deep vein thrombosis - diagnosis
The main method for diagnosing deep vein thrombosis today is ultrasound duplex scanning. With ultrasound scanning, you can determine the location of the thrombus, its size, and condition (is it attached to the walls of the vein or dangling in the lumen - floating).
Doctor Malakhov A.M. performs ultrasound diagnostics of deep veins of the lower extremities
Phlebography and radionuclide scanning are also prescribed to assess venous blood flow. The state of microcirculation is assessed based on rheovasography data.
Surgical methods for treating deep vein thrombosis
If a patient has a severe form of thrombosis of the lower extremities, the most effective treatment method is surgery - thrombolysis. A timely operation makes it possible to restore full blood flow if the diagnosis is deep vein thrombosis. Only timely intervention can completely cure the patient from this serious condition. Thrombolysis is performed only in hospital settings and by very experienced endovascular surgeons. Treatment after surgery is also aimed at the same goal - resorption of blood clots.
In addition to thrombolysis, there are two more surgical methods for treating deep vein thrombosis - thrombectomy with angioplasty and installation of a blood clot trap - a vena cava filter.
Surgical methods for treating deep vein thrombosis
Deep vein thrombosis - treatment in Moscow
Modern Moscow medicine offers several methods of treating deep vein thrombosis, the use of which depends on the severity of the disease. In the early stages, you can get by with thrombolytic drugs if you have deep vein thrombosis. Treatment (Moscow is a city where there are world luminaries in phlebology) must be very qualified. In the later stages, such therapy is dangerous due to the possible separation of the blood clot and the occurrence of thromboembolism of the pulmonary artery. If severe circulatory disorders and deep vein thrombosis are observed, treatment is surgery (thrombectomy).
Deep vein thrombosis - conservative treatment
Conservative treatment can only stop or slow down the progression of the disease. Such therapy can also be prescribed as part of complex treatment.
Principles of conservative therapy:
- compression therapy (elastic compression) – the result of such an effect is the elimination of the mechanisms of progression of varicose veins; without such therapy, conservative treatment is impossible;
- the required level of compression is achieved through the use of special knitwear (special medical product), in this case it is important to choose the correct size of the compression knitwear;
- compression hosiery can relieve swelling, pain and increased fatigue of the lower extremities;
- the desired result is achieved with constant use of elastic compression.
Deep vein thrombosis treatment with medication
This involves a course of treatment with anticoagulants (drugs that prevent blood from clotting). The average duration of such a course is at least 3 months, and sometimes longer. A combination of drugs differing in their mechanism of action is provided. An important stage in the drug treatment of DVT is the selection of blood thinning drugs. To prevent gastrointestinal complications, some medications are administered parenterally.
Pharmacotherapy is often carried out on an outpatient basis. In severe forms of the disease, patients who have suffered thromboembolism of the pulmonary artery or thrombosis of the vena cava are annually hospitalized in the therapeutic or cardiology department for 2-3 weeks, where infusion hemorheological and cardiotonic therapy is carried out.
Summarizing
In recent years, the complexity of the coagulation system has gradually become less mysterious. The discovery of all essential components of the system, the development of mathematical models and the use of new experimental approaches made it possible to lift the veil of secrecy. The structure of the coagulation cascade is being deciphered, and now, as we saw above, for almost every significant part of the system, the role it plays in the regulation of the entire process has been identified or proposed.
Figure 7 shows the most recent attempt to reconsider the structure of the coagulation system. This is the same diagram as in Fig. 1, where parts of the system responsible for different tasks are highlighted with multi-colored shading, as discussed above. Not everything in this scheme is securely established. For example, our theoretical prediction that activation of factor VII by factor Xa allows clotting to respond in a threshold manner to flow rate remains as yet untested experimentally.
Figure 7. Modular structure of the coagulation system: the role of individual coagulation reactions in the functioning of the system.
[1]
It is quite possible that this picture is not yet completely complete. However, progress in this field in recent years gives hope that in the foreseeable future, the remaining unsolved regions of the coagulation circuitry will gain meaningful physiological function. And then it will be possible to talk about the birth of a new concept of blood coagulation, which replaced the old cascade model, which faithfully served medicine for many decades.
The article was written with the participation of A.N. Balandina and F.I. Ataullakhanova and was originally published in Priroda [10].
Deep vein thrombosis - treatment at home
Today, along with traditional methods of treating the disease, traditional medicine is practiced if deep vein thrombosis is determined. Treatment with folk remedies is used as a complement to the main treatment.
The first thing to do is thin the blood. If you have deep vein thrombosis, treatment with traditional methods includes consuming the following products:
- onion and garlic;
- sunflower seeds;
- cocoa;
- beets;
- Apple vinegar;
- tomatoes or tomato juice;
- Hercules;
- oatmeal;
- cranberries;
- oatmeal;
- lemon;
- cherries;
- viburnum.
Blood thinning should be approached with caution so as not to provoke bleeding. It is not recommended to consume fatty and meat products if you have deep vein thrombosis. Photos and results of improper treatment are available on the Internet.
Every day you can eat one spoon of a mixture made from crushed garlic, two tablespoons of unrefined vegetable oil and one tablespoon of honey.
Deep vein thrombosis - reviews from our patients.
Feedback from our patient about the treatment of deep vein thrombosis in
Anita, 38 years old, Moscow.
I would like to thank the clinic staff for their professionalism. With their help, I began to trust traditional medicine again. Before I went to the clinic, I was repeatedly subjected to various medical procedures for deep vein thrombosis in my legs. At first I had varicose veins with a complication, for which I had an operation to “stitch the veins.” As a result of this, I practically became disabled. On the advice of my friends, I turned to the doctors of the MIFC clinic, who returned me to a full life. It’s good that everything worked out without surgical intervention. Anita, 38 years old, Moscow.
Patient's review of the diagnosis of deep vein thrombosis in our center
Andrey, 40 years old, Krasnogorsk.
Due to frequent stressful situations and bad habits, I developed problems with my legs, or rather, poor circulation. My legs often swelled, turned blue, and sometimes hurt when walking. I accidentally saw an article on the Internet about vein thrombosis, and the symptoms described coincided with my feelings. I was just recently recommended to a phlebology clinic, and I decided to go for a consultation. Doctor Malakhov A.M. diagnosed: acute deep vein thrombosis. At first they calmed me down and told me that in this case it would be impossible to do without surgical intervention. Since there was no other choice, I agreed and did not regret it. The operation in the vascular department of the city hospital, where I was urgently hospitalized, to remove the blood clot was successful and without complications. Now my life is not in danger, thanks to the doctors of the MIFC clinic for their professionalism and “humane” attitude towards patients! Andrey, 40 years old, Moscow.
Is it possible to prevent a blood clot: prevention
Sport is the best prevention of thrombosis.
Every year in Russia alone, hundreds of thousands of people die from stroke. The most common type of stroke is ischemic, caused by a blockage of a blood vessel or a blood clot. It occurs 4 times more often than strokes due to vascular rupture. Is it possible to prevent a blood clot?
- Prevention of thrombosis involves thinning the blood. We need to accelerate it so that there is no stagnation.
- Any physical exercise will help you do this . You should exercise at least 30 minutes a day. It is important to walk a lot in the fresh air.
- People who are inactive most often suffer from stroke , so prevention in the form of daily physical activity is mandatory.
As mentioned above, in order to avoid the appearance of blood clots, you need to follow a drinking regime. You should drink at least two liters of water daily. To be completely sure, you can take medications that improve blood circulation. But this should be done only after consulting a doctor.
Remember : Self-medication is dangerous!
Frequently asked questions from our patients on the Internet about deep vein thrombosis
How to understand that there are blood clots in the veins?
Only a specialist, phlebologist or vascular surgeon can reliably understand that there are blood clots in the veins. And even a specialized specialist will need instrumental support and ultrasound examination of blood vessels. You can assume that you have blood clots in your veins based on the following signs:
- Edema.
- Blueness of the skin.
- Soreness, swelling of tissues, redness of the skin along the veins.
If there are blood clots in the veins, how to recognize them, symptoms and treatment?
Blood clots in veins can be recognized using duplex ultrasound scanning. The presence of blood clots in the veins is indicated by the following symptoms: swelling, pain, discoloration of the limb. The best option for diagnosis, as well as subsequent treatment, is to contact a good phlebological center.
How to recognize a blood clot on the leg?
In order to recognize a blood clot on the leg, you must seek professional medical help. As an option, do an ultrasound examination of the vessels of the lower limb. The best solution would be to consult a specialist, a phlebologist.
How to identify blood clots in the legs?
From the point of view of modern diagnostics, the best way to determine blood clots in the legs is ultrasound examination of the vessels of the lower extremities.
A blood clot in a vein, how does it form?
A blood clot in a vein is formed as a result of a complex chain of biochemical reactions, during which a network of insoluble fibrin molecules is formed from fibrinogen molecules. Blood cells are fixed in the latter, creating a dense intravascular structure, which is a thrombus.
How to identify a blood clot?
A thrombus can be detected using various methods, such as computed tomography, magnetic resonance imaging, and good ultrasound. The latter method has the best price-quality ratio and is the gold standard for diagnosing thrombosis.
Regulation of the coagulation system
Figure 6. Contribution of extrinsic and intrinsic tenase to fibrin clot formation in space. We used a mathematical model to investigate how far the influence of a clotting activator (tissue factor) could extend in space. To do this, we calculated the distribution of factor Xa (which determines the distribution of thrombin, which determines the distribution of fibrin). The animation shows the distributions of factor Xa produced by extrinsic tenase (VIIa–TF complex) or intrinsic tenase (IXa–VIIIa complex), as well as the total amount of factor Xa (shaded area). (The inset shows the same thing on a larger concentration scale.) It can be seen that activator-produced factor Xa cannot travel far from the activator due to the high rate of inhibition in the plasma. On the contrary, the IXa–VIIIa complex works far from the activator (since factor IXa is inhibited more slowly and therefore has a greater effective diffusion distance from the activator), and ensures the distribution of factor Xa in space.
[9]
Let's take the next logical step and try to answer the question - how does the system described above work?
Cascade device of the coagulation system
Let's start with the cascade - a chain of enzymes that activate each other. A single enzyme operating at a constant speed produces a linear dependence of product concentration on time. For a cascade of N enzymes, this dependence will have the form tN, where t is time. For the effective operation of the system, it is important that the response is of precisely this “explosive” nature, since this minimizes the period when the fibrin clot is still fragile.
Triggering of coagulation and the role of positive feedbacks
As mentioned in the first part of the article, many clotting reactions are slow. Thus, factors IXa and Xa themselves are very poor enzymes and require cofactors (factors VIIIa and Va, respectively) to function effectively. These cofactors are activated by thrombin, a device where the enzyme activates its own production is called a positive feedback loop.
As we have shown experimentally and theoretically, the positive feedback of factor V activation by thrombin forms the activation threshold - the property of the system not to respond to small activation, but to quickly respond when a large one appears. This ability to switch seems to be very valuable for folding: it helps prevent “false positives” of the system.
The role of the intrinsic pathway in the spatial dynamics of folding
One of the intriguing mysteries that haunted biochemists for many years after the discovery of the essential coagulation proteins was the role of factor XII in hemostasis. Its deficiency was detected in simple clotting tests, increasing the time required for clot formation, but, unlike factor XI deficiency, was not accompanied by coagulation disorders.
One of the most plausible options for unraveling the role of the internal pathway was proposed by us using spatially inhomogeneous experimental systems. Positive feedbacks have been found to be important specifically for the propagation of coagulation. Effective activation of factor X by external tenase on the activator will not help form a clot away from the activator, since factor Xa is rapidly inhibited in the plasma and cannot move far from the activator. But factor IXa, which is inhibited an order of magnitude slower, is quite capable of this (and is helped by factor VIIIa, which is activated by thrombin). And where it is difficult for him to reach, factor XI, also activated by thrombin, begins to work. Thus, the presence of positive feedback loops helps create the three-dimensional structure of the clot.
Protein C pathway as a possible localization mechanism for thrombus formation
Activation of protein C by thrombin itself is slow, but accelerates sharply when thrombin binds to the transmembrane protein thrombomodulin, synthesized by endothelial cells. Activated protein C is capable of destroying factors Va and VIIIa, slowing down the coagulation system by orders of magnitude. The key to understanding the role of this reaction was spatially inhomogeneous experimental approaches. Our experiments suggested that it stops the spatial growth of the thrombus, limiting its size.