Mildronate is a metabolic agent based on meldonium, which has angioprotective, cardioprotective, antianginal and antihypoxic effects. The drug helps activate the compensatory capabilities of the cardiovascular system, significantly increasing its functional readiness. Available in capsules and tablets of 250 mg, 500 mg, as well as in the form of a solution for parenteral (intramuscular, intravenous and parabulbar) administration of 100 mg/ml.
pharmachologic effect
Pharmacodynamics
Meldonium in its chemical structure is an analogue of gamma-butyrobetaine, which acts as a precursor for the synthesis of carnitine in the body. It inhibits the action of the enzyme gamma-butyrobetaine hydroxylase, which reduces the synthesis of carnitine molecules and eliminates the active transport of fatty acids into the cell. Thus, it prevents the accumulation of active forms of under-oxidized forms of fatty acids, which are one of the main agents that can damage cell membranes. This is due to the cytoprotective effect of the drug Mildronate.
In conditions of oxygen starvation of cells that develop during physical activity, meldonium maintains the exchange of oxygen in the cell in balance, balancing its delivery and consumption in the cells. It also prevents disruption of the transport of the main energy molecule ATP to target organs and activates anaerobic glycolysis.
Thus, by influencing the basic components of the metabolism of oxygen, fatty acids and ATP, Mildronate provides vasodilation, angioprotection, and a cardioprotective effect. In conditions of ischemic infarction, the drug significantly slows down the spread of the necrosis zone, and during physical activity it maintains the delivery of a sufficient amount of oxygen, increasing exercise tolerance. Tones the central nervous system, prevents mental exhaustion, and when applied topically, eliminates dystrophic changes in blood vessels.
Pharmacokinetics
When administered orally, Mildronate is effectively absorbed from the gastrointestinal tract. Capsules allow the drug to bypass the aggressive environment of the stomach and enter the intestines, where absorption is fastest. The bioavailability of the drug reaches 78%, and the maximum concentration in plasma is observed 1.5–2 hours after use.
From the systemic bloodstream, meldonium enters the tissues. It has been established that meldonium metabolites penetrate the blood-placental barrier and are also found in breast milk. The protein binding coefficient is 61.7%. Most of the metabolism of the substance occurs in the liver, and excretion is carried out by the kidneys. The half-life of the drug is 4 hours.
Composition of Mildronate and release forms
The composition may vary depending on the form of release.
- Mildronate ampoules contain meldonium and water for injection.
One ampoule contains 5 ml of the drug, which contains 250 mg of the active substance. Available in cardboard packaging of 10 ampoules.
- Mildronate capsules:
- meldonium;
- potato starch;
- calcium stearate;
- colloidal silicon dioxide;
- titanium dioxide and gelatin in the shell.
Capsules come in two types: with 250 mg and 500 mg of active substance. Sold in cardboard packs of 40 and 60 pieces.
- Mildronate in syrup:
- meldonium;
- purified water;
- cherry essence;
- glycerol;
- dyes;
- propylene glycol.
Two packaging: 150 ml and 250 ml. 5 ml (one dose) of the medicine contains 250 mg of the active substance. Available in cardboard packaging with a 5 ml measuring spoon.
Mildronate enters the blood most quickly in ampoules, immediately after administration of the drug. The syrup also has a good absorption rate. But capsules are more convenient to take.
Indications for use of Mildronate
Mildronate is used to maintain cardiac muscle tone in chronic heart failure, cardiomyopathies of any origin, in the treatment of coronary heart disease, heart attack, and angina attacks. It is also used for the treatment of cerebral blood flow disorders, strokes and transient ischemic attacks. In ophthalmological practice it is used for hemophthalmia, dystrophic changes in the blood vessels of the eye, and retinopathy of vascular origin. Reduced performance, chronic fatigue, physical activity in athletes, depletion of the central nervous system due to mental stress are relative indications for the use of Mildronate. Also used to reduce withdrawal symptoms in alcoholism and hangovers.
For the treatment of cardiomyopathy, 500 mg/day orally is prescribed for a course of 12 days.
Chronic heart failure, coronary heart disease, angina attacks and heart attacks, which are accompanied by contractility disorders and bradycardia, suggest the appointment of Mildronate for oral use at 500 mg/day, the dose is divided into two doses. The course of treatment can last 4–6 weeks.
For cerebral circulation disorders, a dose of 500 mg/day is indicated for 4–6 weeks.
To relieve withdrawal symptoms and in case of severe hangover, 4 oral doses of Mildronate are prescribed at a dose of 500 mg/dose. The course of treatment can last up to 7 to 10 days. A loading dose quickly eliminates the symptoms of alcohol intoxication.
In case of a pronounced decrease in performance, physical overstrain and exhaustion of the central nervous system, 500 mg of the drug is prescribed 2 times a day for 1–2 weeks.
To increase energy supply before training and for weight loss, athletes are prescribed 500 mg 1-2 times a day for 14-21 days. However, you should be careful, because meldonium can give a positive result in a doping test.
Injection forms of the drug can be used for intravenous, intramuscular, and parabulbar administration. The injection should be given in the first half of the day, given the stimulating effect of Mildronate.
In case of myocardial infarction, intravenous bolus administration of 0.5–1 g of the substance per day (5–10 ml of the drug, respectively) is recommended. For coronary heart disease, angina attacks, cardiomyopathy and chronic heart failure, 0.5–1 g is prescribed intravenously or 2 injections of 0.5 g intramuscularly. The course of treatment lasts up to 14 days, after which the patient should be transferred to oral forms, the course of which will last 4–6 months.
For hemophthalmia, dystrophic changes in the vessels of the eye, retinopathy of vascular origin, 0.05 g of the substance (which corresponds to 0.5 ml of Mildronate) is administered parabulbarly for a course of 10 days.
Repeated courses for the prevention of the underlying disease can be carried out for any form of the drug 2-3 times a year, but only based on medical indications according to the recommendations of the attending physician. It is not recommended to prescribe the drug independently for heart rhythm disturbances, surges in blood pressure, for the purpose of stimulation or achieving an immunomodulatory effect (for colds, acute respiratory viral infections).
How Mildronate works
The active substance meldonium, acting in several directions at once, establishes a balance between the cells’ need for oxygen and its delivery, removes accumulated toxins and neutralizes their negative impact on the body. Thanks to this, a person taking Mildronate becomes more resilient to mental and physical stress and gets an improved metabolism.
Other results of using Mildronate:
- normalization of cerebral circulation;
- improvement of blood circulation in the area of ischemia;
- slowing down the formation of the necrotic zone;
- elimination of functional disorders of the nervous system;
- reduction of the rehabilitation period in the treatment of heart disease, stroke and alcoholism
Mildronate is easily absorbed and excreted from the body after 3-6 hours. Although traces of it can still be found for several months.
Contraindications for use
- age under 18 years (based on the lack of data from isolated clinical studies on the tolerability of Mildronate in patients under the age of 18);
- hypersensitivity to meldonium or other components that are part of the drug, episodes of anaphylactic reactions or other allergic reactions (itching, swelling, hyperthermia) in the anamnesis;
- increased intracranial pressure caused by impaired outflow of cerebrospinal fluid, impaired venous outflow (in the presence of an obstructed vessel or intracranial tumor);
- end-stage liver and kidney failure: the drug will not be able to undergo transformation in these organs, so conversion and elimination will be impaired and toxicity will increase;
- pregnancy;
- period of lactation and breastfeeding.
Mildronate price
Price of the drug in Russia
You can buy 250 mg capsules in Russian pharmacies for an average of 300 rubles, 500 mg capsules for 650 rubles. The price of Mildronate for intravenous administration is 350 rubles. Price Mildronate Gx 500 mg - about 800 rubles.
Price of the drug on the Ukrainian market
The average price of Mildronate 250 mg tablets is 250 UAH. The price of Mildronate ampoules of 5 ml is 450 UAH. 500 mg capsules cost about 400 UAH per package. Mildronate GX is sold for an average of 450 UAH.
Moreover, Mildronate injections, tablets and capsules in pharmacies in Kharkov or Odessa are somewhat cheaper than in most pharmacies in the capital.
- Online pharmacies in RussiaRussia
- Online pharmacies in UkraineUkraine
- Online pharmacies in KazakhstanKazakhstan
ZdravCity
- Mildronate caps.
500 mg 60 pcs. JSC Grindeks 558 rub. order - Mildronate caps. 250 mg 40 pcs. JSC Grindeks
RUB 254 order
- Mildronate solution IV, IM and parabulb. input 100 mg/ml amp. 5ml No. 10HB Pharma s.r.o.
RUR 348 order
- Mildronate sol. IV and parabulb. input 100 mg/ml amp. 5 ml 10 pcs. HB Pharma s.r.o.
RUB 356 order
- Mildronate solution for IM, IV and parabulb. input 0.1g/ml 5ml 20pcsHB Em Pharma s.r.o.
675 rub. order
Pharmacy Dialogue
- Mildronate 10% ampoules 5ml No. 10Grindex/HM Pharma
RUB 337 order
- Mildronate capsules 500 mg No. 60Grindex
RUR 527 order
- Mildronate capsules 250 mg No. 40Grindex
RUB 232 order
- Mildronate (amp. 10% 5ml No. 10) Sanitas
RUB 337 order
- Mildronate 10% ampoules 5ml No. 10Santonica JSC
340 rub. order
show more
Pharmacy24
- Mildronate 0.5 g/5 ml 5 ml No. 10 solution AT "Grindeks", Latvia
830 UAH. order - Mildronate 500 mg N60 capsules AT "Grindeks", Latvia
409 UAH order
- Mildronate GX 500 mg N60 tablets AT Grindeks, Latvia
442 UAH order
- Mildronate 5 ml N10 solution AT "Grindeks", Latvia/HBM Pharma s.r.o., Slovakina
461 UAH order
PaniPharmacy
- Mildronate capsule Mildronate caps. 0.25g No. 40 Latvia, Grindeks
251 UAH order
- Mildronate GX tablets Mildronate GX tablets 0.5g No. 60 Latvia, Grindeks
487 UAH. order
- Mildronate ampoule Mildronate solution d/in. 10% amp. 5ml No. 10 Lithuania, Sanitas
450 UAH. order
- Mildronate solution d/in.amp.0.5g/5ml 5ml N20
901 UAH. order
- Mildronate capsule Mildronate caps. 0.5g No. 60 Latvia, Grindeks
412 UAH. order
show more
Side effects
Side effects of the drug are classified by frequency of occurrence based on reports of adverse reactions and reviews received in the post-marketing period as follows: very common (1 case in 10), common (1 case in 100); uncommon (1 in 1000), rare (1 in 10,000), very rare (less than 1 in 10,000), unknown (no exact data on the frequency of occurrence). Mildronate is characterized by:
- From the immune system: rarely - allergic reactions, allergic dermatitis, urticaria, angioedema, anaphylactic reactions, shock.
- From the nervous system: often - headache; rarely - changes in sensitivity, paresthesia, hypoesthesia, tremor, tinnitus, dizziness, fainting.
- From the mental side: rarely - psychomotor agitation, unreasonable fear, panic attacks, sleep disturbances.
- From the respiratory system: often - infectious diseases of the respiratory tract, decreased local immunity of the respiratory system; rarely - cryptogenic cough, dyspnea, apnea.
- From the cardiovascular system: rarely - hypertension, hypotension, hypertensive crisis, hyperemia or pallor of the skin, changes in the contractility of the heart, arrhythmia, palpitations, sinus tachycardia, atrial fibrillation, a feeling of discomfort or heaviness in the chest and in the area of the projection of the heart behind sternum, chest pain.
- From the gastrointestinal tract: often - dyspeptic manifestations; rarely - loss of appetite, nausea, vomiting, flatulence, diarrhea, abdominal pain, dry mouth, hypersalivation.
- From the skin and subcutaneous tissue: rarely - rash, itching, dermatitis, papules.
- From the musculoskeletal system: rarely - myalgia, muscle weakness.
- From the kidneys: rarely - pollakiuria.
- General reactions: rarely - swelling, sensations of heat or cold, weakness, asthenic manifestations.
- Changes in laboratory parameters: often - increased levels of C-reactive peptide, dyslipidemia; rarely - eosinophilia, increased wave voltage on the ECG.
Effect of the drug
Meldonium improves metabolic processes in the heart muscle and provides energy to the myocardium, as well as:
- improves blood circulation in the brain;
- normalizes the inflow and outflow of blood in areas with insufficient blood supply;
- slows down the formation of a zone of tissue death;
- brings myocardial performance indicators back to normal;
- provides brain and heart cells with oxygen;
- removes toxins;
- normalizes the functioning of the nervous system;
- shortens the rehabilitation period after heart surgery or stroke;
- helps in the treatment of alcoholism.
Overdose
Subject to the conditions of use, Mildronate does not exhibit toxic properties to specific organs and systems. There have been no documented cases of meldonium overdose resulting in severe consequences.
If the therapeutic dosage is exceeded for hypotension, the following symptoms can be expected: headache, dizziness, general malaise, severe tachycardia, instability of blood pressure.
There is no specific antidote; in case of overdose, symptomatic treatment is carried out aimed at relieving pathological manifestations. In case of severe overdose, strict control of the function of the kidneys and liver is necessary as the organs that interact with the drug the most.
Hemodialysis is not effective in case of an overdose of Mildronate due to the high binding coefficient to blood proteins.
Price, reviews, analogues
The price of Meldonium depends on the number of capsules in the package and the concentration of the main substance. The price for a package of 30 pieces with a dosage of 250 mg costs from 170 rubles, 60 pieces - from 320 rubles, 60 pieces with a dosage of 500 mg - from 550 rubles.
Structural analogues of Meldonium are Mildronate, Idrinol, Cardionate.
Reviews of Meldonia from doctors are generally positive. The drug has shown effectiveness in the treatment of withdrawal symptoms and cardiac pathologies, is well tolerated by patients and increases endurance.
Patients note a surge of strength and faster recovery after training, but it is not recommended to use it in the treatment of diseases of the cardiovascular system without a doctor’s prescription.
Interaction
Due to its profound effect, meldonium often enhances the effect of drugs that are its agonists in terms of the expected effect and mechanism of action. Thus, by combining drugs, greater progress can be made in the treatment of pathology.
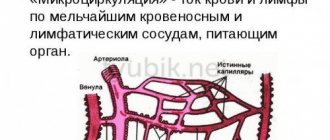
Mildronate enhances the effect of drugs that have angioprotective, antianginal, drugs, cardiotonics, cardiac glycosides. It prolongs the time of their active action, makes the rise and fall of the effect smoother. It is recommended to use Mildronate simultaneously with drugs that improve circulation in the microcapillary bed.
Mildronate significantly enhances the effect of vasodilators, antihypertensive drugs, in particular beta-blockers. With an increase in the therapeutic dosage of the drug meldonium, bradycardia may develop when combined with beta-adrenergic receptor blockers (Concor, Nebivolol).
The simultaneous use of Mildronate and Mexidol leads to mutual potentiation, the membrane protective effect of Mexidol is enhanced, it acquires a pronounced antihypoxic agent, increasing the endurance of the body as a whole and cells in particular under conditions of ischemia. Nonspecific actions of Mexidol are antiepileptic and nootropic effects, which are also enhanced when Mildronate is used.
Actovegin and Mildronate are compatible and are agonists in relation to the antihypoxic effect. These drugs in combination help maintain the integrity of the cell membrane and cell function under conditions of acute oxygen starvation. This action is most relevant in the context of myocardial infarction, to reduce the size of the area of necrosis.
Meldonium promotes better absorption of microelements by the target organ, which is used in cardiology when prescribing Panangin, Asparkam simultaneously with it. This ensures the restoration of electrolyte balance, and therefore the heart rate.
Mildronate, in addition to its effect on striated muscles and energy metabolism, also has the property of improving brain microcirculation, which is why it is used for central nervous system overstrain and chronic fatigue syndrome. Mildronate is compatible with nootropics and enhances their effect, so it can be taken with Glycine, Piracetam, Noopept, and ginkgo biloba preparations. Similar compatibility is observed with drugs that are used to restore cerebral circulation - Phezam, Cytoflavin.
Meldonium can be combined with drugs that have antiplatelet and anticoagulant effects for effective prevention of thrombosis. The medicine is compatible with Cardiomagnyl and acetylsalicylic acid.
Separately, Mildronate’s ability to potentiate the effect of psychotropic substances, in particular antiphobic drugs and anxiolytics, will highlight. The simultaneous use of meldonium with Afobazol, Phenibut, Grandexin is possible.
The use of Mildronate and caffeine is not recommended due to unexpected changes in blood pressure that these drugs together may cause.
Analogues and substitutes
In case of intolerance or other contraindications to taking Mildronate, it is recommended to select a substitute depending on the desired effect.
The most used analogues of the drug are presented in the table.
| Group | Trade names |
| Nootropics (drugs for normalizing metabolic processes in the brain, improving cognitive abilities) |
|
| Metabolic agents with a predominant effect on the cardiovascular system |
|
| Vitamin preparations for pathologies of the nervous system |
|
| Angioprotectors prescribed in neurological practice |
|
The selection of an effective substitute is carried out by the attending physician, taking into account the person’s concomitant diseases.
"Mildronate" is used among athletes as a doping to enhance the body's functioning, stimulate activity and increase muscle mass. For the same purpose, steroids are used - artificial hormones of the adrenal cortex, which accelerate the process of weight gain due to muscles (Danabol, Boldabol, Deca, Andropen).
Analogues of Mildronate
Analogs of a drug based on meldonium are medicines that also contain meldonium as the main active ingredient and are its derivatives (generics) or have a similar effect. But they are not completely similar to Mildronate. Medicines differ not only in price, but also in indications for use, and in greater or lesser intensity of the effect for individual therapeutic purposes.
Which is better: Mildronate or Cardionate?
Cardionate contains the same active ingredient as Mildronate - meldonium. The dosages and indications for use of this drug are the same, but Mildronate in this case is more affordable, and Cardionate has a more complex composition, and therefore a higher chance of developing allergic reactions and unwanted interactions with other drugs.
Which is better: Mildronate or Idrinol?
This drug continues the list of those developed on the basis of meldonium. It is distinguished by its convenient capsule form, otherwise the doses and indications are similar to Mildronate, and the price is sometimes significantly higher than its price. Idrinol is not the drug of choice for maintenance treatment of cardiac pathology and cerebrovascular accidents.
Which is better: Mildronate or Mexidol?
Mexidol is based on the active ingredient ethylmethylhydroxypyridine succinate. Unlike meldonium, this substance also has a nootropic and antioxidant effect - it protects membranes from active free radicals that are formed in cells. A wide range of effects includes stabilization and protection of membranes, normalization of microcirculation, and relief of mental stress. However, this drug does not have an evidence base and does not undergo the most serious studies, unlike Mildronate.
Which is better: Mildronate or Meldonium?
It is obvious that Mildronate as a drug that has been patented and put on the market will be as effective as the pure active ingredient - meldonium. The only difference is that meldonium is more accessible.
Which is better: Mildronate or Riboxin?
Riboxin contains inosine, which is an angioprotector, anabolic and antiarrhythmic agent. It significantly affects energy metabolism, in particular the synthesis and use of ATP. Its indications for use are somewhat broader than those of Mildronate: Riboxin is also used for the treatment of myocarditis, liver diseases, for the prevention of leukopenia, and for glaucoma. But it is contraindicated in patients with disorders of purine metabolism, with gout, which precludes its use for a whole group of patients.
Which is better: Mildronate or Trimetazidine?
This medicine is used exclusively in therapy and cardiology, because it acts selectively on the heart and blood vessels. Trimetazidine improves the energy and oxygen supply of the heart muscle, increases resistance to hypoxia, and prevents the death of cells that are in an intermediate state between life and necrosis - in parabiosis. However, for systemic use, relieving mental stress, and treating cerebral blood flow disorders, Mildronate is better suited, and Trimetazidine is better suited for the heart.
Which is better: Mildronate or Preductal?
Preductal is based on the above-described trimetazidine, and all the properties are characteristic of this drug. But there is a feature that concerns Preductal and its use in otorhinolaryngology - the drug helps compensate for dysfunction of the vestibular apparatus: it reduces the severity of dizziness, tinnitus, and hearing impairment. In other areas, the use of Mildronate is more appropriate.
Which is better: Mildronate or Panangin?
Panangin contains a balanced ratio of potassium and magnesium, which are widely used in cardiological practice. This is necessary to normalize electrolyte balance and proper heart function. But Panangin alone cannot provide the therapeutic effect that is expected from taking a real cardioprotector. However, the simultaneous use of Mildronate and Panangin solves the problem and allows one to achieve results in the treatment of coronary artery disease and myocardial infarction.
Which is better: Mildronate or Elcar?
Elkar contains levocarnitine (L-carnitine), which is one of the main metabolites in the work of skeletal muscles. It is used for a lack of levocarnitine in the body (normally it is synthesized endogenously in sufficient quantities) or for kidney diseases. It is used to restore balance after hemodialysis, for congenital enzymopathies, but has no metabolic effect. Mildronate performs much better in this regard.
Which is better: Mildronate or Actovegin?
Actovegin has pronounced neuroprotective, metabolic and microcirculatory effects. It is used for recovery after acute cerebrovascular accidents and is even more effective for this purpose than Mildronate. The medicine also improves the metabolism of glucose, oxygen, and maintains the energy supply of cells. But due to its composition (it is made from physiological material - calf blood), it may be intolerable to some patients, and the scope of application of the drug outside of neurology and neurosurgery is narrow.
Which is better: Mildronate or Cytoflavin?
Cytoflavin contains inosine and succinic acid. Inosine is very similar to meldonium in its metabolic action, but differs in mechanism. Succinic acid reduces tissue hypoxia and normalizes metabolism and energy metabolism. Mildronate has a much more pronounced and reasonable effect.
Mildronate - a little history
Mildronate is also known as Meldonium. Actually, meldonium is the active ingredient of mildronate.
Meldonium was developed in the 1970s by Ivars Kalvins - then director of the Institute of Organic Synthesis of the Academy of Sciences of the Latvian SSR - one of the leading research institutes in this field in the USSR. It was his scientists who were instructed to make a “product for household use” from meldonium, which then had a military purpose.
Initially, the development was called “mildronate”. It was registered in 1976, and began to be used in 1984. On December 7, 2011, by order of the government of the Russian Federation, meldonium was included in the list of vital and essential drugs.
As of January 1, 2021, meldonium is banned by the World Anti-Doping Agency (WADA) for pre- and non-competition use by athletes. Then, many famous Russian athletes, for example, tennis player Maria Sharapova, were disqualified for using meldonium, although they claimed that they last took it in 2015. The international doping scandal was just beginning to gain momentum...
By the way, L-carnitine, a popular dietary supplement that is freely sold in many countries and is not on the list of prohibited drugs, has properties similar to meldonium.
Alcohol compatibility
It is not recommended to take Mildronate during the acute phase of alcohol intoxication (alcohol intoxication), this can cause unpredictable interactions at the stage of pharmacokinetics and pharmacodynamics. The active substance meldonium undergoes all stages of transformation in the liver using enzyme systems and inhibits them during metabolism. This cross-folds the toxicity of both ethanol and meldonium due to its incomplete conversion.
Inhibition of the protective function of the liver in relation to meldonium and alcohol can lead to a pronounced hepatotoxic effect, increasing manifestations of alcoholic encephalopathy, and the accumulation of toxic breakdown products of ethanol.
It is possible to develop allergic reactions due to the simultaneous use of Mildronate and alcohol.
Reviews of Mildronate
Reviews about Mildronate on forums are mostly positive . The unique mechanism of action of this drug allows it to be widely used to eliminate problems with the cardiovascular system, as well as as a means of increasing performance in healthy people who are subject to frequent physical and intellectual overload.
Both patients in cardiology departments, doctors, and athletes note the fact that Mildronate provokes a tonic effect. Its use significantly improves memory function, accelerates thought processes, increases dexterity, endurance and the degree of body resistance to adverse factors.
Reviews from cardiologists confirm the data of numerous studies that have shown that the use of Mildronate in capsules and in the form of a solution for injections can reduce the incidence of repeated myocardial infarction .
Reviews from patients about Mildronate allow us to conclude that the drug is simply necessary for people whose activities are associated with increased stress on the body, as well as during the recovery period after prolonged alcohol abuse, with pain and burning in the heart, VSD and other pathologies of the cardiovascular system .
The average rating for this product is quite high. However, sometimes you come across negative reviews about Mildronate. It is important to remember that, like any other drug, Mildronate gives a good result only if its dose and concomitant treatment (if necessary) are selected correctly.
Use of Mildronate during pregnancy and lactation
Pregnancy and lactation, as well as the period of breastfeeding, are absolute contraindications to the use of the drug Mildronate. Due to the distribution characteristics and high activity, metabolites perfectly penetrate from the systemic bloodstream into tissues, this ensures the effective effect of the drug on any target organ. But meldonium also penetrates the hematoplacental barrier and accumulates in the fetus.
There is no data on the effect of meldonium on the fetus, but the drug is not recommended for use under 18 years of age due to the development of adverse reactions. No teratogenic effect of meldonium on the embryo was detected, but the concentrations of the drug inside the placenta were higher than permissible. Changes in hemodynamics caused by meldonium can be difficult to tolerate by the body of a pregnant woman.
The drug also passes freely into breast milk in high concentrations and in the form of active forms, which can lead to unforeseen consequences for the baby. If there are vital indications for the use of Mildronate, breastfeeding should be stopped immediately.
Pharmacodynamics and pharmacokinetics
The action of the active substance of the drug is aimed at inhibiting the enzymatic activity of γ-butyrobetaine hydroxylase, which is the last enzyme in the chain reaction of l-carnitine .
Meldonium helps reduce the concentration of free carnitine , prevents the transport of long-chain fatty acids across cell membranes, and prevents the accumulation in cells of activated forms of unoxidized fatty acids, which are derivatives of acylcarnitine and acyl coenzyme .
In ischemic tissues, it restores the balance between the transport of oxygen and its absorption by cells, prevents disruption of the transport of adenosine triphosphate, while simultaneously activating glycolysis, which occurs without additional oxygen consumption.
The result of a decrease in carnitine concentration is increased synthesis of the vasodilator γ-butyrobetaine.
After taking Mildronate tablets per os, the meldonium it contains is quickly absorbed in the digestive tract. The drug is characterized by a fairly high bioavailability index. The latter is approximately 78%.
The concentration of meldonium in the blood plasma reaches its maximum values an hour or two after administration. In the body, meldonium is metabolized to non-toxic products - glucose, succinate, 3-hydroxypropionic acid.
Metabolites are excreted by the kidneys. The half-life (T½), depending on the characteristics of the particular organism and the dose taken, can range from three to six hours.
The drug in injection form is characterized by 100% bioavailability. The concentration of meldonium in the blood plasma reaches its maximum values immediately after administration of the drug. The half-life (T½), depending on the characteristics of the particular organism and the dose taken, can range from three to six hours.
The result of the metabolization of meldonium is the formation of non-toxic metabolites (glucose, succinate, 3-hydroxypropionic acid), which are then excreted from the body by the kidneys.
Benefits or harms of Mildronate for older people
The instructions do not indicate whether Mildronate can be taken by older people. This group of patients is allowed to drink drugs of class C01EB22 only as prescribed by a doctor. Representatives of this age category are often forced to take several medications, so meldonium can cause harm to them due to a drug conflict.
Taking Mildronate should be individually agreed with your doctor. He will control the combination of medications, clarify the absence of contraindications, and if there are no restrictions on treatment, he will allow you to take the drug.
The benefits of Mildronate for men and women
There is information on the Internet that Mildronate increases potency and libido. Doctors deny this, since research results have not confirmed this effect of meldonium.
But the drug has a beneficial effect on the activity of the nervous, cardiovascular and endocrine systems, and increases the body’s tolerance to excessive physical activity. This benefits a person in the sexual sphere. Therefore, doctors can prescribe monotherapy with Mildronate to a man or woman. If the patient has decreased potency/libido, he is prescribed to take the medicine for 10-12 days at a daily dose of 500 mg. If necessary, the course is repeated after two weeks.
Why take Mildronate?
The drug is used only in the treatment of adults. Regardless of the dosage form, the indications for prescribing Mildronate are:
- Heart diseases caused by impaired coronary circulation (in complex therapy).
- Chronic heart failure and cardialgia.
- Reduced performance.
- Pathologies of peripheral arteries.
- The recovery period after surgery to restore the body.
- Encephalopathy.
- Stroke.
- Bronchial asthma and chronic obstructive pulmonary disease.
- II and III stages of alcoholism.
- Violations of the integrity of the vessels supplying blood to the tissues of the eye.
The use of Mildronate slows down the formation of necrotic areas and shortens the rehabilitation period after acute damage to cardiac muscle tissue.
During active training, meldonium restores the balance of oxygen in cells, prevents the accumulation of toxins, and helps restore energy reserves.
The drug is successfully used in sports in healthy people because:
- Increases adaptability to physical activity.
- Improves muscle nutrition, including the heart, and accelerates their recovery.
- Reduces fatigue.
- Increases the effect of sports.
Mildronate is not suitable for gaining muscle mass, but is used to prevent overwork.
Results and discussion
Pre-hospital stage
Since age is a significant non-correctable risk factor for the development of cardiovascular diseases in both men and women, let us consider the data obtained primarily in terms of this indicator (Fig. 2)
.
Figure 2. Number of emergency calls (along the x-axis) in different age groups.
In group 1, the largest number of patients belonged to the age groups 60-69 years (37.5%), 50-59 years and 70-79 years (21.9% each); in group 2, the largest number of patients were in the age groups 50-59 years (36.8%); 70-79 years old (23.0%); 60-69 years old (18.4%). Thus, it was revealed that in the vast majority of cases, AMI and CCI develop in elderly people. This was reflected in the number of EMS calls (Fig. 2)
.
As mentioned above, the main factors in the development of AMI and CCI are atherosclerosis and hypertension. In addition, the influence of ischemic heart disease, stroke, DE, diabetes and obesity may be noted.
Of the concomitant diseases in the anamnesis of the examined patients, the presence of hypertension, an independent and second most important (after age) risk factor for IS and DE, was revealed in 30 (93.8%) patients of the 1st group and 87 (100%) patients of the 2nd group . The duration of elevated blood pressure in patients of group 1 was on average 12.7 years, in patients of group 2 - 11.9 years. At the same time, in both patients with IS and DE, the combination of hypertension with other modifiable risk factors turned out to be common. It is known that the “crisis” course of hypertension is particularly dangerous, since fluctuations in blood pressure, both its increase and decrease, can cause hypoperfusion of the brain substance, which leads to circulatory disorders. A history of hypertensive crisis was noted in 4 (12.5%) patients in the 1st group, and in 14 (16.1%) patients in the 2nd group. A burdened hereditary and family history of these diseases was identified in 29 (90.1%) patients in the 1st group, and in 81 (93.1%) in the 2nd group.
The presented data confirm the importance of hypertension and other correctable risk factors on the formation of IS and DE.
In outpatient settings, patients should constantly receive adequate therapy focused on risk factors. This is reflected in our data on the frequency of treatment with different drugs (Table 2)
taking into account the implementation of the therapy regimen
(Table 3)
.
Thus, in an outpatient setting, some patients received drug therapy regularly, while others received it occasionally. The range of medicines was quite wide. In group 1, 23 (71.9%) patients were treated with medications. Among them, 12 (23.5%) took ACE inhibitors, more often enalapril (13.7%); diroton (5.9%); beta-blockers - 13 (25.5%), more often concor (23.5%); calcium channel blockers - 5 (9.8%); diuretics - 4 (7.8%) - indapamide; angiotensin receptor blockers (ARBs) - 7 (13.7%), more often lozap (11.8%). In group 2, 61 (70.1%) patients were treated with medications: ACE inhibitors - 44 (50.7%), most often monopril (17.5%), enalapril (9.2%), diroton (9.2% ); beta blockers - 13 (14.9%), more often atenolol (9.2%); diuretics - 13 (14.9%), more often hypothiazide (10.3); ARB - 6 (6.8%): lozap, lorista (3.4% each).
The total number of complaints made by patients before and after treatment in groups 1 and 2 differed slightly. In the 1st group it was 194, after therapy 124. In the 2nd - before treatment 936, after treatment 414. During examination, patients of the 1st group presented an average of 6-7 complaints, and patients of the 2nd group - by 10-11; after therapy, the number of complaints decreased to 3-4 and 4-5, respectively. The use of a scoring system that makes it possible to quantify the severity of complaints and thereby corresponding violations makes it possible to more objectively present the effectiveness of drug therapy in the conditions of emergency medical care (Fig. 3, 4)
.
Figure 3. Number of complaints in patients with AMI before and after therapy in emergency medical services.
Figure 4. Number of complaints in patients with CCI before and after therapy. As can be seen from the presented figures, most often patients in group 1 complained of headache (7.7%), nausea (8.2%), chills (8.2%), general weakness (11.9%), weakness in the arm and leg (7.2%), speech impairment (5.2%), numbness in the arm and leg (2.1%).
In group 2, subjective disorders were most often recorded in the form of headache (9.3%), non-systemic dizziness (9.3%), redness of the skin (8.2%), noise in the head (7.6%), decreased memory (6.9%), unsteady gait (4.5%), forgetfulness (2.4%). This suggests that the complaints of patients in group 1 are more related to neurological disorders, and the complaints of patients in group 2 are more specific for DE in general.
After treatment with mildronate in patients with IS, the number of somatovegetative complaints decreased - it decreased to 49.5%, and the number of specific complaints did not change - 14.5 and 13.5%, respectively; in patients with CCI, the number of subjective somatovegetative complaints decreased significantly (25.8%), while typical ones did not change (18.2%). A decrease in varying degrees of severity of the main subjective symptoms (headache, non-systemic dizziness, general weakness, noise in the head, etc.), assessed using a 4-point system, occurred in 100% of patients.
Currently, according to the literature, the risk of stroke in patients with blood pressure above 160/95 mmHg. increases approximately 4 times compared to persons with normal blood pressure, and with blood pressure more than 200/115 mm Hg. - 10 times. However, it should be remembered that an increase in blood pressure may be a stress response aimed at increasing cerebral blood flow in the area of cerebral ischemia, maintaining blood flow in ischemic areas of the brain. A low level of systemic blood pressure is also undesirable, leading, in conditions of impaired autoregulation of cerebral circulation, to cerebral hypoperfusion with the development of small cortical foci of infarction. The study allowed us to obtain our own data (Fig. 5 and 6)
, from which it follows that during therapy, the average SBP in the 1st group decreased by 9.5%, and in the 2nd group - by 6.2%, i.e.
administration of mildronate does not cause a sharp decrease in blood pressure. Figure 5. Average blood pressure and heart rate in AMI during different periods of the examination.
Figure 6. Average blood pressure and heart rate during CCI during different periods of the examination. It is known that heart rate affects life expectancy and an indicator of more than 80 beats/min requires correction. The state of the heart rhythm in the acute period of ischemic stroke and CCI was shown in Fig. 5 and 6
. The average heart rate was: in group 1 - 70.5 beats/min (from 60 to 80); “office” - 81.9 beats/min (from 56 to 120); 15 minutes after treatment - 78.2 beats/min (from 56 to 100). In the 2nd group - usual for the patient - 69.0 beats/min (from 62 to 80); “office” - 76.6 beats/min (from 48 to 110); 15 minutes after treatment - 74.4 beats/min (from 48 to 100). In group 1, tachycardia was registered before treatment in 9 (28.1%) patients; after treatment - it was absent, and in the 2nd group, before treatment it was in 14 (3.7%) patients, after treatment - in 2 (0.5%).
Thus, mildronate, used in the prehospital stage, does not have a pronounced effect on heart rate.
Let us dwell on the direct neurological consequences of the vascular diseases studied. It is known that the diagnosis of IS is based on the development of acute neurological disorders characteristic of cerebral vascular damage, taking into account risk factors. CCI is caused by slowly progressive diffuse damage to the brain as a result of decreased blood flow, which leads to pronounced changes in brain structures and various neurological disorders.
From those shown in Fig. 7 and 8
The data shows that in patients of group 1, symptoms of focal brain damage predominated: hemiparesis, hemiplegia - in 12 (13.7%) patients, dysarthria - in 14 (15.9%), tongue deviation - in 7 (8. 0%), hypoesthesia - in 6 (6.8%), lethargy - in 14 (15.9%).
Figure 7. Distribution of patients according to neurological symptoms in AMI.
Here and in Fig. 8-10: numbers reflect the number of patients. Figure 8. Distribution of patients according to neurological symptoms during CCI. In group 2, the clinical picture was characterized by a large polymorphism of neurological symptoms: hemiparesis - in 18 (4.7%) patients, dysarthria - in 8 (2.1%), agitation - in 32 (8.4%), chills, muscle pain. trembling - in 77 (18.8%), cognitive impairment - in 87 (22.5%). After therapy in group 1, the frequency of corresponding clinical symptoms decreased by 27.2%, smoothness of the nasolabial triangle - to 4.5%, dysarthria - to 4.5%, lethargy - to 3.4%, finger-nose test - to 2. 3%; in group 2, the decrease in clinical manifestations was 57.5%. At the same time, somatovegetative symptoms decreased by 47%, but the neurological deficit practically (consequences of IS) remained unchanged - 10.5%. In IS and CCI, there is a connection between the severity of neurological deficit and the age of the patients: in group 1 it was more pronounced in people over 60 years of age, and in group 2 - in people over 50 years of age.
Thus, during treatment with mildronate, there was a positive dynamics of the neurological condition in both groups 1 and 2. It should be emphasized that we are talking about a decrease in the severity of mainly somatovegetative manifestations, while focal neurological symptoms remained unchanged.
After the administration of mildronate, 15-20 minutes later, the doctor and the patient assessed its effect, and when transporting patients with IS to the hospital, before examination by a neurologist. In the 1st group, patients in 19 (59.4%) cases, and the doctor - in 13 (40.6%), in the 2nd group - in 80 (92.0%) and in 68 (78.2%) %) cases respectively. The proportion of assessment “no changes” in the 1st group was noted by patients in 13 (40.6%) cases, by the doctor - in 19 (59.4%), in the 2nd group - patients noted in 7 (8.0% ) cases, the doctor - in 19 (21.8%).
According to ECG data, in group 1, sinus rhythm was detected in 32 (100%) patients, sinus tachycardia - in 9 (28.1%), ventricular extrasystole - in 2 (6.3%), atrial extrasystole - in 2 (6.3%); in group 2, sinus rhythm was detected in 81 (93.1%) patients, sinus tachycardia - in 8 (9.2%), sinus bradycardia - in 2 (2.3%); atrial fibrillation - in 6 (6.9%). Left ventricular hypertrophy associated with ventricular rhythm disturbances was registered in group 1 - in 23 (71.9%) patients, in group 2 - in 66 (75.9%). The average glucose level in group 1 was 6.9 mmol/l, in group 2 - 6.6 mmol/l. Blood glucose levels exceeding 6 mmol/l were recorded in 7 (21.9%) patients in group 1, and in 18 (20.7%) patients in group 2.
During prehospital treatment, the main drug used for emergency medical treatment in both groups 1 and 2 was mildronate. A favorable profile of its efficacy and safety was established at a dose of 1000 mg in both groups. There were no significant adverse events or deterioration in the general somatic condition of the examined patients. The number of patients with side effects in the study groups was the same: in group 1 - 4 (12.4%) patients, in group 2 - 14 (12.6%). During the administration of the drug, patients complained: in the 1st group - dizziness - 3 (9.3%) patients, general weakness - 1 (3.1%), in the 2nd group - headache - 3 (3). .4%) of the patient, for dizziness - 4 (4.6%), for general weakness - 4 (4.6%). The listed complaints were short-term, since the corresponding disorders disappeared 1-3 minutes after the administration of mildronate, they did not require additional treatment and were not life-threatening.
Additional therapy was prescribed by doctors at the emergency medical service station to 25 (78.1%) patients in group 1, and to all (100%) patients in group 2. In this case, we are talking about antihypertensive therapy with the use of ebrantil (16% of patients), ENAP-R (4.0%), sublingual captopril (8.0%), magnesium sulfate (40.0%) in group 1 . In group 2, the following were used to reduce blood pressure: ebrantil (4.7%), ENAP-R (3.1%), captopril under the tongue (9.4%), magnesium sulfate (42.2%). In groups 1 and 2, glycine was also prescribed in 32.0 and 40.6% of cases, respectively.
Experience in prehospital treatment by EMS station specialists shows that the most important thing is to focus on stabilizing blood pressure. In this case, correction of blood pressure levels should be extremely careful, and nonspecific neuroprotective therapy should be started as early as possible (impact on the early stages of the ischemic cascade).
Description of the drug
The main active ingredient is meldonium. Produced in Latvia, Poland, Lithuania and Slovakia.
Presented in pharmacy chains in two forms:
- A transparent solution for injection, 1 ml of which contains 100 mg of the main substance.
- Gelatin capsules with 250 or 500 mg of meldonium dihydrate. Contents: white powder with a slight odor and sweetish taste.
The solution is sold in ampoules of 5 ml, a cardboard box contains 10 ampoules and instructions for use.
Meldonium is a synthetic drug with an effect similar to the precursor substance hydroxytrimethylaminobutyric acid, related to B vitamins.
The substance is able to improve metabolic processes and provide cells with energy.
Meldonium:
- Increases performance.
- Reduces the manifestations of mental, physical and mental stress.
- Increases the strength of heart contractions.
- Reduces the frequency of angina attacks.
- Increases the body's endurance during physical activity.
The medicine should be stored in a dry place at temperatures up to 25°C for no more than 4 years.