Atrial fibrillation and atrial fibrillation are currently equivalent terms. They have similar causes, clinical manifestations and changes on the electrocardiogram. They can often transform into each other. Atrial fibrillation is understood as a heart rhythm disorder in which the atria and ventricles contract in their own pattern, and not sequentially, so the frequency of contraction of the atria and ventricles is different.
Predisposing factors for the occurrence of atrial fibrillation are: coronary heart disease (CHD), arterial hypertension, heart defects, structural heart diseases, chronic obstructive pulmonary disease, excess weight, diabetes mellitus, sleep apnea, chronic kidney disease, thyroid dysfunction.
There are conservative (antiarrhythmic drugs) and surgical methods for treating atrial fibrillation. More details about them in the article “atrial fibrillation”.
Over the past 30 years, several types of surgical treatment have been developed.
— surgical isolation of the left atrium, — “corridor” procedure, — “labyrinth” operation — a method of surgical ablation.
The most effective among them was the “labyrinth” operation, which was first performed in 1987 by cardiac surgeon J. Cox in St. Louis.
Over the years, this operation has undergone three modifications - Maze-1, Maze-2 and Maze-3. Maze -1 was modified because it was associated with sinus node dysfunction and intraatrial conduction delay. Maze-2 was abandoned due to the extreme complexity of the procedure. And in 1992, J. Cox developed a third option (Maze-3), which combined all the advantages of the previous options and was easy to implement. It is worth noting that this operation is combined and is currently the “gold standard” for correcting mitral valve disease in combination with atrial fibrillation. In its pure form, the “labyrinth” (surgical ablation method) is performed extremely rarely due to its high morbidity.
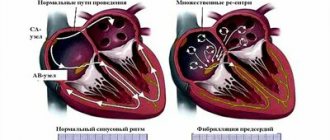
To understand the essence of the “labyrinth” operation, you need to understand the cause of atrial fibrillation.
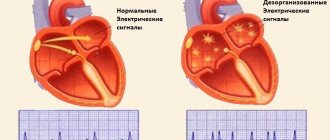
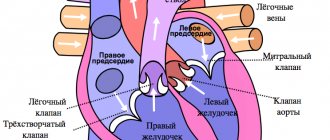
The human heart consists of four chambers, the left and right atrium and the left and right ventricle. Normally, the nerve impulse should go from the sinus node, located in the wall of the right atrium, to the atrioventricular node in the interatrial septum. In this case, the atria and ventricles of the heart contract correctly. With atrial fibrillation, the correct course of the impulse is disrupted. Some of the impulses, as it should be, go to the atrioventricular node, and some return to the sinus node and cause extraordinary contraction of the atria.
The essence of the “labyrinth” operation is to destroy the conduction pathways that are responsible for the occurrence and maintenance of arrhythmia. This is achieved by a surgical “incision and suture” method (blue straight lines in the diagram) through the atria, by excision of the posterior wall of the left atrium along with the pulmonary veins and making multiple small incisions in the right and left atria, forming a so-called “labyrinth”, which does not allows the nerve impulse to return back and cause an extraordinary contraction of the atrium. Simply put, the impulse that wants to return to the sinus node runs into microscopic cuts in the heart and fades away. As a result, the impulse goes where it should normally go, i.e. to the atrioventricular node, which causes the ventricles of the heart to contract and promotes proper contraction of the heart.
Scheme
The “Labyrinth” technique has not found wide clinical application due to the long time of artificial circulation, cross-clamping of the aorta, high risk of bleeding, and lack of experience in performing this technique. Therefore, a number of modifications of this operation have been proposed using various physical methods of ablation of the walls of the atria, replacing the scalpel: radiofrequency, irrigation radiofrequency, ultrasound, cryogenic, laser and microwave exposure.
Contraindications
Contraindications to the labyrinth operation are:
• Sharply increased size of the left atrium. • High value of the cardiothoracic index, with low amplitude of ƒ-waves on the ECG in leads V1. • Pulmonary hypertension. • Kidney and liver failure. • Low left ventricular ejection fraction (less than 30%). • Long-term chronic form of AF in the anamnesis, because in this case, restoration of sinus rhythm after surgery is practically not observed. • General contraindications before cardiac surgery. They depend on the underlying heart disease and are considered by a cardiac surgeon on a case-by-case basis.
Treatment
Conservative treatment of atrial fibrillation (atrial fibrillation)
Atrial fibrillation is a risk factor for ischemic stroke, which develops as a result of the formation of blood clots in the cavity of the left atrium. The first-line treatment for atrial fibrillation is drugs that prevent blood clots. They are prescribed by a doctor, because... monitoring of the blood coagulation system is required. These drugs are indicated for almost all patients who suffer from atrial fibrillation, regardless of whether the arrhythmia is constantly present or occurs in attacks (paroxysmal form of arrhythmia). The risk of stroke is the same both in the presence of a chronic form of arrhythmia and in a paroxysmal form of arrhythmia.
In patients with paroxysmal atrial fibrillation, the issue of preventing the occurrence of arrhythmia attacks is addressed. If the attack occurs for the first time, antiarrhythmic drugs are not prescribed. Medications may be recommended to control heart rate and improve tolerance to repeated episodes of arrhythmias. Antiarrhythmic drugs are also not prescribed if the patient’s arrhythmia attacks are asymptomatic and do not reduce his quality of life. If rhythm disturbances recur and paroxysm tolerance deteriorates, the cardiologist-arrhythmologist, together with the patient, decides on the prescription of antiarrhythmic drugs or surgical treatment of arrhythmia (catheter ablation).
If a prolonged attack of atrial fibrillation develops, which does not go away on its own, it is necessary to contact a specialist cardiologist-arrhythmologist, who will choose the most suitable method for stopping the arrhythmia for the patient. A technique for medicinal restoration of normal heart rhythm has been developed, as well as a procedure for restoring rhythm using electrical cardioversion. To restore the rhythm, a certain medication preparation is necessary, the regimen of which will be determined by the doctor, based on the individual characteristics of the course of the disease. With the advent of the latest highly effective antiarrhythmic drugs, preference is given to drug restoration of rhythm.
When transforming the paroxysmal form of atrial fibrillation into a chronic form, the main task is to control the heart rate. In the presence of tachysystole (high heart rate), drugs are prescribed that reduce the heart rate, the primary of which are beta-blockers. An integral part of the treatment of atrial fibrillation is the treatment of the disease that provoked the rhythm disturbance - coronary heart disease, heart failure, arterial hypertension, disorders of the thyroid gland and others.
It is necessary to contact a cardiologist-arrhythmologist if:
- development of an attack of atrial fibrillation for the first time in life,
- development of another attack of arrhythmia that cannot be controlled by usual means,
- ineffectiveness of previously prescribed antiarrhythmic therapy.
Preparing for surgery from the patient's side
Before the operation, the patient must perform a number of examinations in the clinic at his place of residence:
• Examination by the attending physician • Laboratory tests (clinical and biochemical blood tests, urine analysis) • 12-lead electrocardiogram (ECG) • Echocardioscopy is necessary to assess structural and functional changes in the heart (condition of valves, cardiac muscle, pericardium, pulmonary artery diameter, pressure in the pulmonary artery, mechanical complications of myocardial infarction, heart tumors, etc.); • X-ray of the chest organs in 4 projections; • Coronary angiography to assess the patency of the arteries that supply blood to the heart muscle; • Cardiac catheterization may be required to determine the pressure in the chambers of the heart, and transesophageal echocardioscopy.
A very important question before surgical treatment is to replace anticoagulant therapy, if necessary; on the eve of hospitalization, antiplatelet agents are canceled if the patient receives them.
Hospitalization is carried out in the cardiac surgery department of a multidisciplinary clinic.
The day before surgery, the patient is consulted by an anesthesiologist. Checks height, weight, presence of chronic diseases, allergies to medications, and examines the patient. In the evening, the patient is denied dinner. Before going to bed, you are only allowed to drink. In the morning before the operation, breakfast is canceled, and drinking is also prohibited. Premedication is performed.
Material and methods
In the Department of Cardiothoracic Surgery of the Federal State Budgetary Institution Northwestern Federal Medical Research Center named after. V.A. Almazov" from 2012 to 2021, using the modified MAZE IV technique, 72 patients with valvular heart defects and concomitant persistent AF were operated on. The average age of the patients was 55.7±10.2 years, the male/female ratio was 39/61%, and the average left atrium (LA) size was 53.6±7.3 mm. The effectiveness of restoration and maintenance of sinus rhythm was assessed through daily ECG monitoring at control points 6 days, 3, 6 and 12 months after surgery.
Surgical technique
:
1 - access to the heart is carried out through a median sternotomy;
2 - connection of the heart-lung machine is carried out using the bicaval technique, the superior vena cava (SVC) is cannulated through the anterior wall at a distance of at least 1 cm from the level of its entry into the right atrium (RA). For the convenience of cannulation of the SVC, as well as to increase its mobility, the SVC is isolated extrapericardially to the level of the innominate vein entering it;
3 - isolation and mobilization of the inferior vena cava (IVC) extrapericardially to the level of its passage through the diaphragm;
4 — placing tourniquets around the ERV and IVC. The level of tourniquets around the SVC should be located between the level of cannulation and the level of the innominate vein to allow unobstructed placement of the branches of the bipolar electrode when forming a line of RFA exposure from the RA incision to the SVC. At this level, the azygos vein also flows into the SVC, which must be taken into account to avoid damage to it;
5 - antegrade blood isothermal cardioplegia through the aortic root until ventricular asystole;
6 - transverse atriotomy of the RA in the direction from the atrioventricular groove to the border ridge with its intersection. The distance from the PP section to the level of the confluence of the UVC should exceed the distance to the level of the confluence of the IVC by approximately 3 times;
7 - suturing the mouth of the coronary sinus with a purse-string suture (prolen 4/0-5/0), installing a cannula into the coronary sinus for retrograde cardioplegia under visual control, followed by its fixation with a purse-string suture. This manipulation makes it possible to conduct sessions of retrograde isothermal blood cardioplegia with great reliability and control of the correct delivery of the cardioplegic solution to the myocardium. In this case, after tightening the purse-string suture, it is necessary to carry out maximum dislocation of the cannula toward itself in order to locate the self-inflating olive directly at the mouth of the coronary sinus and adequately distribute the cardioplegic solution into those large veins of the heart that flow into the coronary sinus most distally;
8 - retrograde isothermal blood cardioplegia under control of pressure in the coronary sinus. The optimal delivery rate of the cardioplegic solution is 220–300 ml/min at a pressure in the coronary sinus of 20–45 mm Hg. The minimum volume of administered cardioplegic solution is not less than 500 ml, the maximum interplegic interval is 15 minutes;
9 — separation of epicardial tissue in the area of Waterstone’s interatrial groove, longitudinal atriotomy of the LA anterior to the mouths of the right pulmonary veins (PV);
10 — revision of the LA cavity, including the appendage, in order to avoid performing manipulations in the presence of intracavitary thrombi with the risk of their fragmentation and uncontrolled dislocation;
11 — pairwise selection of the right and left PVs, placing tight fittings around them for the convenience of further positioning of instruments for RFA;
12 — isolation of the mouths of the left L.V. The heart is moved anteriorly from the pericardial cavity by the surgeon's hand by the apex. One of the branches of the bipolar electrode is positioned behind, the other anterior to the pair of left PVs using a tight fit performed during the isolation of the PVs. The jaws of the bipolar electrode are clamped with the maximum possible grip of the LA wall, while the instrument arc should also be bent towards the LA. These actions make it possible to increase the area of the isolated surface of the L.P. areas adjacent to the mouths of the LV. After snapping the jaw of the bipolar electrode, repeated visual control of the level of tissue clamping is carried out. It is important to check whether the entire line of intended influence is located between the working surfaces of the electrodes, without going beyond the contour of the branch. After this, RF energy is applied to the tissue. After the device indicates transmural tissue damage, the jaws are separated. The formed conduction block line is visualized from both the endocardial and epicardial surfaces of the atrium wall. The electrode branches are shifted parallel to this line by 2-3 mm and the procedure is repeated again. The optimal repetition rate for LA walls is 3;
13 — insulation of the base of the LP ear;
14 - formation of a line of the conduction block from the left auricle to the upper left left side. To do this, the wall of the LA appendage is cut 5-6 mm in length, and one of the bipolar electrode branches is inserted through the resulting hole, which is directed to the upper left LA. After snapping the jaws, it is important to check that their working surface necessarily intersects the insulation line of the base of the left eyelet, and then the line formed when isolating the mouths of the left left. In this case, to ensure reliable closure of the conduction block lines, it is necessary to advance the electrode branch 10-15 mm along the length of the pulmonary vein from its mouth (Fig. 1);
Rice. 1. Formation of a line of the conduction block from the left left ear to the upper left left side. Intraoperative photography. The branch of the bipolar electrode is passed through a hole in the apex of the left PV appendage (a) and sequentially crosses the previously formed line of the conduction block at the base of the appendage (b) and the line around the left PVs (c), capturing 10-15 mm of the length of the PV wall (d).
15 — suturing the hole in the left ear;
16 — completion of isolation of the mouths of the right L.V. The jaws of the bipolar electrode and the RFA are positioned in a manner similar to when performing isolation of the left PV. The peculiarity is that in this case the impact is only on the posterior wall of the left atrium in the area of the PV orifices, since the line of the conduction block along the anterior wall is the atriotomy incision;
17 - formation of a block line along the roof of L.P. It is carried out by passing the branches of a bipolar electrode from the upper corner of the atriotomy incision of the left atrium to the left upper PV, followed by triple RFA. To simplify the placement of branches, additional “blunt” or “sharp” isolation of the roof of the left atrium towards the left upper PV may first be required, especially in cases where there is pronounced epicardial tissue in this area. In addition, for the convenience of positioning the branches and greater reliability of closing the lines of the conduction block, the atriotomy incision of the left atrium should be significantly (up to 2-3 cm) extended to the roof of the left atrium compared to what is necessary when performing an isolated surgical procedure on the mitral valve. With large LA sizes, without extending the incision, the length of the branches may not be enough to reach the conduction block line formed around the left PVs. As in the case of forming a line from the appendage of the left PV to the upper left PV, when performing this intervention, the branch of the bipolar electrode should, with a 10-15 mm margin, intersect the line of the conduction block formed around the left PV, i.e., actually directly capture the wall of the ostial part of the left upper PV;
18 — formation of a conduction block line from the lower corner of the atriotomy incision of the left PV to the mouth of the lower left PV with the intersection of the conduction block line formed around the left PV. It is carried out in a manner similar to that when forming a block line on the roof of L.P. For the convenience of its implementation, the atriotomy incision should be extended by 2-3 cm to the posterior wall of the left atrium in the direction of the appendage, depending on the size of the left atrium;
19 — formation of a conduction block line in the area of the mitral isthmus. This manipulation is performed in 2 steps. First, a short (about 2-2.5 cm long) open conduction block line is formed using a bipolar RFA electrode from the lower edge of the atriotomy incision of the left atrium in the direction of the fibrous annulus of the mitral valve (Fig. 2). In the second step, this line is extended to the fibrous ring of the mitral valve at the border of segments P2 and P3 of the posterior leaflet using a transmural incision of the atrium wall (Fig. 3, color insert). The main and fundamental points of this incision are: dissection of the entire thickness of the LA myocardium to visually distinguishable epicardial tissue (having dielectric properties) or the lumen of the coronary sinus; closedness of the line to the fibrous ring of the MV, on the one hand, and the line formed at the first appointment. An alternative to these manipulations can be considered a continuous incision through the entire thickness of the left atrium wall from the lower corner of the atriotomy incision of the atrium to the fibrous ring of the mitral valve, however, this can create greater difficulty in suturing the left atrium and a greater risk of difficult-to-treat bleeding;
Rice. 2. The first technique when forming a line of electrical conduction block in the area of the mitral isthmus. Intraoperative photography.
Rice. 3. The second technique when forming the line of the electrical conduction block in the area of the mitral isthmus. The epicardial tissue and the wall of the coronary sinus are visualized. Intraoperative photography.
20 — suturing the incision in the area of the mitral isthmus with a double-row suture (prolen 5/0);
21 — suturing of the LA appendage from the side of the LA cavity with a double-row suture (prolen 4/0);
22 — formation of a conduction block line from the middle of the atriotomy incision of the RA to the apex of the RA appendage;
23 — formation of the conduction block line from the lateral corner of the atriotomy incision of the RA to the SVC. In this case, the working surfaces of the bipolar electrode jaws are closed along the maximum lateral surface of the RA to avoid impact on the sinus node. Taking into account the smaller thickness of the tissues of the wall of the RA, the frequency of RF exposure can be reduced to 2. In this case, the branches of the electrode should be carried out up to the level of cannulation of the SVC, i.e., at a distance of at least 1 cm from its entry into the cavity of the RA. In cases where the distance from the lateral angle of the atriotomy incision of the RA to the SVC is greater than the length of the electrode branches or there is a difference in the thickness of the RA wall that does not allow for close contact of the RA wall with the working surface of the electrode, the formation of this line can be performed in portions using a small incision in the RA wall in projections of the expected line of influence. Lines of the RA wall conduction block must be performed during retrograde cardioplegia sessions to reduce the time of aortic cross-clamping;
24 — formation of the conduction block line from the lateral corner of the atriotomy incision of the RA to the IVC. This line is formed similarly to the previous one and, as a rule, is 3 times or more shorter than it, which does not require additional techniques;
25 — extension of the atriotomy incision of the RA from the medial angle to the fibrous ring of the TC for 1-2 hours (Fig. 4). The method of forming this incision is identical in its fundamental aspects to that in the area of the mitral isthmus. The incision should be made through the entire thickness of the RA wall to the visualized epicardial tissue and be clearly closed on the fibrous ring of the T.K. This incision eliminates the possibility of pathological rotation of the electrical impulse around the fibrous ring of the TC during the development of typical atrial flutter. Together with the atriotomy incision of the RA and the line of the conduction block formed in the direction of the IVC, it forms a functional analogue of the line of the conduction block in the area of the cavatricuspid isthmus;
Rice. 4. Extension of the atriotomy incision of the right atrium from the medial angle to the fibrous ring of the tricuspid valve for 1-2 hours. The valve leaflet is held with a hook, the epicardial tissue is visualized. Intraoperative photography.
26 — suturing an analogue of the cavatricuspid isthmus incision with a double-row suture (prolen 5/0);
27 — correction of the heart valve apparatus;
28 — suturing of atriotomy incisions;
29 — a mandatory step in completing the procedure is the suturing of two pairs of epicardial electrodes to enable atrioventricular temporary cardiac pacing until the patient’s own heart rhythm is completely restored.
Surgery through the patient's eyes
In the operating room, the anesthesiologist puts the patient under anesthesia; after the administration of the drugs, there may be slight short-term dizziness, a feeling of chills, or a slight fever. Otherwise, the patient falls asleep unnoticed and wakes up in the intensive care unit (ward). The operation is performed under general anesthesia, so the patient does not feel anything.
Operation “labyrinth” is a combined surgical intervention, i.e. is performed during another heart operation (for example, CABG, for the correction of heart defects), so the time of the procedure cannot be specified precisely; it is different in each specific case, depending on the nature of the operation. On average, the duration is from 2 to 4 hours. In any case, it feels like it lasts a few seconds for the patient.
Forecast
The prognosis is favorable. According to various estimates, sinus rhythm is restored in 88% to 98% of cases. Approximately 2% of patients require postoperative use of antiarrhythmic drugs. The fatal outcome, according to various authors, ranges from 1% to 16%, with an average of approximately 7.5%. In the long-term prognosis, the study revealed two main complications:
• Development of sinus node dysfunction, which required implantation of a pacemaker or, in milder cases, restriction of patients in physical activity. • Postoperative left atrial dysfunction.