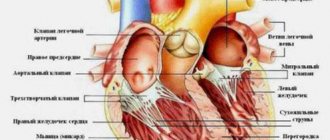
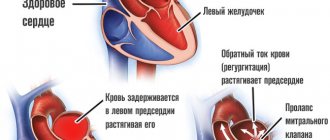
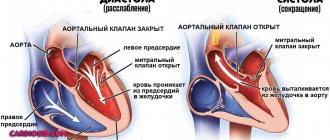
The left atrial appendage is a muscular bursa connected by a lumen to the left atrium and is part of the normal anatomy of the heart. However, in most cases, it is the left atrial appendage that is the main source of blood clots and thrombotic complications in patients with atrial fibrillation.
Atrial fibrillation (AF) is a major risk factor for the formation of blood clots (thrombi), which can block blood flow to the brain and lead to cerebral infarction (stroke).
How the left atrial appendage is associated with stroke in patients with AF
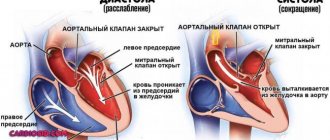
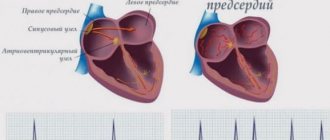
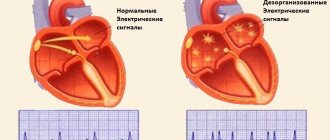
With AF, disruptions occur in the conduction system of the heart and irregular electrical impulses occur in the upper parts of the heart (atria), which leads to their trembling and irregular contraction. Irregular heartbeats lead to decreased blood flow, rapid heart rate, difficulty breathing, and shortness of breath. These irregular heartbeats lead to an increased risk of developing blood clots. The left atrial appendage has a long, tubular shape and connects to the left atrium. During AF, blood can pool in the atrial appendage and lead to the formation of blood clots. When the heart rhythm returns to normal, these blood clots can fly out of the appendage into the left atrium and then travel through the blood throughout the body, causing blockage of arteries in the brain and leading to the development of a stroke.
Uniqueness of the operation
Surgically isolated left atrial appendage has an advantage over conservative treatment methods in patients with persistent or paroxysmal atrial fibrillation. After surgery, 70% of patients maintain sinus heart rhythm during a long observation period. Clinical signs of the disease are significantly reduced, and overall well-being improves.
This operation is unique and is performed in only a few institutions in Russia. In our center, this operation is performed by the head of the department of x-ray surgical methods of diagnosis and treatment - Sergey Vladimirovich Korolev.
Specialists of the cardiac surgery department of the Federal Scientific and Clinical Center of the Federal Medical and Biological Agency successfully use advanced methods of treating cardiac arrhythmias. Our clinic performs unique minimally invasive operations, as well as high-quality diagnostics of various forms of arrhythmia, coronary heart disease and other cardiovascular diseases.
Our center provides services for mammary coronary bypass surgery, thoracoscopic isolation of the ostia of the pulmonary veins, the posterior wall of the left atrium and suturing of the appendage. As a result of these procedures, hundreds of patients annually manage to prevent a number of serious complications: such as stroke, heart attack, heart failure.
You can obtain more detailed information about other methods of treating cardiovascular diseases at the Cardiology Center of the Federal Scientific and Clinical Center of the Federal Medical and Biological Agency of Russia.
How is left atrial appendage occlusion performed?
The procedure is performed using an endovascular, minimally invasive technique in a cath lab. By puncturing the femoral vein (usually on the right), the x-ray surgeon inserts a thin, flexible and long tube (catheter) into the right side of the heart. Next, puncture of the interatrial septum is performed and special instruments are carried out to the mouth of the left atrial appendage. During the entire procedure, X-ray images, as well as transesophageal echocardiography (TEE-CG) data, are used to monitor the implementation of instruments and their correct placement in the cavities of the heart.
Thoracoscopic isolation of the left atrium
This type of operation is performed in situations where traditional catheter ablation is impossible or ineffective. The impact on the heart is carried out through the inserted thoracoscope. Through 6-8 punctures in the chest area (symmetrically on the right and left), electrodes are inserted. High-frequency current destroys the source of arrhythmia. At the same time, in case of a blood clot, the left atrial appendage is sutured to prevent re-formation of clots, as well as reduce the risk of strokes and heart attacks.
The procedure is performed under anesthesia. Its distinctive features:
- high efficiency (75-96%);
- low morbidity;
- no need for artificial blood circulation and long-term hospitalization;
- short rehabilitation period, low risk of complications;
- rare relapses (less than with other methods).
A relative contraindication for treatment is severe heart disease (decompensated ischemic disease, valvular pathology), requiring more extensive surgical interventions.
Preparing for the study
Before the procedure, a consultation with a cardiologist and/or a neurologist is necessary, who will tell you in detail about all stages of the study, possible results and complications. A detailed allergy history is also collected to determine if there is an allergy to medications and/or contrast used during the procedure.
Your doctor will tell you which medications you are taking to stop taking on the day of your procedure. The patient should not independently decide to stop taking medications and can do this only after agreement with the cardiologist. It is advisable to avoid taking liquids and food several hours before the procedure.
What is the Amplatzer Cardiac Plug Occluder?
The Amplatzer Cardiac Plug is a device specifically designed for non-surgical, low-impact closure of the left atrial appendage.
The device is folded into a thin catheter (~4 mm in diameter) and delivered folded to the left atrial appendage. Next, the occluder is released from the catheter and takes on the shape as shown in the figure.
The occluder is securely fixed to the delivery cable and, if necessary, the x-ray surgeon can repeatedly remove the occluder again into the lumen of the catheter until he is sure that the occluder is securely fixed in the cavity of the ear. Only after this is the occluder disconnected from the delivery device.
Amplatzer Cardiac Plug is manufactured at the plant in Minnesota (USA) from a special alloy Nitinol (nickel-titanium alloy). Nitinol is absolutely not susceptible to corrosion, its strength exceeds both titanium and steel, and also has a special property of “shape memory”, when, when straightened, it acquires its original shape as shown in the figure.
Lung vessels
The pulmonary trunk (truncus pulmonalis) with a diameter of 30 mm emerges from the right ventricle of the heart, from which it is delimited by its valve. The beginning of the pulmonary trunk and, accordingly, its opening are projected onto the anterior chest wall above the place of attachment of the cartilage of the third left rib to the sternum. The pulmonary trunk is located anterior to the remaining large vessels of the base of the heart (aorta and superior vena cava). To the right and behind it is the ascending aorta, and to the left is the left ear of the heart. The pulmonary trunk, located in the pericardial cavity, is directed in front of the aorta to the left and posteriorly and at the level of the IV thoracic vertebra (cartilage of the II left rib) is divided into the right and left pulmonary arteries. This place is called the bifurcation of the pulmonary trunk (bifurcatio tninci pulmonalis). Between the bifurcation of the pulmonary trunk and the aortic arch there is a short arterial ligament (ligamentum arteriosum), which is an overgrown arterial duct (ductus arteriosus).
The right pulmonary artery (a.pulmonalis dextra) with a diameter of 21 mm follows to the right to the gate of the right lung behind the ascending aorta and the terminal section of the superior vena cava and anterior to the right bronchus. In the region of the right lung hilum, in front of and under the right main bronchus, the right pulmonary artery divides into three lobar branches. Each lobar branch in the corresponding lobe of the lung is in turn divided into segmental branches. In the upper lobe of the right lung, there is an apical branch (r.apicalis), posterior descending and ascending branches (rr.posteriores descendens et ascendens), anterior descending and ascending branches (rr.anteriores descendens et ascendens), which follow into the apical, posterior and anterior segments of the right lung.
The branch of the middle lobe (rr.lobi medii) is divided into two branches - lateral and medial (r.lateralis et r.medialis).
These branches go to the lateral and medial segments of the middle lobe of the right lung. The branches of the lower lobe (rr.lobi inferioris) include the upper (apical) branch of the lower lobe [r.superior (apicalis) lobi inferioris], heading to the apical (upper) segment of the lower lobe of the right lung, as well as the basal part (pars basalis). The latter is divided into 4 branches: medial, anterior, lateral and posterior (rr.basales medialis, anterior, lateralis et posterior). They carry blood to the basal segments of the same name in the lower lobe of the right lung.
The left pulmonary artery (a.pulmonalis sinistra) is shorter and thinner than the right, passes from the bifurcation of the pulmonary trunk along the shortest path to the gate of the left lung in the transverse direction in front of the descending aorta and left bronchus. On its way, the artery crosses the left main bronchus, and at the hilum of the lung is located above it. According to the two lobes of the left lung, the pulmonary artery is divided into two branches. One of them breaks up into segmental branches within the upper lobe, the second - the basal part - with its branches supplies blood to the segments of the lower lobe of the left lung.
The branches of the upper lobe (rr.lobi superioris) are sent to the segments of the upper , which give off the apical branch (r.apicalis), anterior ascending and descending (rr.anteriores ascendens et descendens), posterior ( r.posterior) and lingual r .lingularis) branches. The superior branch of the lower lobe (r.superior lobi inferioris), as in the right lung, follows into the lower lobe of the left lung, to its upper segment. The second lobar branch - the basal part (pars basalis) is divided into four basal segmental branches: medial, lateral, anterior and posterior (rr.basales medialis, lateralis, anterior et posterior), which branch in the corresponding basal segments of the lower lobe of the left lung.
In the lung tissue (under the pleura and in the area of the respiratory bronchioles), small branches of the pulmonary artery and bronchial branches extending from the thoracic aorta form systems of interarterial anastomoses. These anastomoses are the only place in the vascular system in which blood can move along a short path from the systemic circulation directly to the pulmonary circulation.
The figure shows the arteries corresponding to the segments of the lungs.
Right lung
Upper lobe
- apical (S1);
- rear (S2);
- anterior (S3).
Average share
- lateral (S4);
- medial (S5).
Lower lobe
- upper (S6)
- ;mediobasal (S7);
- anterobasal (S8);
- lateralobasal (S9);
- posterobasal (S10).
Left lung
Upper lobe
- apical-posterior (S1+2);
- anterior (S3);
- upper reed (S4);
- lower reed (S5).
Lower lobe
- upper (S6);
- anterobasal (S8);
- lateralobasal, or laterobasal (S9);
- posterobasal (S10).
PULMONARY VEINS
Venules begin from the capillaries of the lung, which merge into larger veins and form two pulmonary veins in each lung.
Of the two right pulmonary veins, the upper one has the larger diameter, since blood flows through it from the two lobes of the right lung (upper and middle). Of the two left pulmonary veins, the inferior vein has the larger diameter. At the gates of the right and left lungs, the pulmonary veins occupy their lower part. In the posterior upper part of the root of the right lung is the main right bronchus, anterior and inferior to it is the right pulmonary artery.
- LVLV - left superior pulmonary vein
- RSPV - right superior pulmonary vein
- ILV - inferior pulmonary vein
- RPA - right pulmonary artery
- LPA - left pulmonary artery
At the top of the left lung is the pulmonary artery, posterior and inferior to it is the left main bronchus. In the right lung, the pulmonary veins lie below the artery, follow almost horizontally and on their way to the heart are located behind the superior vena cava, the right atrium and the ascending aorta. Both left pulmonary veins, which are somewhat shorter than the right ones, are located under the left main bronchus and are also directed to the heart in the transverse direction, anterior to the descending aorta. The right and left pulmonary veins, perforating the pericardium, flow into the left atrium (their terminal sections are covered with the epicardium).
What happens after the occluder installation procedure
Because the occluder procedure is minimally invasive, recovery is likely to be quick and easy. Many patients are discharged from the hospital within the next 48 hours, with subsequent medication recommendations to continue treatment and recovery in an outpatient setting. It is necessary to conduct a control TEE-CG 3 and 6 months after installation of the occluder to monitor the process of endothelialization of the installed device. Endothelialization is the growth of the occluder with connective tissue and, in fact, its ingrowth into the wall of the heart. This is a normal and desirable process. In 99% of cases, complete endothelialization of the occluder occurs within several months. The patient returns to his normal lifestyle within the first month.
Is it possible to travel with an implanted device? Will there be problems going through the metal detector at airport security?
The metal parts of the Amplatzer Cardiac Plug are very small and will not normally trigger an alarm in an airport metal detector frame. However, for your comfort and peace of mind, you will be given a special card confirming the fact that the occluder has been installed.
Will an MRI interfere with or disrupt the occluder?
Most modern devices do not in any way affect the operation of the occluder, and the presence of an occluder does not affect the operation of the devices. However, it is best to alert staff to the presence of implanted devices before undergoing any medical procedure. Magnetic resonance imaging (MRI) is acceptable and the Amplatzer Cardiac Plug will not affect the performance of an MRI in any way, even at 3 Tesla. It is necessary to inform the staff of the MRI department about the presence of an implant.
Is the procedure possible for pregnant women and nursing mothers?
The risk of exposure of a child to X-ray radiation and the benefits of treatment must be weighed, and the correct and most effective tactics must be adopted. If it is necessary to implant a device during pregnancy, all possible measures will be taken to minimize radiation exposure to the fetus and mother.
There is no evidence of the effect of installing an occluder on the lactation process in nursing mothers.
Rehabilitation period after installation of an occluder in the left atrial appendage
Intensive observation and monitoring is carried out for 6 hours after implantation:
- blood pressure;
- heart rate;
- blood oxygen saturation;
- neurological status.
The patient is discharged from the hospital provided hemodynamics are stable and there is no risk of complications. At the same time, during the rehabilitation period it is important to control the process of implantation of the occluder into the tissue. During the first week, the remaining elements of the ear are thrombosed. Endothelialization then occurs over several weeks. Scarring is completely completed after six months.
Until complete healing, all patients must continue treatment with anticoagulant drugs. The regimen and combination of drugs are selected individually, taking into account individual indications and contraindications. Discontinuation of medications in this group is carried out under the supervision of a doctor and depends on the quality of the closure of the ear. Only after scarring is complete, patients are transferred to continuous use of aspirin (at a dose of 75 mg).
Transesophageal ultrasound is performed on the 45th day and six months later. If a hole is detected, coagulants are continued with subsequent observation and examination every 6 months until the fistula is completely closed.
Possible complications associated with the Amplatzer Cardiac Plug installation procedure
There are some potential risks associated with the placement of an occluder, as well as additional risks associated with the vein puncture procedure itself. It is necessary to consult with a radiologist about the possible risks of implanting the device.
Potential risks include, but are not limited to the following:
- air embolism (an air bubble that can move through the vessels and block the work of some of them);
- allergic reactions to contrast;
- allergic reaction to anesthetic drugs;
- cardiac arrhythmias (the occurrence of irregular heart rhythms);
- bleeding;
- heart failure;
- cardiac tamponade (rupture of the heart muscle);
- death;
- fever;
- hypertensive or hypotensive reactions of the body;
- infections;
- multiple organ failure;
- myocardial infarction (heart attack);
- perforation of the heart cavity or vessel;
- pericarditis (excess fluid in the pericardial sac);
- renal failure/renal dysfunction;
- cerebrovascular accidents (temporary or permanent);
- arterial thrombosis;
- valvular regurgitation or insufficiency.