The heart is a muscular pump that ensures continuous movement of blood through the vessels. Together, the heart and blood vessels make up the cardiovascular system. This system consists of the systemic and pulmonary circulation. From the left side of the heart, blood first moves through the aorta, then through large and small arteries, arterioles, and capillaries. In the capillaries, oxygen and other substances necessary for the body enter the organs and tissues, and from there carbon dioxide, metabolic products, are removed. After this, the blood turns from arterial to venous and again begins to move towards the heart. First along the venules, then through smaller and larger veins. Through the inferior and superior vena cava, blood again enters the heart, only this time into the right atrium. A large circle of blood circulation is formed.
Venous blood from the right side of the heart is sent through the pulmonary arteries to the lungs, where it is enriched with oxygen, and returns to the heart again - this is the pulmonary circulation.
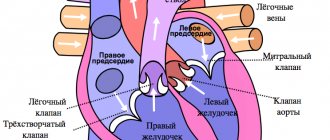
Inside, the heart is divided by partitions into four chambers. The two atria are divided by the interatrial septum into the left and right atria. The left and right ventricles of the heart are separated by the interventricular septum. Normally, the left and right parts of the heart are completely separate. The atria and ventricles have different functions. The atria store blood that flows into the heart. When the volume of this blood is sufficient, it is pushed into the ventricles. And the ventricles push blood into the arteries, through which it moves throughout the body. The ventricles have to do more hard work, so the muscle layer in the ventricles is much thicker than in the atria. The atria and ventricles on each side of the heart are connected by the atrioventricular orifice. Blood moves through the heart in only one direction. In the systemic circle of blood circulation from the left side of the heart (left atrium and left ventricle) to the right, and in the small circle from the right to the left.
The correct direction of blood flow is ensured by the valve apparatus of the heart:
Valves:
- tricuspid
- pulmonary
- mitral
- aortic
They open at the right time and close, preventing blood flow in the opposite direction.
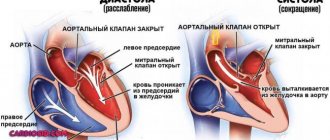
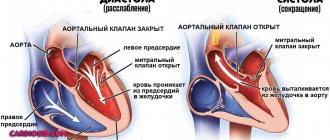
Aortic valve
Closes the entrance to the aorta. It also consists of three valves, which look like crescents. Opens when the left ventricle contracts. In this case, blood enters the aorta. When the left ventricle relaxes, it closes. Thus, venous blood (poor in oxygen) from the superior and inferior vena cava enters the right atrium. When the right atrium contracts, it moves through the tricuspid valve into the right ventricle. Contracting, the right ventricle ejects blood through the pulmonary valve into the pulmonary arteries (pulmonary circulation). Enriched with oxygen in the lungs, the blood turns into arterial blood and moves through the pulmonary veins to the left atrium, then to the left ventricle. When the left ventricle contracts, arterial blood enters the aorta through the aortic valve under high pressure and spreads throughout the body (systemic circulation).
The heart muscle is called the myocardium
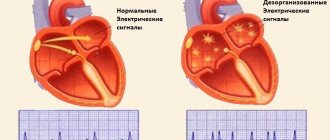
There are contractile and conductive myocardium. The contractile myocardium is the actual muscle that contracts and produces the work of the heart. In order for the heart to contract in a certain rhythm, it has a unique conduction system. The electrical impulse to contract the heart muscle occurs in the sinoatrial node, which is located in the upper part of the right atrium and spreads through the conduction system of the heart, reaching every muscle fiber
First, both atria contract, then both ventricles, thereby ensuring the flow of blood to all organs and tissues of the body. The heart muscle has two membranes (external and internal). The inner lining of the heart is called the endocardium. The outer lining of the heart is called the pericardium.
CT scan of the left atrium with assessment of the pulmonary veins shows:
- heart muscle;
- thrombosis of the left atrial appendage;
- left atrial myxoma and developmental anomalies of the pulmonary venous system;
- coronary vessels of the heart;
- main vessels (aorta, pulmonary trunk) and their branches.
The method is fast and accessible; high quality visualization of blood vessels and tissues of the heart; layer-by-layer viewing of structures in all planes; non-invasiveness; the ability to create a 3D model of the vessels and chambers of the heart.
MSCT of the left atrium with assessment of the pulmonary veins is performed to assess the anatomy of the left atrium; calculation of left atrium parameters; assessment of the anatomy of the pulmonary veins of the heart; calculation of pulmonary vein parameters
Conduction system of the heart
The heart, like any organ, has its own nervous system. The nervous system of the heart has several levels. The first and main pacemaker of the heart is the sinus node, located in the right atrium. It is subject to the atrioventricular node, which is located on the border between the atria and ventricles and quite often slows down the heart rate set by the sinus node. Then the nerve impulse goes to the ventricles of the heart along the branches of the Hiss bundle, which are divided into the smallest nerve endings - Purkinje fibers.
How to prepare for MSCT of the left atrium with assessment of the pulmonary veins?
The last meal should be taken 3-4 hours before MSCT. If necessary, you can take the medicine with water. You must arrive for the procedure half an hour before the procedure, as it will take time to install a catheter in a peripheral vein in order to administer a contrast agent intravenously. In addition, the patient fills out a questionnaire.
Since the procedure involves the use of a contrast agent, it is necessary to determine the level of urea and creatinine in the blood. The patient should also have the results of a biochemical blood test with him.
If the patient is taking beta blockers, the doctor should be told about this. Medicines can be used when the heart rate becomes more than 60 beats per minute to slow it down. Otherwise, if interference occurs, it is impossible to conduct research.
To increase the accuracy of MSCT, the doctor needs to familiarize himself with the results of ultrasound, CT or MRI if the patient has previously undergone these procedures. In addition, the patient must remove objects containing metal from the chest area.
The procedure is contraindicated for pregnant women; people with severe calcification of the coronary arteries, intractable arrhythmia, tachycardia, allergy to iodine, renal failure, severe diabetes mellitus, thyroid diseases. There are also relative contraindications for MSCT associated with taking metformin-containing drugs and nephrotoxic drugs; a person's inability to hold their breath; inappropriate behavior; overweight.
Publications in the media
Anomalous pulmonary vein drainage (ADPV) is a congenital heart disease characterized by the drainage of all pulmonary veins into the right atrium or the main veins of the systemic circulation. Thus, total ADLV or partial ADLV are distinguished.
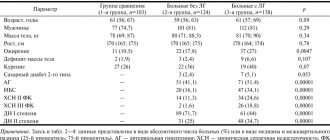
Statistical data • Total ADLV makes up 2.6% of all CHDs, partial ADLV - 1.5% • The combination of total ADLV with atrial septal defect (ASD) occurs in 0.7–9% of all CHDs, partial ADLV with ASD - in 5, 7% • The right lung is most often abnormally drained (97.2%) • The most common type of ADLV is supracardiac (75% partial and 45% total ADLV).
Etiology: factors that form congenital heart disease (see Tetralogy of Fallot).
Pathogenesis • Changes in hemodynamics with partial ADPV are similar to those with ASD (see Atrial septal defect) and almost do not depend on the anatomical type of the defect (see below) • With total ADPV, blood from all veins of the body enters the right atrium, and the child’s life is impossible without the presence of communication between the systemic and pulmonary circulation • Most often, such a shunt is an open foramen ovale or a true secondary ASD, the size of the latter determines the speed of development and severity of pulmonary hypertension and, therefore, the prognosis in the natural course.
Classification of anatomical types of partial ADLV
• Supracardial type - drainage of individual pulmonary veins or their common collector into the superior vena cava or its tributaries •• Right superior pulmonary vein ® superior vena cava •• Accessory right superior pulmonary vein ® superior vena cava •• Left superior pulmonary vein ® left brachiocephalic vein •• Left superior pulmonary vein ® left brachiocephalic vein ® right atrium (separate from the superior vena cava) •• Right superior pulmonary vein + right inferior pulmonary vein ® accessory superior vena cava
• Cardiac type - drainage of individual pulmonary veins or their common collector into the right atrium or coronary sinus •• Right superior pulmonary vein + right inferior pulmonary vein ® right atrium (isolated or in combination with ASD) •• Left superior pulmonary vein + left inferior pulmonary vein ® coronary sinus
• Subcardial type - drainage of individual pulmonary veins or their common collector into the inferior vena cava •• Right inferior pulmonary vein ® inferior vena cava •• Left inferior pulmonary vein ® inferior vena cava
• Mixed type.
Clinical picture and diagnosis. Diagnosis is almost entirely based on these instrumental methods, since the pathogenesis and clinical picture of the defect are identical to those of ASD, and also due to the frequent combination of ASD and ADLV.
Complaints: see Atrial septal defect.
Objectively: see Atrial septal defect.
Instrumental diagnostics
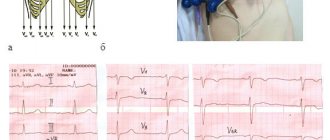
• ECG •• Signs of hypertrophy and overload of the right sections •• Most often, the ECG is normal.
• X-ray of the chest organs •• Strengthening the pulmonary pattern •• Bulging of the arches of the pulmonary artery and the right chambers of the heart •• In the most common variant of ADPV (drainage of the right superior pulmonary vein into the superior vena cava), the expansion of the shadow of the lower segment of the superior vena cava and the root of the right lung is determined •• With the supracardial type of total ADLV, radiological data are highly specific ••• With the most common variant of total ADLV (drainage of the common collector into the left brachiocephalic vein or accessory superior vena cava), the shadow of the heart has a configuration resembling the number 8, or the figure of a snow woman (Snellen sign - Albers) ••• Total ADLV into the superior vena cava leads to an aneurysm of its central section, the latter is visible in the form of a local protrusion along the right contour of the shadow of the vascular bundle •• Cardial and subcardial types of ADLV do not have specific signs •• Sometimes a homogeneous spotting of the pulmonary pattern resembles a picture miliary pulmonary tuberculosis •• When the right inferior pulmonary vein flows into the inferior vena cava in the anteroposterior projection, the shadow of an abnormally running vessel is visualized, resembling the shape of a “Turkish saber” (symptom of the “Turkish saber”).
• EchoCG •• Absence of the orifices of the pulmonary veins in the left atrium •• Diagnosis of the defect is practically based only on the registration of pathological flows from the pulmonary veins according to the study in the color Doppler mapping mode •• EchoCG is a highly sensitive method for diagnosing ADPV, but in most cases the anatomical variant of ADPV is diagnosed erroneously or incompletely •• All adults and children of the older age group undergo transesophageal echocardiography •• For the rest, see Atrial septal defect.
• Radionuclide angiocardiography (first pass method or equilibrium) •• Registration of pathological flow from the pulmonary veins and its quantitative assessment •• Diagnosis of concomitant dysfunction of the contractile function of the right ventricle.
• Probing of the cavities of the heart •• Pressure in the cavities of the heart is usually within normal limits or slightly increased in the right atrium •• With the supracardial type of APPV - increased pO2 and blood oxygen saturation in the superior hollow; in the case of subcardial - in the inferior vena cava •• Changes in blood oxygen saturation in the right chambers of the heart are not specific for ADLV •• The most specific sign is passing a probe from the inferior vena cava or right atrium into the pulmonary vein •• Diagnosis of concomitant ASD - see Atrial septal defect .
• Right atriography and ventriculography, phlebography of the superior vena cava, left atriography, angiopulmonography •• Receipt of contrast that has passed through the pulmonary circulation into the vena cava or right atrium (practically the only sensitive method for diagnosing total ADPV) •• The most specific sign is that the contrast of the orifice is abnormal drained pulmonary vein when a catheter is inserted into its lumen •• Diagnosis of concomitant ASD - see Atrial septal defect.
Drug therapy: not developed, there is no need for the prevention of infective endocarditis.
Surgery
• Indications: see Atrial septal defect.
• Contraindications: see Atrial septal defect.
• Methods of surgical treatment •• In case of partial ADPV - complete anatomical correction of the defect through numerous options for replantation of the mouths of the pulmonary veins, their common collectors, additional superior vena cava or coronary sinus draining the pulmonary veins into the left atrium •• Elimination of concomitant ASD - see Defect interatrial septum •• With the cardiac variant of partial ADLV and with total ADLV - the creation of an artificial or expansion of a natural ASD with subsequent isolation using an autopericardial or synthetic patch of the created defect and the mouths of abnormally drained veins into the left atrium.
Specific postoperative complications: sick sinus syndrome, increased pulmonary venous hypertension with inadequate provision of outflow tracts from the pulmonary veins.
Prognosis • With partial ADPV is similar to that with ASD (see Atrial septal defect) • With total ADPV, less than 50% of children survive to 3 months and less than 20% to 1 year • Patients who survive the first few weeks usually have severe cardiomegaly, cardiac insufficiency and pulmonary hypertension • Patients who survive up to 1 year without surgery usually have very large ASDs and survive until 10–20 years, after which some of them develop irreversible pulmonary hypertension • Presence of obstruction in the outflow tract from abnormally draining pulmonary veins reduces life expectancy by 3.5-5 times • Overall hospital mortality with surgical correction of total ADLV - 6-25% (on average 13%), partial ADLV - 2-4% • Hospital mortality with radical correction of subcardial type of ADLV in newborns - 30–40%, cardiac and supracardial - 5–10% • In 85% of patients, complete correction of hemodynamics occurs within 1 year after surgical treatment of partial ADLV • Long-term mortality after correction of total ADLV almost completely depends on the immediate (hospital) period, subsequent lethal cases are caused by other diseases.
Synonyms: Abnormal drainage of the pulmonary veins; Anomaly of the connection of the pulmonary veins; Complete anomalous drainage of the pulmonary veins; Incomplete anomalous drainage of the pulmonary veins.
Abbreviations. APLV is anomalous drainage of the pulmonary veins.
ICD-10 • Q26 Congenital anomalies [malformations] of large veins