Mandeep Singh, MD; David R. Holmes, Jr, MD; A. Jamil Tajik, MD; and Gregory W. Barness, MD
Nearly 8 million people in the United States have CHD. Unfortunately, the number of patients with coronary artery disease in whom surgical or endovascular interventions cannot be performed continues to grow. For such patients, when drug therapy is ineffective, EECP has become a new, non-invasive, outpatient treatment method that improves the quality of life, reducing the number of episodes of myocardial ischemia, allowing for an increase in the level of physical activity. We report the case of a 56-year-old woman suffering from severe coronary artery disease despite maximum possible medical therapy, who underwent EECP sessions because other types of revascularization were not possible. After a course of EECP therapy, she had a significant decrease in episodes of angina pectoris and complete disappearance of signs of myocardial ischemia according to myocardial stress scintigraphy. This case confirms that EECP is a safe and effective treatment for reducing symptoms of myocardial ischemia in patients for whom standard percutaneous or surgical myocardial revascularization is not an option.
CABG - coronary artery bypass grafting; IHD; ECG - electrocardiography; EECP = Enhanced external counterpulsation.
Standard therapy for patients with symptomatic CAD currently includes pharmacological treatment (β-blockers, calcium channel blockers, nitrates), percutaneous coronary intervention, and surgical revascularization. Limitations of each of these strategies include procedure-related mortality and morbidity, drug side effects, restenosis after percutaneous interventions, and time-dependent graft failure after coronary artery bypass graft surgery. And although most patients are indicated for drug therapy, many of them do not experience complete disappearance of the symptoms of the disease, only with the use of drugs. In addition, there is an increasing number of patients who are not suitable candidates for traditional revascularization procedures. Severe damage to the distal coronary bed limits the possibility of revascularization. Among patients who undergo revascularization and require repeat procedures, the mortality and morbidity associated with repeat procedures is significantly higher, which is often a reason to exclude them from candidates for repeat revascularization procedures. Therefore, these patients do not experience optimal symptomatic relief.
Recently, therapeutic options have been expanded to include transmyocardial revascularization or percutaneous laser myocardial revascularization, minimally invasive CABG surgery, and transcutaneous electrical nerve stimulation. A variety of therapeutic approaches have also been used, including the use of growth factors.
Newer treatments are still in experimental development or have had very limited clinical studies. Many new devices are used invasively, which is associated with a significant risk of complications. Enhanced external counterpulsation (EECP) has been introduced as a noninvasive, nontraumatic procedure for the outpatient treatment of patients with CAD. EECP increases perfusion pressure in the coronary arteries as a result of diastolic enlargement and reduces myocardial oxygen demand. We present a case report of a patient who was treated with EECP, as well as a review of the literature and information regarding the current state of EECP technology.
Disease history
A postmenopausal woman, 56 years old, suffering from hypertension, hyperlipidemia, and with a 10-year history of insulin-dependent diabetes mellitus, with refractory angina pectoris class IV, despite the use of optimal antianginal therapy, including diltiazem (slow-releasing form) 300 mg/day; atenolol, 100 mg/day; oral nitrates, 60 mg/day. Since 1996, she suffered from severe coronary artery disease with attacks of typical angina pectoris; she had previously undergone coronary angiography with percutaneous intervention on the right coronary artery. Due to persistent angina and severe coronary artery disease, she underwent CABG surgery in July 1996, with mammarocoronary bypass grafting of the anterior descending coronary artery (LAD) and venous bypass grafting of the first diagonal branch, the intermediate artery, and the right coronary artery.
After 1 year, angina pectoris recurred. Angiography revealed a lesion of the left artery trunk and a 3-vessel lesion with occlusion of venous shunts. The shunt to the RCA was well patent, 80% of the narrowing of the venous shunt into the RCA was eliminated by stenting.
2 months after the procedure, the patient developed a clinical picture of progressive angina pectoris between exertion and rest, and within a year of coronary intervention, she was hospitalized with a diagnosis of unstable angina. She complained of frequent attacks of angina pectoris, which occurred both at rest and with minimal physical activity. She took 1 to 4 sublingual nitroglycerin tablets daily. During exercise (ATP) scintigraphy of the myocardium with the waist, a decrease in blood pressure during exercise was detected, accompanied by chest pain and changes in the ECG.
Defects in perfusion of the apical, inferior and inferoseptal segments with a decrease in myocardial reserve were revealed. Repeated coronary angiography revealed 50% stenosis in the distal part of the LCA and 75% stenosis in the proximal third of the LAD. The disease progressed in both venous grafts and coronary arteries, including both severe LAD disease, distal graft insertion sites, and the RCA. The shunt to the RCA artery was occluded. Echocardiography showed normal left ventricular systolic function. A repeat revascularization procedure was not considered due to severe distal lesions. Despite active drug treatment with aspirin, nitrates, angiotensin-converting enzyme blockers, beta-blockers, statins, vitamins E and C, the patient continued to complain of frequent attacks of resting and low-tension angina. Further intensification of drug therapy was impossible due to the fact that blood pressure was at a borderline level (90/70 mmHg) and sinus bradycardia was observed. Therefore, the patient was referred to EECP sessions.
Material and methods
In the period from 2007 to 2013, at the Department of Cardiac Surgery No. 2 of the Rostov Regional Clinical Hospital (Rostov-on-Don), more than 5,000 coronary bypass surgeries were performed, of which 60 patients underwent CABG in combination with TMLR. Informed consent was obtained from each patient. The criteria for inclusion in the study were diffuse lesions of the coronary artery, atherosclerotic changes in the distal bed or the presence of small coronary arteries unsuitable for bypass surgery, and preservation of viable myocardium in the surgical area. If direct revascularization of at least one coronary artery was possible, CABG was performed, which was supplemented by TMLR in those areas that were not accessible to revascularization. Exclusion criteria were severe chronic obstructive pulmonary disease (forced expiratory volume in 1 s <55% of the predicted value); acute period of myocardial infarction; severe rhythm disturbances; decompensated heart failure.
The study group consisted of 52 men and 8 women aged from 46 to 81 years (average age 65.9±7.3 years). Most patients ( n
=44) had angina pectoris IV F.K. The general summary characteristics of the patients are presented in Table. 1.
Table 1. General characteristics of patients Note.
LVEF - left ventricular ejection fraction, MI - myocardial infarction, CHF - chronic heart failure. To objectify the condition, patients underwent coronary angiography and Holter ECG monitoring. Determination of angina class was carried out according to the recommendations of the Canadian Cardiovascular Society and the modified Bruce protocol. Before surgical treatment, patients were unable to perform stress tests (treadmill test) due to severe angina. To diagnose myocardial viability, myocardial scintigraphy and echocardiography were performed. All operations were performed in a standard manner: under conditions of artificial circulation and pharmacocold cardioplegia with Custodiol solution. In all cases, CABG was first performed, then TMLR was performed after the application of shunts and restoration of blood flow in the coronary artery under conditions of artificial circulation (CPB). In all cases, laser revascularization was performed using the domestic ECG-synchronized laser device Perfocor. After positioning the manipulator handle above the surface of the heart, a pulse was applied, synchronized with the patient’s ECG relative to the R wave. Penetration of the entire thickness of the myocardium using a 500 W CO2 laser occurred in one pulse, which was confirmed by the appearance of a pulsating stream of blood from the transmyocardial channel, as well as by transesophageal echocardiography data in in the form of gas bubbles in the cavity of the L.Zh. In this case, no rhythm disturbances occurred, and bleeding from the canal stopped on its own or by pressing with a gauze pad or napkin for 2 minutes. An active drain was installed in the pleural cavity, and the chest was sutured using standard techniques.
In all patients, in-hospital, long-term and overall mortality rates were assessed, as well as the dynamics of such clinical parameters as angina FC, the need for nitroglycerin, exercise tolerance, myocardial contractility, LVEF, myocardial perfusion. The dynamics of quality of life were determined using SF-36 questionnaires, and the psycho-emotional and physical state of patients was assessed at various times after surgery. Survival rates, freedom from recurrence of angina and myocardial infarction in the long term were calculated.
Patients were under clinical observation in the immediate postoperative period (in hospital or 30 days outpatient) and long-term period: 3, 6 and 12 months after surgery and then selectively up to 10 years after surgery (on average 7.5±0.9 years ). At each interval, patients were assessed for major adverse cardiac events, angina class, LVEF, and the need for repeat revascularizations.
In the first hours after surgery, invasive hemodynamic monitoring, ECG monitoring, blood gas composition, acid-base status and biochemical parameters, including enzyme markers of myocardial necrosis, were carried out. Analysis of the ECG in the first hours after TMLR showed that, despite the effect of the laser on the myocardium, there was no evidence of myocardial damage or ischemia.
EECP treatment protocol
All potential patients to receive EECP sessions had detailed medical documentation, in addition, they underwent a thorough physical examination, during which attention was paid to symptoms of the disease, previous revascularization procedures, and documentation confirming CAD (Table 1). Special precautions were taken to identify whether patients had any contraindications to this procedure (Table 2).
The principle of operation of EECP is to sequentially fill and deflate air from cuffs wrapped around the patient's calves and thighs. The inflation and deflation of air in the cuffs is synchronized with the cardiac cycle, and is controlled by ECG signals, which are processed by a microprocessor (Fig. 1). As a result of compression of the muscles of the lower extremities, diastolic pressure in the aorta increases, which leads to an increase in perfusion pressure in the coronary arteries. Sessions usually last 1 hour per day, the total course consists of 35 hours over 7 weeks.
Table 1. Indications for EECP treatment
| Symptoms of myocardial ischemia (corresponding to FC III-IV), despite optimal drug therapy. The patient is not a candidate for surgical or percutaneous revascularization. IHD is confirmed by the presence of: stenosis >70% in at least 1 of the main epicardial coronary arteries and/or bypass, or scintigraphic or echocardiographic evidence of myocardial infarction and/or myocardial ischemia, or a history of myocardial infarction. |
Table 2. Relative contraindications to EECP
| Persistent, persistent ischemia. Decompensated congestive heart failure. Severe pathology of the valve apparatus. Uncontrolled arterial hypertension (> 180/110 mmHg). Malignant arrhythmias. Severe pathology of peripheral vessels. A history of phlebitis, deep venous thrombosis, severe varicose veins, or trophic ulcer. Bleeding diathesis, including continued use of warfarin with INR >2.0. Increased risk of bleeding (within 7 days after angiography or other invasive procedure). Pregnant or fertile women who do not use a reliable method of contraception in order to avoid pregnancy. |
Endovascular diagnostic methods
It is impossible to imagine medicine of the 21st century without endovascular methods of diagnosis and treatment. Currently, X-ray endovascular surgery is a highly effective and low-traumatic method of treatment for coronary disease, vascular and valvular pathology, and congenital heart defects. Endovascular surgery occupies its niche in almost all areas of modern medicine (oncology, urology, gynecology, etc.). The development of endovascular treatment methods promotes close cooperation between endovascular surgeons, cardiologists, cardiac surgeons and other related specialties. Such cooperation led to the development of a new direction - the so-called hybrid surgery.
Over the past decades, the number of clinics engaged in endovascular surgery has increased significantly in our country. The nineties of the 20th century became the heyday of endovascular surgery. Catheter technology has reached a high level of development and is constantly being improved. High-tech innovations appear almost every year. Some of them are destined to remain in the history of interventional cardiology, and some are destined to become the operator’s perfect tool. And only extensive experimental and clinical experience will be able to determine the place of each method in endovascular surgery.
Today, X-ray endovascular interventions represent an alternative to almost any open surgery for pathology of the heart and blood vessels. The range of endovascular interventions performed is enormous.
Federal State Budgetary Institution National Medical Research Center of Sports Sciences named after. A.N. The Ministry of Health of the Russian Federation is one of the pioneers of domestic endovascular surgery. It was here in 1982 that the cornerstone of many schools on endovascular diagnostics and treatment was laid. Today, the departments of the Federal State Budgetary Institution National Medical Research Center for Cardiovascular Agricultural Sciences named after. A.N. The Ministry of Health of the Russian Federation, engaged in the provision of x-ray endovascular medical care, is equipped with the most modern equipment, not inferior to leading clinics in Europe and the USA, including all available methods of intravascular imaging and invasive assessment of intracoronary physiology. Every year, the center performs more than 15,000 diagnostic endovascular interventions and more than 3,000 thousand endovascular operations in patients with coronary heart disease, vascular pathology, congenital and acquired heart defects . The staff of the departments is represented by first-class international specialists who have extensive experience in performing these operations in complex clinical cases.
Cardiac ischemia
Coronary heart disease is an acute or chronic myocardial damage that occurs as a result of a decrease or cessation of arterial blood supply to the heart muscle, which is based on pathological processes in the coronary artery system. IHD is a widespread disease. One of the leading causes of mortality, temporary and permanent disability throughout the world. In the structure of mortality, cardiovascular diseases are in first place, of which IHD accounts for about 40%.
Etiology of IHD
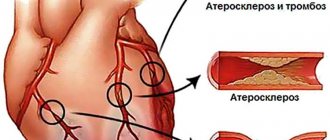
The leading etiological factor in the development of coronary heart disease is atherosclerosis of the coronary arteries. Atherosclerosis develops consistently, in waves and steadily. As a result of the accumulation of cholesterol in the artery wall, an atherosclerotic plaque is formed. Excess cholesterol leads to an increase in plaque size and obstructions to blood flow. Symptoms worsen with the growth of atherosclerotic plaque, which gradually narrows the lumen of the artery. A decrease in the lumen area of the artery by more than 90-95% is critical, causing a decrease in coronary blood flow and deterioration of well-being even at rest.
Clinical picture of IHD
The first description of angina pectoris was offered by the English physician William Heberden in 1772: “... pain in the chest that occurs while walking and forces the patient to stop, especially while walking soon after eating. It seems that this pain, if it continues or intensifies, can take a person’s life...” Usually, symptoms of the disease first appear after 50 years. At first they occur only during physical activity.
The classic manifestations of coronary heart disease are:
- Pain behind the sternum, often radiating to the lower jaw, neck, left shoulder, forearm, hand, back. Often occurs in cold weather.
- The pain is pressing, squeezing, burning, suffocating. The intensity varies.
- Triggered by physical or emotional factors. At rest it stops on its own.
- Lasts from 30 seconds to 5-15 minutes.
- Quick effect of taking nitroglycerin.
Diagnosis of IHD
Selective coronary angiography is considered the “gold standard” in the diagnosis of obstructive lesions of the coronary arteries of the heart.
This X-ray contrast study is used to find out whether the narrowing of the vessel is significant, which arteries and how many of them are affected, in what place and over what extent.
Treatment of coronary artery disease
Treatment of this pathology is primarily aimed at restoring normal blood supply to the myocardium and improving the quality of life of patients. Unfortunately, purely therapeutic (conservative) treatment methods are not always effective. Today, the generally accepted methods of treating coronary artery disease with proven and undeniable effectiveness are surgical revascularization of the myocardium - coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) or “stenting”.
The first coronary artery stenting in humans was performed by J. Puel et al. in March 1986 in Toulouse (France) and almost simultaneously with them U. Sigwart et al. in Lausanne (Switzerland).
Today, in the era of high technologies in the field of pharmaceuticals and medical engineering, modern intracoronary stents make it possible to achieve good results both in the early and late periods after PCI.
This operation is performed under local anesthesia . An artery puncture (radial or femoral) is performed in the wrist or thigh area, an introducer (catheter) is installed, and a guiding catheter is inserted through it along a guide under the control of an angiographic unit.
The catheter is inserted into the left or right coronary artery, and then a series of images are taken. Through the installed catheter, a microconductor is passed into the artery through the affected area. A coronary stent is guided and installed to the affected area via a microconductor.
Today, the range of percutaneous coronary interventions has expanded significantly from the treatment of simple local narrowings to multivessel revascularization of the myocardium (including the trunk of the left coronary artery) and recanalization of chronically occluded (completely closed vessels) coronary arteries. Today, stents with a special bioabsorbable coating have been introduced into clinical practice ,
allowing to reduce the frequency of restenosis (overgrowth) and stent thrombosis, thereby minimizing the risk of recurrent heart attacks in the patient after stenting. Intravascular imaging techniques have also been widely introduced, providing real-time images of the coronary arteries in the patient during surgery, comparable to data obtained using a light microscope. All of the above makes it possible to obtain intraluminal images of the coronary arteries in real time. This allows the surgeon to obtain a “jewelry” result when installing a stent in the coronary vessels.
Intraoperative shuntoraphy
At the Federal State Budgetary Institution National Medical Research Center for Sports Agriculture named after. A.N. , intraoperative shuntography was introduced into routine practice
. Intraoperative bypass grafting is an angiographic study at the final stage of coronary artery bypass surgery. Using anigography, the patency of all placed bypasses is checked directly in the cardiac surgery operating room. If a lesion or malfunction of the shunt is detected, the operating surgeon has the opportunity to eliminate it directly in the operating room, thereby achieving an “ideal” result of a major cardiac surgery.
Federal State Budgetary Institution National Medical Research Center of Sports Sciences named after. A. N. Bakuleva Ministry of Health of the Russian Federation is the only center in the Russian Federation and one of the few centers in the world where intraoperative bypass grafting is performed simultaneously with coronary bypass surgery.
Congenital heart defects (CHD)
The second half of the twentieth century was marked by stunning advances in virtually all aspects of pediatric cardiovascular surgery.
Currently, using X-ray endovascular treatment methods, it is possible to eliminate such congenital heart defects as atrial septal defect (ASD), patent ductus arteriosus (PDA) or ductus Botallus, patent foramen ovale (POO), ventricular septal defect (VSD), coarctation of the aorta. Over the past decade, these interventions have become the “standard” procedure with virtually zero mortality. X-ray endovascular treatment methods are the method of choice for such critical conditions of newborns as critical valvular stenosis of the aorta and pulmonary artery. The implementation of balloon atrioseptostomy, proposed in 1966 by Rashkind and Miller, in newborns with complex congenital heart disease incompatible with life led to a sharp decrease in mortality among this severe category of patients.
Today, at the Federal State Budgetary Institution National Medical Research Center for Cardiovascular Agricultural Sciences named after. A.N. Bakulev Ministry of Health of the Russian Federation performs a full range of endovascular interventions for congenital heart defects in patients of all age groups,
including in newborns with critical malformations of the heart and blood vessels.
Many operations were performed for the first time in world practice, and the developed techniques are unique.
The most common are patent ductus arteriosus, atrial septal defect, coarctation of the aorta, valvular stenosis of the aorta and pulmonary artery.
Coarctation of the aorta
Coarctation of the aorta is a congenital narrowing of the aorta in the area of transition of the arch into the descending part of the aorta, and sometimes in the thoracic or abdominal region. The frequency of this defect ranges from 6.3% to 15% among all congenital heart defects (CHD). The defect is rarely isolated; more often it is combined with other congenital defects.
Clinic
Patients complain of dizziness, heaviness and headache, increased fatigue, nosebleeds, possible pain in the heart, as well as weakness and pain in the lower extremities, cramps in the leg muscles, and chilly feet. Women may experience menstrual irregularities and infertility. On examination, good physical development is noted with a disproportion of the muscular system: the muscles of the upper half of the body are hypertrophied with relative hypotrophy of the muscles of the pelvis and lower extremities, the legs are cold to the touch. When palpating the intercostal arteries, their increased pulsation is noted.
Of decisive importance in the diagnosis of aortic coarctation is determining the nature of the pulse in the arms and legs. There is no or sharply weakened pulsation in the femoral arteries, and at the same time a tense pulse in the upper extremities. Systolic blood pressure in the arms of patients with isolated severe coarctation of the aorta reaches high numbers up to 190-200/90-100 mm Hg.
Diagnostics
Diagnosis is suggested by clinical examination (including blood pressure measurements in all 4 extremities), confirmed by chest x-ray and ECG, and based on the results of two-dimensional color flow echocardiography and Doppler studies. In older patients - using CT or MR angiography.
The most informative method for detecting aortic coarctation is
two-dimensional echocardiography (EchoCG).
Endovascular treatment In our country, the first TLBAP for aortic coarctation was performed by Yu.S. Petrosyan et al. in 1985 at the Institute of Cardiovascular Surgery of the USSR Academy of Medical Sciences. The operation is performed under sedation through a small puncture of the femoral artery. A catheter and balloon are passed through the puncture to the site of narrowing of the aorta. Next, the narrowed section of the aorta is expanded with a balloon of the appropriate size, restoring the lumen and blood flow through the aorta. Federal State Budgetary Institution "NMITs SSH im. A.N. Bakuleva" has extensive experience in the treatment of aortic coarctation.
Angiogram of a patient with aortic coarctation performed via arterial access:
a- aorthoraphy before balloon angioplasty, arrows indicate the membrane in the area of the aortic isthmus;
b — opening of the balloon (the arrow indicates the constriction corresponding to the area of coarctation of the artery);
c- aortography after balloon angioplasty - narrowing of the aortic isthmus has been eliminated.
Stenting of aortic coarctation in children weighing more than 15 kg
Stenting of coarctation/recoarctation is indicated in children weighing more than 15-20 kg. It must be remembered that stenting of aortic coarctation is performed with special stents, which can be further expanded as the child grows.
Drawing. Stenting of aortic coarctation
A – aortography visualizes a narrowing of the isthmus
B — after stenting of aortic coarctation.
VALVE AORTIC STENOSIS IN NEWBORNS
Valvular aortic stenosis is a congenital heart defect in which the cusps of the aortic valve are fused. Without surgical intervention, the mortality rate among newborns with congenital valvular aortic stenosis is extremely high - almost 85-90% of patients die during the first month of life.
Symptoms
Newborns with severe aortic stenosis become irritable, eat poorly, sweat during feeding, have trouble breathing, have unnaturally pale or grayish skin, cold palms and soles, decreased urine output, and an increased heart rate.
Treatment
Balloon dilatation (expansion) of the aortic valve is one of the treatment methods
Experience of the Federal State Budgetary Institution "NMITSSSH im. A.N. Bakulev"
National Medical Research Center for Cardiovascular Surgery named after. A. N. Bakuleva.
Drawing. Stages of performing transluminal balloon valvuloplasty of aortic valve stenosis.
a — angiography of the ascending aorta: a stream (arrow) of non-constricted blood is visible, indicating stenosis of the aortic valve;
b — left ventriculography (the arrow indicates the flow of uncontrasted blood from the LV, also indicating aortic valve stenosis);
c – positioning of the balloon in the projection of the aortic valve. Radiopaque balloon marks (arrows) are located above and below the aortic valve annulus;
Atrial septal defect
Atrial septal defect (ASD) is a congenital heart defect (CHD), characterized by the presence of a communication (hole) between the right and left atria, which causes the existence of an arteriovenous discharge between them. The risk of developing an ASD in an unborn child is significantly higher in those families where there are relatives with congenital heart disease. In addition to hereditary conditions, viral diseases of the pregnant woman (rubella, chickenpox, etc.), endocrinopathies, taking certain medications and alcohol during pregnancy, occupational hazards, gestational complications (toxicosis, threat of miscarriage, etc.) can lead to the occurrence of ASD.
Complaints
Dyspnea and palpitations are the most common early symptoms of the disease in large ASDs in children, but usually during the first months of life hemodynamic compensation and regression of the clinical picture occur. Subsequently, in most children, ASDs are asymptomatic, and patients have no complaints. Children often have an asthenic physique with noticeable pallor of the skin.
Diagnosis
It is recommended to diagnose ASD using transthoracic echocardiography (EchoCG) using color Doppler mapping, which is the main diagnostic tool in diagnosing ASD, determining its size, location, volume and direction of blood shunting.
Today, endovascular treatment methods with appropriate anatomy are the method of choice in the treatment of ASD. Defects are closed using special occluder devices. The occluder is a double-disk device: the left disk opens in the left atrium, the right disk opens in the right atrium. The defect remains between the two disks. The operation is performed under local anesthesia through the femoral vein. The duration of the operation is 40-60 minutes. After the operation, the patient is transferred to the ward and can be discharged the next day.
Drawing. Various modifications of occluders for closing atrial septal defect.
Patent ductus arteriosus
Patent ductus arteriosus (PDA) is a vessel through which pathological communication between the aorta and the pulmonary artery (PA) remains after birth. Comments: Normally, the PDA is necessarily present in the fetus, but closes soon after birth, turning into an arterial ligament.
Risk factors for patent ductus arteriosus are premature birth and prematurity, family history, the presence of other congenital heart diseases, infectious and somatic diseases of the pregnant woman.
PDA usually occurs in premature infants and is extremely rare in infants born at term.
Complaints of patients with PDA are nonspecific. Clinical manifestations depend on the size of the duct and the stage of hemodynamic disorders. The course of the defect varies from asymptomatic to extremely severe. With large duct sizes, the latter manifests itself already from the first weeks of life with signs of heart failure and retardation in physical development. In young children, when screaming (or straining), cyanosis may appear, which is more pronounced on the lower half of the body, especially on the lower extremities. It is typical that cyanosis disappears after the load is stopped.
Diagnostics
The main diagnostic method is ultrasound and auscultation. Auscultation reveals a “machine” noise characteristic of the defect in the second or third intercostal space to the left of the sternum, radiating into the interscapular space and vessels of the neck.
Treatment
The main treatment method for PDA is endovascular intervention. Small ducts are closed using spirals, large ones (more than 3 mm) - using occluders. In 1992, P. Cambier was the first in the world to use a coil for embolization of the patent ductus arteriosus. The operation is performed through the femoral artery or vein without opening the chest. The duration of the operation is 30-40 minutes. The patient is transferred to a ward under observation and can be discharged after a day. The implanted devices are MRI-compatible, meaning MRI examinations can be performed after 6 months.
A B
Drawing. A - Patent ductus arteriosus. B - after closing the duct with a spiral.
Large ducts are closed using special devices called occluders.
Drawing. Occluder for closing the patent ductus arteriosus.
Patent foramen ovale
The patent foramen ovale (PFO) is a short interatrial canal (average length 5 mm) located exactly on the axis of blood flow coming from the inferior vena cava. In 25-30% there is no complete anatomical occlusion, and the oval window remains open or, more precisely, openable. This is called LLC and, as a rule, is not considered a deviation, but rather a variant of the norm. In most cases, LLC remains asymptomatic and does not manifest itself. The most obvious manifestations of PFO are paradoxical arterial embolisms, the most serious of which are the resulting strokes .
Indications for closing an LLC:
Migraine (with aura), sleep apnea, stroke, diving, high-altitude pulmonary edema, LLC is closed using special occluders. The operation is performed under local anesthesia, accessed through the femoral vein. An occluder is passed through the installed catheter, which closes the LLC using two disks (“sandwich”).
Drawing. Various devices for closing a patent oval window
Vascular pathology
Endovascular methods of treating blood vessels and veins is one of the most interesting and rapidly developing specialties of medicine with a steady increase in the number of operations. Endovascular treatment methods are used when all major vessels and veins are affected (carotid, renal, subclavian arteries, vessels of the lower extremities).
Vascular pathology
X-ray endovascular methods of diagnosis and treatment occupy a leading position in the treatment of vascular pathology, and their share is steadily growing every year. These methods are most actively used in the pathology of the carotid, brachiocephalic, renal arteries, and vessels of the lower extremities.
Stenting of the internal carotid artery
According to the American Heart Association (AHA/ASA), approximately 6.5 million strokes occur annually in the United States. About 20% of all ischemic strokes occur due to atherosclerotic stenosis of the carotid arteries, usually located in the area of their division into the external and internal arteries. Atherosclerosis of the ICA is one of the main pathologies of the ICA, which poses a threat of developing cerebral stroke due to blockage of cerebral vessels, followed by disability or leading to death.
Clinic:
If
patency is impaired, the following manifestations occur:
- stroke
- dizziness
- noise in ears
- darkening of the eyes
- weakness
Often arterial stenosis precedes an acute disruption of cerebral blood supply. Pathology can be recognized by the following symptoms:
- nausea and vomiting
- headache
- numbness of the limbs or one side of the face
- difficulties with coordination
- speech problems
Diagnostics:
- USDG
- CT – angiography
- Angiography
Surgery:
One of the treatment methods for internal carotid artery (ICA) stenosis is ICA stenting. The method consists of installing a high-tech, biocompatible, metal (cobalt-chromium alloy) frame in the narrowing zone with complete restoration of the artery lumen, which prevents the development of acute disorders of cerebral blood supply (TIA and stroke).
This operation is performed under local anesthesia using access through the femoral or radial arteries (through the thigh or arm). Through an installed catheter, a special self-expanding stent of various designs is passed and implanted into the affected area of the carotid artery. In order to reduce the number of complications, special filter traps are used in all operations. After the operation, the patient is transferred to the ward and discharged the next day.
Clinical examples:
- Stenosis of the left ICA 85%
- Stenosis of the right ICA 95%
Renal artery stenting
The blood supply to the kidneys is provided by the renal arteries, which arise from the largest vessel in the body (the aorta). With renal artery stenosis, the patient develops so-called renovascular hypertension. Progression of stenosis over time can lead to the development of renal failure.
Diagnostics:
- USDG
- CT – angiography
- Angiography
Surgery:
Renal artery stenting is an effective and safe procedure. The method consists of installing a high-tech, biocompatible, metal (cobalt chromium alloy) frame into the narrowing zone, under local anesthesia, with complete restoration of the artery lumen, which prevents further progress of organ ischemia (decreased function), and also leads to stabilization of blood pressure.
Stenting of lower extremity arteries
Pathology:
Atherosclerosis of the arteries of the lower extremities is one of the main pathologies, which poses a threat to the development of ischemia of the lower extremities, leading to decreased function of the lower extremities, as well as trophic tissue damage.
Clinic:
If patency is impaired, the following manifestations occur:
- Pain in the legs when walking - “intermittent claudication”
- Numbness, cold feet
- Hair reduction
- Dystrophy of the nail plates
- Trophic ulcers
- Dry gangrene
- Wet gangrene
- Dysfunction of the pelvic organs
Diagnostics:
- USDG
- CT – angiography
- Angiography
Surgery:
Stenting of the arteries of the lower extremities is an effective and safe procedure. The method consists of installing a high-tech, biocompatible, metal (cobalt-chromium alloy) frame into the narrowing zone, under local anesthesia, with complete restoration of the lumen of the artery, which prevents the development of chronic and acute disorders of the blood supply to the lower limb, and also eliminates the clinical picture of the disease.
Subclavian artery stenting
Atherosclerosis of the subclavian artery develops most often in the initial part of this artery and may be accompanied by the development of symptoms of circulatory disorders in the arm or cerebral circulatory failure due to the phenomenon of vertebral-subclavian steal, when blood flows through the vertebral artery into the subclavian artery, as a result of which the development of cerebral ischemia is possible .
Clinic:
If its patency is impaired, the following manifestations occur:
- Pain, fatigue, coldness, parasthesia, numbness of the upper limb
- Dizziness
- Instability
- Ataxia
- Nystagmus, diplopia, hemianopsia
- Hearing loss
Diagnostics:
- USDG
- CT – angiography
- Angiography
Surgery:
Subclavian artery stenting is an effective and safe procedure. The procedure is painless and takes place under local anesthesia.
Stenting is performed through a small puncture in the femoral or radial artery (through the arm). Through an installed catheter, a special stent of the appropriate size is passed into the affected area of the subclavian artery and implanted. Next, the stent is deployed and the narrowed area is reinforced with complete restoration of the lumen.
Endoprosthetics of the abdominal aorta
Anatomy:
The aorta is the largest vessel in the human body. It departs directly from the heart, giving off main branches to all organs and tissues in the body. Anatomically, the aorta is divided into the ascending section (aortic arch) and the descending section (thoracic section and abdominal section).
Pathology:
An aortic aneurysm is usually called a lumen formed in it that is twice (or more) the normal diameter of the vessels. The defect appears as a result of the destruction of the elastic fibers (filaments) of the central shell, as a result of which the remaining fibrous tissue elongates, thereby expanding the diameter of the vessels and leading to thinning and tension of their walls. As the disease progresses and the subsequent increase in the size of the lumen, there is a possibility of rupture of the aortic aneurysm with massive internal bleeding, leading to death in 2 out of 5 cases.
Clinic:
An abdominal aortic aneurysm manifests itself in the form of dull, aching and gradually increasing pain in the abdomen. Unpleasant sensations, as a rule, occur to the left of the navel and radiate to the back, sacrum and lower back. If such symptoms are detected, you should consult a doctor, otherwise the abdominal aortic aneurysm may rupture.
Most often, with an abdominal aortic aneurysm, the first signal is attacks of pain. They occur unexpectedly and often radiate to the lower back, groin area or legs. The pain lasts for several hours and is difficult to respond to medications. When the aneurysm becomes inflamed, the temperature may rise. Sometimes blueness and coldness of the fingers are observed.
Diagnostics:
- USDG
- MRI
- CT – angiography
- Angiography
Surgery:
Abdominal aortic replacement is an effective and safe procedure. The method consists of installing a high-tech, biocompatible, coated metal (cobalt-chromium alloy) frame into the expansion zone, under local anesthesia, completely excluding the aneurysmal expansion from the bloodstream, which prevents further increase in the size of the aneurysm, the formation of blood clots, and also prevents aortic rupture.
Drawing
Abdominal aortic aneurysm before and after stenting
Transcatheter aortic valve implantation
Transcatheter aortic valve implantation (TAI) has opened new possibilities for the treatment of patients with severe disease (narrowing) of the aortic valve. Transcatheter aortic valve implantation (TAVI) is a modern minimally invasive transcatheter method of replacing the fortal valve with an artificial biological one.
Aortic valve stenosis
- a severe pathology, it ranks second among all acquired structural heart diseases, and significantly increases the risk of sudden cardiac death. Such patients experience severe heart failure and shortness of breath even with minimal exertion, attacks of angina in the form of chest pain, sudden weakness, peripheral edema and fainting. Just 15 years ago, the main method of treating this pathology was open surgery on a stopped heart. However, in modern medical practice, an alternative minimally invasive transcatheter approach of aortic valve implantation (TAI) is increasingly being used, when a biological prosthesis is passed through the opening in the common femoral artery and implanted in the position of the aortic valve. In 80% of cases, the operation does not require general anesthesia; the patient is conscious and in contact with the doctor. The operation time varies from 30 to 70 minutes, and the patient's stay in the intensive care unit is reduced to 24 hours. Modern endovascular valve prostheses have proven their effectiveness in many multicenter studies conducted by leading clinics in Europe and the USA, and the design of the models used allows us to minimize the risks of developing adverse consequences in the postoperative period while achieving maximum clinical effect.
Drawing. Transcatheter aortic valve implantation
Treatment and period after the course of EECP
EECP therapy did not start by chance. At the beginning of treatment, the patient required assistance in getting to her treatment session due to angina pectoris. During treatment, she noted a decrease in angina attacks, accompanied by an increase in physical activity (Table 3). At the end of the 7-week course of treatment, she experienced no restrictions in physical activity, including 60 minutes of cycling or treadmill training. She continued to take the same angina medications as before EECP.
Rice. 1. The mechanism of diastolic enhancement and afterload reduction during EECP use. ECG-dependent sequential diastolic activation of “milk” venous blood of the lower extremities and arterial blood from the periphery to the main vessels of the system was noted (stage 1-3). All cuffs are simultaneously deflated immediately before systole (step 4).
Subsequently, an improvement in myocardial perfusion was observed, as shown by scintigraphy data during the adenosine test; no signs of myocardial ischemia or myocardial infarction were detected (Fig. 2). 9 months after the end of the course of treatment, the patient remained active, angina attacks were less than once a month, and immediately stopped with rest or after the use of sublingual nitroglycerin.
Table 3. Clinical picture during treatment.
| Week 1 1 attack of angina at rest Physical activity limited to hotel room Week 3 1 attack of angina at rest Walking around hotel room Week 5 2 short attacks of mild angina Patient begins small exercise program Week 7 No angina Walking /ride a bike for 60 minutes a day |
Fig. 2 Comparison of resting and stress perfusion images obtained before (left) and after (right) EECP treatment. There is a decrease in myocardial ischemia caused by adenosine in the inferior wall (arrow), inferior septal segment (asterisk), and apical segment of the LV (plus sign) after treatment. The marks are on the image taken before treatment. Redist - redistribution.
Discussion
The presented case is an impressive example of the capabilities of EECP used to reduce the symptoms of myocardial ischemia. At the beginning of therapy, the patient was bedridden due to severe angina. After 7 weeks of EECP treatment, she was able to exercise daily without angina and had no evidence of myocardial ischemia on repeat myocardial scintigraphy. This positive clinical result was maintained for 9 months after treatment.
There is growing evidence of the effectiveness of EECP used for angina for 2 decades. This procedure, first proposed by Kantrowitz and Kantrowitz, is non-invasive and non-traumatic, it increases diastolic blood pressure and perfusion pressure in the coronary arteries, and also helps unload the left ventricle, similar to intra-aortic counterpulsation. The first hydraulic counterpulsation devices were cumbersome and have been modified over several years and now include computerized, ECG-controlled pneumatic compression cuffs that are wrapped around the lower extremities, which improves diastolic augmentation and reproducibility. The use of new EECP devices has demonstrated long-lasting reductions in symptoms of myocardial ischemia and improved quality of life in a heterogeneous group of patients with coronary artery disease.
EECP in the treatment of angina pectoris
The results of several studies have been published that report experience with the use of EECP in patients with angina refractory to drug therapy. These patients were not suitable candidates for percutaneous or surgical revascularization. Studies have shown a significant reduction in the Canadian Society of Cardiology classification of angina; the number of nitroglycerin tablets used daily; increasing tolerance to physical activity; and a decrease in myocardial ischemia according to objective data (Table 4). The disadvantages of these studies are that they involved a small number of patients and there was no control to rule out the placebo effect. A recent multicenter, randomized, placebo-controlled EECP trial randomized 139 patients with angina and documented CAD to active versus hemodynamically inactive counterpulsation. The active counterpulsation group showed a significant increase in the time to the development of ST segment depression during exercise, as well as its duration. The number of angina attacks also decreased significantly in the main group (Table 5). No serious complications were observed, and the impact on quality of life was maintained for 1 year. Radionuclide perfusion scintigraphy showed a decrease or disappearance of perfusion defects after a course of EECP. Also, EECP could increase perfusion from the initial level, which was proven by positron emission tomography data.
Table 4. Results of the first non-randomized EECP studies
| Author | Number of patients | Number of treatment sessions (hour) | Reduction in angina symptoms (%) | Perfusion improvement (%) |
| Zeng et al., 1983 | 200 | 12 | 97 | N.A. |
| Lawson et al., 1992, 1995 | 18 | 36 | 100 | 78 |
| Karim et al., 1995 | 38 | 36 | 86 | 78 |
| EECP=enhanced external counterpulsation; NA=not available. | ||||
Table 5. Change in exercise tolerance in MUST-EECP*
| Control group | EECP Group | ||||
| Options | № | Average (SE) changes (sec) | № | Average (SE) changes (sec) | R |
| Duration of load | 58 | 26(12) | 57 | 42(11) | .30 |
| Time to development of ST segment depression | 56 | — 4 (12) | 56 | 37 (11) | .01 |
| *MUST-EECP= Multicenter EECP Study | |||||
Alternative techniques for surgical revascularization
Modern medicine has various means of minimally invasive revascularization. They are alternatives to classical surgical methods of excision of arteries (arteriectomy) - but are by no means methods of alternative medicine. These means have been adopted by official medicine, which today practically no longer gives preference to the classical means of vascular surgery.
Modern minimally invasive techniques are commonly called endovascular surgery. She is given preference. Endovascular surgery meets the interests of patients: the treatment is less painful, less traumatic, and postoperative rehabilitation is faster. The only question is whether this or that surgical clinic has equipment for minimally invasive endovascular operations and therapeutic actions, and whether its staff has the necessary experience.
Endovascular procedures involve the use of vascular catheters placed along the internal lumen of the artery not through an external incision, but through small “punctures” made in the shallowest areas of the artery (for example, in the groin). Attachments on catheters make it possible not only to catch a blood clot to remove it out, but also to expand the vessel from the inside (balloon dilatation) and implant a stent (a vascular frame that prevents its narrowing).
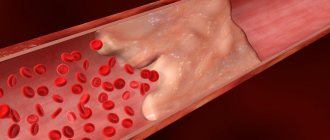
The modern technique of interventional thrombolysis (chemical decomposition of a blood clot) is based on the catheter administration of decomposing substances that treat the body of the blood clot. If the position of the blockage site is sufficiently “convenient,” substances for thrombolysis (streptokinase, urokinase, rt-PA drug) can be administered directly at the site where the artery is punctured.
Thrombectomy immediately frees the internal lumen of the artery. This means that blood flow is immediately restored. It takes time for the blood clot to chemically break down. Therefore, thrombolysis can be performed when the artery is not completely blocked. Or with simultaneous bypass of the blocked area (establishment of temporary bypass blood flow).
Mechanism of action
The exact mechanism by which EECP reduces symptoms in patients with chronic angina and other syndromes is still unclear. The acute hemodynamic effect is similar to that observed with intra-aortic balloon counterpulsation, with a decrease in afterload and an increase in aortic diastolic pressure. In addition, preload may be increased. Recent advances in the study of the physiology of the coronary circulation have made it possible to suggest mechanisms that explain the beneficial effects of EECP. During ischemia, increased coronary blood flow and shear stress caused by increased diastolic pressure may be sufficient to stimulate angiogenesis and collateral formation. In vitro, increased shear stress activates tyrosine kinase in the endothelium. The tyrosine receptor causes phosphorylation in a group of submembrane proteins, which, in turn, leads to changes in the actin cytoskeleton. This modification of the actin cytoskeleton is a key factor in the migration of endothelial and smooth muscle cells, potentially leading to the formation of new vessels in the ischemic myocardium.
In addition to angiogenesis and collateral formation, increased shear stress induced centrally or peripherally during EECP may contribute to improved vasomotor function. It has been demonstrated that shear stress increases the production of the endothelial relaxant factor NO. Down-regulation of endothelin-1 levels during pneumatic external pulsation has recently been demonstrated. Down – regulation of endothelin-1 levels may increase coronary artery dilatation and improve myocardial perfusion. It may also contribute to the anti-ischemic effects of EECP. Thus, these factors play a major role in the response of arterial smooth muscle cells and vascular dilatation. The participation of various growth factors, especially vascular endothelial growth factors, is also observed here. These changes are thought to be important in maintaining angiogenesis and collateral formation and may explain the beneficial effects even after a course of EECP has been completed.
results
Endarterectomy from the RCA basin was performed in 4 (56.2%) patients. Of these patients, hypokinesis of the LV inferior wall before surgery was observed in 25 (34.2%) cases. Regression of hypokinesis of the posterior wall of the LV was noted in 8 (32%) patients. LAD endarterectomy was performed in 21 (28.8%) patients (Fig. 5).
Rice. 5. Endarterectomy from the LAD from two septal branches. Of these patients, disturbances in the kinetics of the interventricular septum before surgery were observed in 3 (4.1%) patients. In 2 (2.7%) patients, hypokinesis of the anterior wall of the LV regressed. In 4 patients who underwent endarterectomy from the OB, hypokinesis was not observed.
Overall in-hospital mortality was 4.1% (3 patients). The causes of death were perioperative myocardial infarction ( n
=1), multiple organ failure due to sepsis and respiratory distress syndrome (
n
=2).
In the early postoperative period, 44 (60.3%) patients required prolonged cardiotonic support (dopamine 5 mg/kg/min, adrenaline 0.01 mcg/kg/min for more than 12 hours). Intra-aortic balloon counterpulsation (IABP) was used in 2 (2.7%) patients. Acute cerebrovascular accident was recorded in 1 (1.4%) patient. In 11 (15.1%) patients, posthypoxic encephalopathy was recorded with complete regression of symptoms during hospital treatment. Seven (9.6%) patients were on prolonged mechanical ventilation (more than 48 hours). One (1.4%) patient developed bleeding, requiring resternotomy and additional hemostasis. The characteristics of patients in the postoperative period are presented in Table. 4.
Table 4. Characteristics of the postoperative period
Bibliography
1. Schub C. Stable angina pectoris, 3: medical treatment. Mayo din Proc. 1990:65:256-273. 2. Stone PH, Gibson RS. Glasser SP, et al. ASIS Study Group. Comparison ot'propranolol, diltiazem, and nifedipine in the treatment of ambulatory ischemia in patients with stable angina: differential effects on ambulatory ischemia, exercise performance, and anginal symptoms. Circulation. 1990:82:1962-1972. 3. RITA-2 Trial Participants. Coronary angioplasty versus medical therapy for angina: the Second Randomised Intervention Treatment of Angina (RITA-2) trial. Lancet. 1997:350:461-468. 4. Chaitman BR. Rosen AD. Williams D.O. et al. Myocardial infarction and cardiac mortality in the Bypass Angioplasty Revascularization Investigation (BARI) randomized trial. Circulation. 1997:96:2162-2170″ 5. Alien KB, Dowling RD, Fudge TL, et al. Comparison of transmyocardial revascularization with medical therapy in patients with refractory angina. N En^l .1 Mcd. 1999:341:1029-1036. 6. Frazier OH. March R.J. Horvath K.A. Transmyocardial revascularization with carbon dioxide laser in patients with end-stage coronary artery disease. N Enyl J Med. 1999:341:1021-1028. 7. Whitlow PL, Knopf WD. O'Neill WW. Kaul U. Londero H, Shawl F. Six month follow-up of percutaneous transmyocardial revascularization in patients with refractory angina [abstract]. J Am Coil Cardiol. 1999;33(suppl):29A. 8. Benetti F, Mariani MA. Sani G. et al. Video-assisted minimally invasive coronary operations without cardiopulmonary bypass: a multicenter study../ Thorac Surg. 1996:112:1478-1484. 9. Boonstra PW, Grandjean JG, Mariani MA. Improved method for direct coronary grafting without CPB via anterolateral small thoracotomy. Ann Thorac Surg. 1997:63:567-569. 10. Holubkov R, Zenali M, Akin JJ. Erb L, Courcoulas A. MIDCAB characteristics and results: the CardioThoracic Systems (CTS) registry. EiirJCardiothoracSurs. 1998:14(suppl 1):S25-S30. 11. Mariani MA. Boonstra PW, Grandjean JG, et al. Minimally invasive coronary artery bypass grafting versus coronary angioplasty for isolated type C stenosis of the left anterior descending artery. J Thorac Cardiovasc Surf;.1997:114:434-439. 12. Alderman EL. Levy JH. Rich JB, et al. Analyzes of coronary graft patency after aprotinin use: results from the International Multi-center Aprotinin Graft Patency Experience (IMAGE) trial. J Thorac Cardiovasc Surf;. 1998; 116:716-730. 13. Chauhan A. Mullins PA. Thuraisingham SI, Taylor G, Fetch MC, Schofield PM. Effect of transcutaneous electrical nerve stimulation on coronary blood flow. Circulation. 1994;89:694-702. 14. DeJongste MJ, Hautvast RW, Hillege HL, Lie KI. Working Group on Neurocardiology. Efficacy of spinal cord stimulation as adjuvant therapy for intractable angina pectoris: a prospective, randomized clinical study. J Am Colt Cardiol. 1994;23:1592-1597. 15. Yeung AC, Hayase M, Fitzgerald P, et al. Percutaneous in-situ coronary artery bypass (PICAB): current development status and preliminary results of a novel myocardial revascularization technique [abstract]., I Am Coil Cardiol. 1999:33(suppl):47A. 16. Carter AJ. Kornowski R. Lamson T, et al. Percutaneous in-situ coronary venous arterial bypass: initial results of retrograde myocardial perfusion in a porcine model [abstract]. J Am Coil Cardiol. 1999;33(suppl):49A. 17. Symes JF. Losordo DW, Vale PR, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann Thorac Sui-s. 1999:68:830-836. 18. Arora RR, Chou TM. Jain D. et al. The Multicenter Study of Enhanced External Counterpulsation (MUST-EECP):effect of EECP on exercise-induced myocardial ischemia and anginal epi.sodes.7Aw Coil Cardiol. 1999:33:1833-1840. 19. Lawson WE. Hui J.C. Soroff HS, et al. Efficacy of enhanced external counterpulsation in the treatment of angina pectoris. Am J Cardiol. 1992:70:859-862. 20. Lawson WE, Hui JCK, Zheng ZS, et al. Three-year sustained benefit from enhanced external counterpulsation in chronic angina pectoris. Am J Cardiol. 1995:75:840-841. 21. Zheng ZS. Li TM, Kambic H, et al. Sequential external counterpulsation (SECP) in China. Trans Am Soc Artif Intern Organs. 1983:29:599-603. 22. Karim S, Sani A, Karo-Karo S. et al. Enhanced external counterpulsation in the treatment and rehabilitation of coronary patients in Indonesia. Asian Cardiovasc Thorac Ann. 1995;3: 26-28. 23. Kantrowitz A, Kantrowitz A. Experimental augmentation of coronary flow by retardation of the arterial pressure pulse. Surgery. 1953:34:678-687. 24. Moulopoulos SD, Topaz S, Kolff WJ. Diastolic balloon pumping (with carbon dioxide) in the aorta—a mechanical assistance to the failing circulation. Am Heart.I. 1962:63:669-675. 25. Jacobey JA. Taylor WJ, Smith GT. Gorlin R. Harken DE. A new therapeutic approach to acute coronary occlusion. II: opening dormant coronary collateral channels by counterpulsation. Am J Cardiol. 1963:11:218-227. 26. Masuda D, Nohara R, Hirai T, et al. The new therapeutic approach with the enhanced external counterpulsation in patients with chronic stable angina: evaluation of myocardial flow and flow reserve by N-13 ammonia PET [abstract]. Circulation. 1999; 100(suppl):l-732. 27. Sessa WC. Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994:74:349-353. 28. Garlichs CD. Zhang H. Werner D, John A, Tragner P. Daniel WG. Reduction of serum endothelin-1 levels by pneumatic external counterpulsation [abstract]. Can J Cardiol. 1998; 14(suppl): 87F. 29. Zarins CK. Zatina MA. Giddens D.P. Ku DN. Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vase Surg. 1987:5:413-420. 30. Tseng H, Peterson TE, Berk BC. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ Res. 1995;77:869-878.