Sodium (Na) is the major extracellular electrolyte responsible for plasma osmotic pressure. It determines the amount of almost all body fluids (circulating and deposited blood, cerebrospinal fluid, fluids of the serous cavities, etc.), and is part of the bicarbonate and phosphate buffers (regulation of ASR). Na is necessary for the transmission of nerve impulses between neurons and neuromuscular synapses. All of the above causes the development of severe disorders when the electrolyte balance is disturbed. There are no exact statistics on the prevalence of hypernatremia.
Causes of hypernatremia
This condition quite often has a multifactorial origin. Conditionally, a physiological cause (not related to any disease) can be considered intense physical activity and dietary disorders - oversalting of food, insufficient consumption of drinking water or prolonged intake of mineral water. Pathological causes of hypernatremia are:
- Fluid deficiency.
Water loss can occur through the skin (sweating), the gastrointestinal tract (nausea, vomiting), and in the urine (diabetes mellitus or diabetes insipidus, polyuric stage of acute renal failure, glomerulonephritis). Failure to drink adequate amounts of water (for example, while comatose) can also cause hypernatremia. - Excess sodium intake.
More often it is provoked by the uncontrolled administration of hypertonic solutions of NaCl, bicarbonates or other solutions containing Na. Hypernatremia is caused by drinking sea water and using dialysis fluids containing high concentrations of Na. - Reduced sodium excretion.
Sodium retention in the body occurs due to increased secretion of renin in the juxtaglomerular apparatus of the kidneys or mineralocorticoids in the adrenal glands (primary and secondary hyperaldosteronism). The causes are congestive heart failure, liver and kidney diseases, and Conn's syndrome. - Taking medications.
Hypernatremia is often potentiated by diuretics, especially those that increase blood osmotic pressure (mannitol). Hypernatremia also develops during treatment with drugs that block the effect of antidiuretic hormone on the kidneys (vaptans, demeclocycline).
Hypernatremia
Home Medical Encyclopedia Directory of Diseases G
Hypernatremia—serum sodium concentration greater than 145 mEq/L; clinically manifested if this indicator is above 155 mEq/l. Hypernatremia always implies hypertonicity of all body fluids, because An increase in the osmolarity of the extracellular fluid causes the movement of water from the intracellular space, which leads to an increase in intracellular osmotic activity and cell dehydration.
Etiology
- Extrarenal causes
- Excessive sodium intake (usually sodium overdose)
- Reduced water consumption
- Increased losses through the skin (hyperhidrosis, burns)
- Increased losses through the gastrointestinal tract (diarrhea, prolonged vomiting)
- Renal causes
- Osmotic diuresis. The presence of osmolarly active nonabsorbable solutes in the glomerular filtrate prevents the reabsorption of water and sodium and leads to increased water loss in the kidneys. Hyperglycemia with glucosuria is a common cause of osmotic diuresis. Since water loss is relatively greater than sodium loss, serum sodium concentration increases progressively during osmotic diuresis.
- Diabetes insipidus (see Diabetes insipidus).
Clinical picture - Pathology of the central nervous system. In young children and the elderly, generalized central nervous system depression develops, including lethargy, coma, and seizures. Due to ruptured veins, intracerebral hemorrhages may occur.
- Reduction of extracellular volume. Although 2/3 of the water deficit is covered by intracellular fluid, the volume of extracellular fluid is also reduced to a small extent (dry skin and mucous membranes, thirst).
- Impaired urine output. If fluid loss is due to the kidneys (i.e., urine output is inappropriately high compared to plasma osmolarity or extracellular fluid volume), then polyuria may occur. If the kidneys are intact and fluid loss is due to extrarenal causes, urine volume usually decreases.
- Children
- Hypernatremia may develop in low birth weight newborns. Mortality is high
- Hypernatremia can develop due to improper preparation of baby food.
- Elderly.
Hypernatremia can be caused by the administration of loop diuretics. Laboratory diagnostics - Dehydration test. The ability to concentrate urine can be tested after overnight dehydration to identify the patient's kidney fluid loss
- Water restriction begins at 8 pm and continues for 14 hours, after which urine osmolarity should exceed 800 mol/kg. Then the patient is injected subcutaneously with ADH (5 units of aqueous solution of vasopressin). Urine osmolarity should no longer increase after this procedure.
- However, if urine osmolarity after water deprivation is below 800 mol/kg or if it increases by more than 15% after administration of ADH, this indicates the presence of some degree of ADH deficiency
- If urine osmolarity after water deprivation does not exceed 300 mo/kg, and there is no further increase after the administration of ADH, then there is some form of nephrogenic diabetes insipidus
- Plasma ADH study. In nephrogenic diabetes insipidus, urine osmolarity may not be a true reflection of ADH release, and plasma ADH levels should be measured
- Assessment of osmolarity and composition of urine.
To assess polyuria, a study of the composition of solutes in the urine is informative. A urine osmolarity below 200 mo/kg suggests a primary defect in water conservation. When urine osmolarity is above 200 mol/kg with polyuria, osmotic diuresis is assumed. After measuring urine osmolarity, the presence of sodium, glucose and urea in the urine should be analyzed to determine the etiology of diuresis. Differential diagnosis - Diabetes insipidus
- Hyperosmolar nonketoacidotic coma
- Abuse of salt
- Dehydration of the hypertensive type.
- Diet. Depending on the etiology of the disease - limiting table salt, increasing the amount of fluid; for diabetes insipidus - a diet with limited proteins and a sufficient amount of fats and carbohydrates, food is prepared without salt (the patient is given 5-6 g / day in crystalline form), vegetables, fruits, juices, milk and lactic acid products are recommended.
- For hypovolemia
- 0.9% NaCl solution or Ringer's solution 10-20 ml/kg IV for 1-2 hours. If the BCC decreases by 10% or more, the dose can be repeated
- 5% glucose solution with 0.5 N NaCl solution until diuresis normalizes.
- For hypernatremia
- Hypotonic solutions (NaCl or glucose). Hypernatremia lasting less than 24 hours is corrected within 24 hours; with longer-term hypernatremia, in order to avoid the development of cerebral edema, the serum sodium content is reduced to normal within 48 hours, i.e. no faster than 0.5 mEq/l/h {0.5 mmol/l/h) or 20 mEq/l/day (20 mmol/l/day)
- Loop diuretics, then, in addition to hydration therapy, intravenous infusion of 5% glucose solution with the addition of potassium chloride
- To prevent hypocalcemia, add 50 mg/kg calcium in the form of 10% calcium gluconate solution to solutions for intravenous administration.
- If acidosis develops, sodium bicarbonate (sodium bicarbonate) 550 mEq/L is added to the infusion fluid. When acidosis and hypocalcemia are combined, calcium deficiency is first eliminated
- If necessary, potassium supplements and phosphates are prescribed.
- For neurogenic diabetes insipidus
- For moderate hypernatremia - desmopressin (adiuretin SD) for adults 10-40 mcg intranasally in 1-3 doses; children 5-30 mcg once at night or in 2 divided doses
- In case of severe hypernatremia (>155 mEq/l) or with damage to the central nervous system, adiurehrin 5-10 units s.c.
- For nephrogenic diabetes insipidus
- Chlorothiazide (hydrochlorothiazide) 10 mg twice a day
- Chlorpropamide 100-250 mg every morning.
Alternative drugs. For nephrogenic diabetes insipidus - NSAIDs.Precautionary measures
- With rapid elimination of hypernatremia, pulmonary edema may develop
- In diabetes insipidus, the administration of large volumes of 5% glucose solution leads to hyperglycemia and, as a consequence, to increased diuresis
- When administering a large volume of fluid in neurogenic or nephrogenic diabetes insipidus, to avoid glucosuria, instead of a 5% solution, you can administer a 2.5% glucose solution.
Age characteristics
DRUG THERAPY Drugs of choice
| print version | This information is not a guide to self-treatment. A doctor's consultation is required. |
Pathogenesis
An increase in plasma sodium leads to an increase in plasma osmolarity. Due to changes in the osmotic concentration gradient, fluid moves from the cells into the extracellular space (bloodstream and interstitium). Due to dehydration, cells shrink and are destroyed. Against the background of a violation of the relationship between the intra- and extracellular content of Na ions, a change in the membrane potential occurs in the direction of lowering the excitability threshold.
These mechanisms underlie the occurrence of neuropsychiatric and neuromuscular symptoms. Sodium is a sympathetic cation, that is, it increases the sensitivity of cells to catecholamines and other vasopressor mediators. Therefore, its accumulation in vascular smooth muscle cells causes an increase in vascular tone and an increase in blood pressure.
Classification
Conventionally, hypernatremia is divided into moderate and severe, focusing on clinical symptoms, since there are no clear numerical indicators for dividing this condition by degree. Depending on the volume of circulating blood, the following types of hypernatremia are distinguished:
- Hypovolemic.
Develops with water deficiency and large losses of Na. Occurs with kidney disease, diabetes and taking osmotic diuretics. - Isovolemic.
Characterized by fluid loss without Na deficiency. Occurs with diabetes insipidus. - Hypervolemic.
Caused by excess Na and water. The main cause of this form is considered to be the administration of large volumes of hypertonic solution or bicarbonate.
According to the rate of development, hypernatremia can be:
- Acute –
lasting up to 48 hours. - Chronic –
lasting more than 48 hours.
Symptoms of hypernatremia
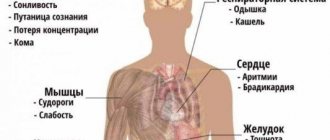
The clinical picture directly depends on the rate of increase in sodium concentration. With slowly developing hypernatremia, due to the inclusion of compensatory mechanisms (stimulation of the secretion of vasopressin and natriuretic peptides), an absolutely asymptomatic course can be observed. The most characteristic sign of rapidly onset hypernatremia is thirst.
Other early symptoms include decreased appetite, nausea and vomiting. The central nervous system is particularly affected. Excessive irritability, increased tendon reflexes, and impaired sense of balance occur. Confusion and panic attacks are possible. Characterized by muscle rigidity, myoclonic twitching, painful spasms.
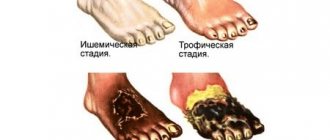
Due to high blood pressure, a patient with hypernatremia experiences heaviness in the back of the head and possible throbbing pain. In case of significant fluid loss (hypovolemic form), blood pressure decreases, which causes dizziness, darkening of the eyes, and sometimes loss of consciousness. In the hypervolemic form, swelling of the lower extremities is often observed.
Why is hypernatremia dangerous?
In the human body, sodium is the main cation in the extracellular fluid, accounting for up to 50% of its osmolality. In blood plasma, its concentration is within a narrow range (135 – 145 mmol/l) even with a significant difference in the amount of fluid consumed, which reflects the rigidity of regulation of the osmotic pressure of liquid media. This regulation is ensured by the work of a self-regulating functional system consisting of a complex of highly sensitive osmoreceptors, antidiuretic hormone (ADH), aldosterone and natriuretic peptides.
The body's daily need for sodium in terms of table salt is from 3.5 to 5 grams, but modern people consume much more - from 10 to 15 grams daily, and a significant part is due to the unconscious addition of salt to food and the consumption of ready-made products with an abnormal amount of table salt in the composition. In a healthy body under natural conditions, even this amount of sodium is not a threat, and its excretion remains equivalent to intake. In other situations, hypernatremia develops.
Hypernatremia is an increase in plasma sodium concentration above 145 mmol/l. Early symptoms of this condition are tachycardia, arterial hypertension and the appearance of edema. Considering the strict framework of sodium homeostasis, deviation of its concentration from the norm is extremely dangerous and is associated with disruption of neuromuscular conduction and functions of higher nervous activity - for example, when reaching 170 mmol/l, stupor develops, 180 mmol/l - convulsions and coma, and overcoming a threshold of 200 mmol/l is considered incompatible with life.
Hypernatremia results from an imbalance in the intake and excretion of water and sodium. Depending on the predominant cause, the patient’s volumetric status changes accordingly. Taking into account that the concentration of sodium in most environments of the body is lower than plasma, it is fair to say that prolonged losses of any fluid other than pancreatic juice and small intestinal secretions can lead to hypovolemic hypernatremia.
Most often, water loss is accompanied by sodium loss, but water deficiency is more pronounced in situations such as vomiting or diarrhea, profuse sweating during fever, the polyuric stage of acute renal failure, when using osmotic diuretics, or when the patient has diabetes insipidus.
Diabetes insipidus (DI) is a disease manifested in the inability of the kidneys to concentrate urine and retain water, i.e., impaired ADH function. There are two main forms of ND: central, in which the primary inhibition of the regulation of ADH synthesis due to lesions of the central nervous system, and nephrogenic - in which the synthesis of ADH is not impaired, but a pathological response of the kidneys to its effect is observed, which is sometimes a side effect of therapy with certain groups of drugs ( aminoglycosides, antifungal agents, contrast agents, vasopressors), as well as with concomitant hypokalemia. This disease requires confirmation using two diagnostic tests - an increase in urine osmolality by less than 30 mOsm/l with a complete ban on fluid verifies ND, and then a differential is carried out. diagnosis between two types of ND using stimulation with 5 units of a synthetic analogue of ADH - in the presence of a central form of ND, urine osmolality will increase up to 50%.
Insufficient fluid intake may be of a nutritional nature: dysregulation of the thirst mechanism or the inability to eliminate it due to depression of consciousness. Special attention should be paid to compiling the daily and cumulative water balance. Hypovolemia can also be observed when replacing fluid losses with hypotonic solutions, since all the delivered fluid will not be retained in the bloodstream.
Isolated water losses are rare. More often they are iatrogenic in nature, such as the patient’s prolonged stay on mechanical ventilation with inadequate parameters (tachypnea) or without established humidification in the circuit, which is especially important in the presence of a tracheostomy.
Hypertonic hypernatremia can develop with excess sodium intake: for example, when using hypertonic solutions. It is important to pay attention to the nutrition of patients with installed tubes - when feeding concentrated formulas without dilution with water, the risk of developing hypernatremia increases significantly.
Hypernatremia in the diuretic phase of the postoperative period is an expected phenomenon, especially if pronounced sequestration of extracellular fluid occurred in the early postoperative period.
In a complex system of homeostasis, disturbances in electrolyte or water balance cannot occur in isolation - the development of any of these disorders means the launch of a chain reaction of the formation of a global pathology of water and electrolyte balance, which, as it progresses, will affect all fluid environments of the body. It follows from this that all critically ill patients require constant monitoring of volume status, water balance and plasma electrolyte concentrations in order to select and timely correct rational therapy.
There are currently no specialized measures to prevent the development of hypernatremia. To reduce the risk, you should follow general preventive recommendations:
- regular screening medical examinations for early detection of pathologies and abnormalities of water and electrolyte balance
- reducing table salt consumption to 3.5-5.0 g/day
- balanced and nutritious nutrition
- monitoring blood pressure and strictly following the therapy prescribed by the doctor
Spanish Aristarkhov P. I.
Complications
Hypernatremia has a very high incidence of adverse effects, including fatal ones. These include cerebral edema, thrombosis of cranial vessels, coma. Complications are also possible due to improper treatment, since with chronic hypernatremia, organic osmotically active substances (osmolytes) begin to accumulate in neurons as a compensatory mechanism.
When hypotonic solutions are administered too quickly, osmolytes do not have time to leave the neurons, as a result, water rushes into the cells and brain edema develops. Sometimes, with a long course, electrolyte-steroid cardiomyopathy with necrosis is observed. Arterial hypertension increases the risk of complications such as myocardial infarction and stroke. Isolated cases of rhabdomyolysis followed by acute renal failure in patients with hypernatremia have been described.
POLYTRAUMA / POLYTRAUMA
Samatov I.Yu., Weinberg A.L., Mezhin A.V., Streltsova E.I., Vereshchagin E.I.
FSBEI HE "Novosibirsk State Medical University" of the Ministry of Health of Russia, GBUZ NSO "State Novosibirsk Regional Clinical Hospital", Novosibirsk, Russia
CORRECTION OF HYPERNATRAEMIA IN PATIENTS WITH SEVERE BURN INJURY
Target
– development of an effective algorithm of actions for hypernatremia in burn patients.
Material and methods.
A retrospective study assessed the incidence of hypernatremia (serum Na more than 150 mmol/l) in 165 patients (age 15-70 years) with severe burn injury (II-III-IV degree burns over 40% of the body area), as well as outcomes in two approaches to intensive therapy of hypernatremia.
In group I, correction of hypernatremia was carried out using hypoosmolar solutions and large doses of saluretics with intravenous bolus administration. In group II, correction was carried out using enteral rehydration (drinking water 20 ml/kg/day), spironolactone (250-300 mg/day) and furosemide (60-100 mg/day intravenously as a continuous infusion). High-volume continuous hemofiltration (HVCVVH) was performed for extrarenal indications in both groups when sodium levels were more than 160-163 mmol/L and conservative therapy was ineffective. Results
.
Correction of hypernatremia using enteral rehydration, spironolactone (250-300 mg/day), prolonged titrated administration of furosemide in small doses was significantly safer and more effective compared to the generally accepted method using hypoosmolar solutions and large doses of saluretics. In 70% of cases, hypernatremia was eliminated by conservative methods. Renal replacement therapy methods were used in 16 patients when conservative therapy was ineffective, but the best results were observed with early initiation of the procedure (plasma Na not more than 160-163 mmol/l). Conclusion.
The proposed conservative tactics for correcting hypernatremia made it possible to effectively and safely control the water-salt balance in the acute period of severe burn injury in most patients.
Keywords
: burn injury; hypernatremia; renin-angiotensin system; intensive therapy; high volume hemofiltration
Hypernatremia (HN) represents a serious problem in the treatment of patients with severe burn injury, which is associated with a high incidence of GN in these patients and high mortality in the group of patients with burn disease complicated by GN [1-3]. In a study [3], a hypernatremic state was recorded in 37.5% of patients with severe burn injury. The authors proved that GN worsens the prognosis and increases the risk of death in burn patients. Thus, the onset of GN was observed on days 5 ± 1.4, and mortality in the group with GN was 20%. The infusion/diuresis ratio in the group of patients with GN was several times lower compared to the group of patients in whom GN was not recorded. The authors indicate that GN in burn patients may be of iatrogenic origin due to inadequate and excessive crystalloid infusion therapy along with increased fluid losses [4, 5]. Thus, patients with severe burn injury have serious fluid and electrolyte imbalance. Statistical analysis showed that in patients with GN, the volume of excreted fluid was significantly higher on days 3-7 after burn injury compared to patients with normal serum sodium levels. In general, GN develops under two conditions: increased sodium intake or water deficiency. The first group of reasons, as already mentioned, includes excessive administration of sodium solutions and primary or secondary hyperaldosteronism [6]. A decrease in total water is one of the causes of GN, but not the only one. Among the factors contributing to this condition, the following are noted: 1) iatrogenic: excessive use of osmo- and saluretics; 2) movement of water into the cell; 3) plasma loss during burn shock and evaporation of water from the wound; 4) intestinal, pulmonary losses; 5) renal (tubular) failure; 6) sequestration of fluid into the “third space”, etc. [16]. Thus, according to these researchers, the optimal strategy for correcting GN is intravenous administration of hypoosmolar solutions [3]. GN was treated with 5% glucose. The authors also indicate the possibility of using a 0.45% sodium chloride solution. When assessing ways to prevent and correct GN in critically ill patients, the fundamental principles of water-salt metabolism are taken into account [8]: 1. Normal kidneys reabsorb or excrete water to maintain normal plasma osmolality of 275-290 mOsm/L. The regulator of plasma osmolality is vasopressin, and its release by osmoreceptors [9, 10]. Hypotension, hypervolemia, pain, acidosis, hunger are triggers for the release of vasopressin [11]. 2. To maintain osmotic balance, water moves freely between the intracellular and extracellular sectors under the influence of osmolality. 3. Rapid intercellular movement of water can cause cellular damage. Compensatory mechanisms for maintaining a normal volume of intracellular fluid are activated only after 48-72 hours and include both the accumulation of intracellular electrolytes (“fast adaptation”) and organic osmotically active substances (“slow adaptation”) [5].
Therefore, sodium reduction is recommended no faster than 0.5 mmol/l/h in order to avoid the development of cerebral edema, since during GN, brain cells are in a state of dehydration and rapid fluid administration can lead precisely to cellular hyperhydration [12]. Basic recommendations for correction
GN in burn patients comes down to calculating the deficit of water and electrolytes, the rate and duration of administration of infusion solutions and monitoring. There are acute and chronic disorders of water-salt metabolism: if the water-electrolyte imbalance persists for more than a day, they speak of a chronic condition. Undoubtedly, eliminating water imbalance is a top priority. In this case, it is necessary to calculate water deficiency using the formula: % theoretical water content in the body × current weight of the patient × ([Na] in blood plasma / norm [Na] – 1). An extremely important task is to monitor Na every 4-8 hours when correcting GN. Generally accepted recommendations are the use of hypotonic solutions (0.45% NaCl solution), bolus administration of saluretics, and spironolactone is mentioned in a few publications, but without clear recommendations [1, 3, 5]. Meanwhile, the safety of intravenous use of hypoosmolar solutions is questionable. The fact is that intravenous administration of hypoosmolar solutions, despite its apparent simplicity, is the most dangerous. Compensatory cells of the central nervous system retain electrolytes (fast adaptation) and accumulate organic osmotically active substances (slow adaptation). Therefore, rapid correction of GN or the use of hypo-osmolar solutions can provoke a deterioration in neurological status, including cerebral edema [5]. Bolus administration of large doses of saluretics also raises a number of questions. The most common method of controlling fluid balance is the use of furosemide. Furosemide is known to be a loop diuretic that has natriuretic and chloruretic effects by blocking the reabsorption of Na+ and Cl−. During the period of action, Na+ excretion increases significantly, but after its cessation, the excretion rate decreases below the initial level (rebound syndrome). The phenomenon is caused by a sharp activation of renin-angiotensin and other antinatriuretic neurohumoral regulation units in response to massive diuresis; stimulates the arginine-vasopressive and sympathetic systems. Thus, due to the rebound phenomenon during bolus administration, furosemide may contribute to the development of GN. Meanwhile, there is evidence that it is small doses of furosemide that provide the natriuretic effect [13]. In view of this circumstance, it seems relevant to assess the effect of furosemide on the development and course of GN when using small doses with titrated administration. A clearly underappreciated method is the use of high doses of spironolactone. Meanwhile, GN in extreme situations can be a consequence not only of dehydration, but also of sodium retention due to activation of the renin-angiotensin system. A number of studies on hypernatremia in CNS damage have unambiguously confirmed the role of pain, blood loss, hypoxia, and trauma in the subsequent activation of the renin-angiotensin system and sodium retention, even with limited infusion therapy and refusal to force diuresis [5]. The vascular effects of the sympathetic nervous system in response to pain, hypovolemia, injury, and/or changes in renal blood flow in critical illness cause activation of the renin-angiotensin-aldosterone system. In addition, in response to stress depletion of the glucocorticoid fraction, mineralocorticoids partially perform their function, so secondary hyperaldosteronism syndrome may develop in critically ill patients [7]. Thus, the questions remain: (1) is GN an iatrogenic effect or a consequence of hyperaldosteronism caused by burn shock, and (2) which method of correcting GN is effective and safe: sodium excretion or water administration? In addition, the question of the appropriateness and timeliness of continuous renal replacement therapy (CRRT) in the acute period of burn injury for extrarenal indications and for GN in particular is important.
Goal of the work -
development of an effective algorithm of actions for hypernatremia in burn patients.
Tasks:
1. Assess the effectiveness of various conservative methods for relieving GN. 2. To evaluate the effectiveness of continuous renal replacement therapy in the correction of GN and, based on the analysis of effectiveness, to clarify the indications for initiating CRRT for burn disease.
MATERIAL AND METHODS
In total, the retrospective study included 165 patients of both sexes with severe burn injury, hospitalized in the burn injury intensive care unit of the State Regional Clinical Clinical Hospital. The work was approved by the local ethical committee and complies with the “Rules of clinical practice in the Russian Federation” (Order of the Ministry of Health of the Russian Federation dated June 19, 2003 No. 266). Inclusion criteria: age 15-70 years, total area of burn injury II-III degree. more than 40%, or II-IV degree. area more than 20%, or II-III st. area of more than 20% + burn of the upper respiratory tract, stay in the ICU for burn injury for more than 3 days. GN was considered severe when the Na level was more than 150 mmol/L. All patients were on respiratory support, and 92% received inotropes/vasopressors. In 18 adult patients, PiCCO monitoring was used for additional monitoring of central hemodynamics, volumetric status and assessment of fluid accumulation in the interstitium of the lungs. Conservative methods for correcting GN in group I (2009-2011) included hypoosmolar solutions (5% glucose) with calculation of the required volume using the formula: % theoretical body water content × current weight of the patient × ([Na] in blood plasma / norm [Na] ] - 1). Considering the high risk of increasing neurological dysfunction and gas exchange disorders in patients with GN, the intravenous administration of hypoosmolar solutions has been limited since 2012. However, the introduction of water into the stomach in small volumes has shown to be safe, so the first main method of conservative correction is the introduction of drinking water per os at a dose of 20 ml/kg/day. At the same time, taking into account the leading role of hyperaldosteronism in the development of GN, we used spironolactone at a dose of 250-300 mg/day. Next, additional furosemide was prescribed titrated at a dose of 0.5-1.5 mg/kg/day. Such an introduction made it possible to avoid the “ricochet” effect, which in itself can increase sodium retention in the body. In addition, small titrated doses of furosemide make it possible to avoid dehydration and control fluid balance with an accuracy of ±1 ml/kg/day. In a number of cases, when these two methods were ineffective or impossible within 24 hours, the use of titrated small doses of furosemide was the only conservative method for correcting GN.
Thus, in the second group of patients (group II), in order to correct GN, the following were used: 1. Administration of water into the stomach 20-30 ml/kg/day in 4-6 doses. 2. Spironolactone at a dose of 250-300 mg/day. 3. Furosemide was administered intravenously titrated at a dose of 0.5-1.5 mg/kg/day.
If conservative methods for correcting GN were ineffective (Na > 160-163 mmol/l), continuous renal replacement therapy was initiated, in which hypernatremia was considered the main extrarenal indication for its initiation and implementation. For extended types of renal replacement therapy, the MultiFiltrate device was used, the following methods were used: HV-CVVH, CVVH, CVVHDF, paed-CVVH. During the procedures, sodium profiling was used in the substitute solution with an acceptable Naplasma/Naasubstitute difference of no more than 10 mmol/l. For the hybrid technology (slow low efficiency daily hemodialysis - SLEDD, sustained low efficiency daily dialysis), the Fresenius 5008 artificial kidney device was used, and the HDF method was used. Ultrafiltration for negative water balance was used depending on the degree of hyperhydration at an average rate of 1-3 ml/kg/hour. The convective dose of hemofiltration was 35-60 ml/kg/hour, UltraFlux AV hemofilters were used, and bicarbonate solutions (HF-23 and HF-42) were used as a substitute. The duration of one SLEDD procedure ranged from 6 to 10 hours, and a bicarbonate buffer and hi-flax membrane were also used. Prolonged anticoagulation was achieved by heparin infusion with titration of its rate to a target increase in APTT by 1.8-2 times. The main criteria for initiating CRRT were conditionally extrarenal, namely the increase in hypernatremia over 160-163. Statistical analysis was carried out using the statistical software package STATISTICA v.10 (StatSoft, USA). For comparative analysis, Fisher's exact test and χ2 test with Yates correction were used. The critical level of significance when testing hypotheses was taken equal to 0.05 (p < 0.05).
RESULTS
Total for the period 2009-2011. 92 patients were included in the study (group I). In all cases, hypoosmolar solutions (5% glucose) were used to correct hypernatremia with the required volume calculated using the formula: % theoretical body water content × patient’s current weight × ([Na] in blood plasma/normal [Na] – 1). Deaths – 41, i.e. overall mortality was 45% (Table).
Table. Mortality in severe burn injury depending on the tactics of hypernatremia correction
| Groups | Overall mortality (%) (n = 92/73) | Mortality of patients with GN (%) (n = 30/20) | Mortality of patients with GN in the first 14 days (%) (n = 30/20) |
| Group I | 45% (41 patients) | 73% (22 patients) | 60% (18 patients) |
| Group II | 41% (30 patients) | 60% (12 patients) | 25% (5 patients) |
| p – Fisher's exact test | p = 0.6382 | p = 0.3662 | p = 0.0213 |
| p – χ2 test (chi-square) with Yates correction | p = 0.7165 | p = 0.4960 | p = 0.0321 |
Note:
differences between groups were accepted as significant at p < 0.05.
Hypernatremia was observed in 33% of patients and occurred on days 4-6 after receiving a severe burn injury. Mortality in the group of patients with GN was 73%, which is consistent with the data of other authors. It is characteristic that the proportion of patients with GN who died in the first 14 days from the moment of injury, i.e. when the fatal outcome may be associated specifically with GN, was 60%. In a later period, death was caused mainly by septic complications of burn disease with the development of multiorgan disorders. In total in the period 2015-2017. The study included 73 patients with severe burn injury (group II). The overall percentage of patients with hypernatremia was 27%, and this complication also occurred on the 4-6th day after receiving a severe burn injury. Thus, there are practically no differences between the groups in these indicators. We did not note any significant differences in overall mortality rates in the analyzed groups. However, among the patients in whom we recorded the development of GN (33% and 27%, respectively), there was a decrease in mortality (73% in group I and 60% in group II). In addition, there was a significant, more than 2-fold, reduction in mortality (60% and 25%, respectively) in patients with GN who died in the first 14 days after injury, that is, when the death can be directly related to the development of GN -states. When using this conservative regimen for relieving GN in the second group, not a single case of polyuria, hypokalemia or impaired creatinine and urea clearance was observed.
Clinical example
Patient D. was admitted to the ICU for burn injury at the State Republican Regional Clinical Hospital on 10/01/2017 with the DS: “Burn injury stage II-III-IV, S burn 60%.” On the 6th day, despite conservative measures for the prevention and treatment of GN (water 20 ml/kg per os, spironolactone 300 mg/day), the sodium level was 162 mmol/l. Titrated furosemide 100 mg/day (1.3 mg/kg/day) was started. After 24 hours, the sodium concentration in the blood serum was close to optimal (154 mmol/l), and the rate of decrease in sodium concentration was 8 mmol/24 hours. At the same time, urea excretion was 611 mmol/day (with a norm of 250-570), daily diuresis was 1500 ml (2 ml/kg/day), i.e. within normal limits. The glomerular filtration rate is 96 ml/min, the degree of tubular reabsorption is 98.9%. Over the next 2 days, GN stopped. Thus, the use of small titrated doses of furosemide, at a dose not exceeding 1.5 mg/kg/day, was accompanied by increased sodium excretion without forcing diuresis and maintaining optimal concentration function. No rebound effect was noted. If conservative methods were ineffective, CRRT was used according to the protocols described in the “Material and Methods” section. In all cases of CRRT use, a decrease in blood sodium levels to normal levels was noted, which was accompanied by regression of signs of MODS. It became possible to provide full nutritional support and control electrolyte and hydrobalance both in cases of CRRT and in cases of SLEDD technology. During the period 2009-2011. CRRT was initiated in 10 patients out of 30 in whom the development of GN was noted (33%), 7 survived. In all three cases of death, a late start of CRRT was noted both in terms of the timing of the burn disease and in plasma sodium levels (Na > 170 mmol/l ). During the period 2015-2017. CRRT was initiated early, also in a third of patients – in 6 out of 20 cases when conservative correction of GN was impossible (30%). 3 patients died more than 14 days after injury as a result of late septic complications of burn disease. An analysis of each clinical case showed that the best results were obtained with early initiation of RRT - within 7 days from the moment of injury. The best results were obtained in patients with a large area of dermal burns and critically increasing GN (plasma Na > 163 mmol/l). Monitoring central hemodynamics and volumetric status using PiCCO technology is also optimal. SLEDD technology can be used as an economically more acceptable alternative to CRRT. However, with a late onset (Na > 170 mmol/l, 12-14 and later days of burn disease) with the development of sepsis and the impossibility of surgically removing the scab by this time, positive results from the use of CRRT methods were not noted. Overall mortality among patients with GN in the second group decreased by 13% compared to the first group. The most important argument in favor of the proposed strategy is a more than twofold reduction in mortality (60% and 25%, respectively) among patients with GN in the first 14 days after receiving a burn injury, i.e. during the period when the negative impact of GBV is most pronounced. In the overwhelming majority of cases, the cause of death of these patients was late purulent-septic complications during the period when the water-salt imbalance had already been eliminated.
CONCLUSIONS:
1. Conservative methods of eliminating hypernatremia (water load 20-30 ml/kg/day per os + spironolactone 200-300 mg/day + furosemide 0.5-1.5 mg/kg/day titrated intravenously) were safe and effective with timely correction (145 mmol/l < Na < 163 mmol/l). Small doses of furosemide with titrated administration (60-100 mg/day or 0.5-1.5 mg/kg/day) help reduce serum sodium levels (8-10 mmol/l/day) during GN. In this case, there is no “ricochet” effect, the level of potassium in the blood serum does not change, the degree of reabsorption and the volume of diuresis do not change. 2. The use of CRRT for GN in burn patients is the most justified extrarenal indication for this method. The greatest effectiveness of RRT was noted when the procedure was started early (Na < 163 mmol/l no later than 7 days from the moment of injury). However, with a late onset (Na > 170 mmol/l and with the development of purulent-septic complications of burn disease), positive results from the use of CRRT methods were not noted.
Funding and conflict of interest information
The study had no sponsorship. The authors declare that there are no obvious or potential conflicts of interest related to the publication of this article.
LITERATURE:
1. Ushakova TA, Alekseev AA. Hyperosmolar syndrome in burn trauma. Laboratory Diagnostics.
2015;
(6): 44-48. Russian (Ushakova T.A., Alekseev A.A. Hyperosmolar syndrome in burn injury // Laboratory diagnostics. 2015. No. 6. P. 44-48) 2. Maggiore U, Picetti E, Antonucci E, Parenti E, Regolisti G , Mergoni M et al. The relation between the incidence of hypernatremia and mortality in patients with severe traumatic brain injury. Crit.
Care Med .
2009; 13(4): R110-R115 3. Namdar T, Stollwerck PL, Stang FH, Kolios G, Lange T, Mailänder P. et al. Progressive fluid removal can avoid electrolyte disorders in severely burned patients. Ger.
Med. Sci .
2011; 57(3): 30-49 4. Snyder NA, Feigal DW, Arieff AI. Hypernatremia in elderly patients: A heterogeneous, morbid, and iatrogenic entity. Ann.
Intern. Med. 1987;
107(3): 309-319 5. Adrogue HJ, Madias NE. Hypernatriemia. N.Engl.
J. Med. 2009;
20: 1493-1499 6. Fistal EYa, Speranskiy II, Samoylenko GE, Arefyev VV. Pathogenesis, classification and treatment of edema syndrome in burn injury. Combustiology
.
2008; (34): 18-26. Russian (Fistal E.Ya., Speransky I.I., Samoilenko G.E., Arefiev V.V. Pathogenesis, classification, diagnosis and treatment of edema syndrome in burnt patients // Combustiology. 2008. No. 34. P. 18-26 ) 7. Zaychik ASh, Churilov LP. Common Pathology. Part 2. Basics of pathological chemistry. Saint Petersburg: ELBI-SPB, 2000. 688 p. Russian (Zaychik A.Sh., Churilov L.P. Fundamentals of general pathology. Part 2. Fundamentals of pathochemistry. St. Petersburg: ELBI-SPB, 2000. 688 p.) 8. Lin M, Liu SJ, Lim IT. Disorders of water imbalance. Emerg. Med. Clin. North Am
.
2005; 23(3): 749-770 9. Abramow M, Beauwens RC, Cogan E. Cellular events in vasopressin action. Kidney Int.
Suppl. 1987;
21: S56-S66 10. McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL et al. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J Neuroendocrinol.
2004;
16(4): 340-347 11. Robertson GL. Antidiuretic hormone: normal and disordered function. Endocrinol. Metab.
Clin. North Am. 2001;
30(3): 671-694 12. Kahn A, Brachet E, Blum D. Controlled fall in natremia and risk of seizures in hypertonic dehydration. Intensive Care Med.
1979;
5: 27-31 13. Mahabir RN, Bacchus R. Diuretic and clinical effects of low-dose furosemide in congestive heart failure patients. J Clin.
Pharmacol. 1976; 16(10): 510-517
View statistics
Loading metrics...
Links
- There are currently no links.
Diagnostics
Patients with this condition are supervised by resuscitators together with specialized specialists (endocrinologist). During examination, pay attention to hyperreflexia, muscle tone, and clarity of consciousness. It is important to determine the type of hypernatremia, since the course of different types of pathology is different. They focus on symptoms such as signs of dehydration (dry skin and mucous membranes, decreased turgor) or peripheral edema and pressure indicators.
Before obtaining laboratory results, differentiation of hypernatremia is carried out primarily from hyponatremia due to very similar clinical symptoms. To confirm the diagnosis and establish the cause of the condition, the following techniques are used:
- Laboratory research.
Serum sodium concentration and osmolarity are measured. In a biochemical blood test, the levels of glucose, urea, creatinine and liver transaminases (ALT, AST) are assessed. If primary hyperaldosteronism is suspected, the renin-aldosterone ratio is examined. The specific gravity, osmolarity of urine, the content of sodium, protein and glucose in it are studied. - Instrumental research.
Determining the volume of the vascular bed by measuring central venous pressure in the subclavian veins provides invaluable assistance in clarifying the type of hypernatremia. To diagnose diseases that could cause the pathology, an abdominal CT scan and echocardiography are performed.
Material and methods
We examined 150 patients aged from 30 to 80 years with severe atherothrombotic or cardioembolic IS (more than 14 points on the NIHSS scale) with a disease duration at the time of hospitalization of no more than 12 hours. Diagnosis II. was established on the basis of the clinical picture and in all cases was confirmed by X-ray CT and MRI data of the brain. Treatment was carried out in the Regional Vascular Center. All patients received the most unified basic therapy for IS in accordance with the recommendations of the European Stroke Organization (ESO, 2008) and the standards of the Ministry of Health of the Russian Federation, aimed at normalizing homeostasis, central and cerebral hemodynamics, and preventing complications.
All patients were monitored the concentration of sodium and other ions in the blood plasma up to 2 times a day for 5 days. The level of plasma osmolarity was determined on the 1st, 3rd and 5th days of stroke. For patients with hypo- and hypernatremia, the level of sodium in the urine was determined. We studied the level of ADH on the 1st day of stroke. Volume and circulating blood volume (CBV) were assessed by studying the level of hematocrit, central venous pressure for patients without signs of pulmonary hypertension, and in some cases using plethysmography.
The dependence of the probability of death on sodium concentration and plasma osmolarity on the 1st, 3rd, and 5th days of the disease was studied using the nonlinear regression method. Differences between models were determined for fixed segments of the initial indicator, Fisher and Student tests were used, differences were recognized as significant when p
<0.05. Associations between stroke severity as assessed by the NIHSS scale, stroke outcome, and plasma sodium concentration and osmolarity were also examined. A comparison was made of the course of stroke and mortality in hypovolemia in comparison with normo- and hypervolemia.
Treatment of hypernatremia
All patients with acute conditions are transferred to the intensive care unit. Patients with chronic hypernatremia can be treated in a general ward. The main condition for successful therapy is constant monitoring of water balance (taking into account the amount of fluid injected and excreted) and the concentration of blood electrolytes. Treatment includes the following main areas:
- Replenishment of fluid deficiency.
In the hypovolemic form of hypernatremia, it is necessary to restore the proper level of free water in the body. Conscious patients are given oral hydration. For vomiting, mental disorders, and coma, intravenous administration of a hypotonic NaCl solution (0.5%) is used, which also reduces the concentration of sodium in the blood. - Active decrease in plasma Na .
To enhance sodium excretion, loop diuretics (furosemide) are indicated. Additionally, a 5% solution of dextrose or glucose with insulin is used. The sodium level is reduced gradually (in the acute form - no more than 1 mmol/hour, in the chronic form - no more than 8 mmol/day), since a high rate of correction is associated with the risk of cerebral edema. - Treatment of the underlying disease.
Particular attention is paid to hypernatremia that has developed against the background of diabetes insipidus (central, nephrogenic or drug-induced), since if there is a deficiency of antidiuretic hormone without replacement therapy, treatment will be ineffective. Synthetic analogues of ADH (desmopressin) are recommended.
Hypernatremia: symptoms, causes, treatment
- Due to osmotic diuresis Typically, the patient exhibits signs of a hypovolemic state.
- The most common cause of osmotic diuresis is hyperglycemia and glucosuria against the background of uncompensated diabetes mellitus. Hyperosmolar hyperglycemic state (HHS) is commonly observed in elderly patients with type 2 diabetes mellitus and is associated with mortality in approximately 11% of cases. The disease course includes hyperglycemia, dehydration, and hyperosmolarity without ketoacidosis.
- Loop diuretics, such as furosemide and torsemide, may cause renal sodium loss; they form an isosmotic solution of diuresis, which leads to an impairment of the concentrating ability of the kidneys, which reduces the reabsorption of water.
- Increased urea production (eg, due to a high-protein diet) and mannitol administered intravenously can also lead to osmotic diuresis.
- Any obstruction of urinary output (eg, due to benign prostatic hypertrophy, prostatitis, prostate cancer, nephrolithiasis, bladder tumors, urethral stricture) often results in post-obstructive diuresis once the obstruction is relieved. Consequently, there is a loss of free water, which can lead to hypernatremia.
- As a rule, the patient exhibits signs of a normovolemic state. Hypernatremia, secondary to nonosmotic renal water loss, is usually caused by central diabetes insipidus, which is characterized by delayed vasopressin secretion, or by renal diabetes insipidus, which results from resistance to the action of vasopressin.
Non-renal losses
May occur due to unnoticeable water loss, such as evaporation from the surface of the skin. These patients show signs of a normovolemic or hypovolemic state (eg, in cases of severe burns, patients usually show signs of a normovolemic state within the first 48 hours, after which a transition to a hypovolemic state occurs). Unnoticeable water losses can also occur as a result of losses in the gastrointestinal tract. These patients show signs of a hypovolemic state.
- Unnoticeable losses may increase with fever, exercise, heat, and severe burns. Severe burns, usually of thermal origin, cause an increase in capillary permeability and the release of fluid from the vessels into other fluid spaces of the body in the first 24 hours after the burn (the patient is in a normovolemic state). Over time, damage to the skin causes increased evaporation, increasing unnoticeable water loss. With regard to heat and sweat production, the concentration of sodium in sweat decreases with prolonged profuse sweating, which increases the loss of dissolved water.
- The most common cause of gastrointestinal hypernatremia is severe diarrhea, and the patient usually exhibits signs of a hypovolemic state. Osmotic diarrhea caused by ingestion of lactulose or sorbitol, carbohydrate malabsorption (most often as a result of canker sores, bowel resection, lactose intolerance, or pancreatitis), and viral gastroenteritis result in water loss that exceeds sodium and potassium losses. This differs from secretory diarrhea, in which stool osmolality is equal to plasma osmolality, and is manifested by a decrease in volume with normal or low plasma sodium concentration. A patient with prolonged vomiting, regardless of the cause, may lose more water than sodium, resulting in decreased interstitial fluid volume and hypernatremia.
- Intestinal fistulas (fistulas are areas that connect 2 epithelial areas) can also cause unnoticed gastrointestinal losses; may occur as a complication of Crohn's disease.
Deficiency of water consumption
Usually the result of limited access to water or a weakened thirst mechanism. The patient exhibits signs of a hypovolemic state.
- People who may have limited access to water include young children, people with disabilities, people with cognitive impairment, post-operative patients, nursing home patients, and intubated ICU patients.
- Weakening of the thirst mechanism as a result of primary adipsia is rare and is caused by damage to the hypothalamic osmoreceptors that regulate the feeling of thirst. It may be associated with a variety of pathological changes, including vascular occlusions, tumors, congenital hypothalamic lesions, and granulomatous diseases (eg, sarcoidosis).
- Inadequate breastfeeding without additional nutrition can lead to severe hypernatremia, potentially life-threatening in a young child.
Increased sodium concentration
Hypernatremia due to elevated sodium concentrations is rare. Sodium overload caused by external causes is often associated with severe hypernatremia (plasma sodium concentration >170 mmol/L [170 mEq/L]). These patients usually show signs of a hypovolemic state. Reasons include:
- Unintentional administration of hypertonic sodium chloride (eg, for irrigation of hydatid cysts) or sodium bicarbonate (eg, for severe metabolic acidosis)
- Administration of isotonic sodium chloride solution (saline) to a patient with diabetic ketoacidosis (DKA) with osmotic diuresis
- Replacing sugar with salt in formula (accidental or intentional)
- Excessive ingestion of salt (for example, using Epsom salt as an inducer for vomiting or as a gargle).
Excess mineralocorticoid hormones
Such patients show signs of a hypervolemic state.
- Cushing's syndrome, regardless of primary or secondary etiology, causes a significant increase in cortisol, which increases serum glucose levels, which often leads to uncontrolled diabetes and hypernatremia (due to sodium retention from fluid retention).
- Primary hyperaldosteronism leads to increased sodium reabsorption, resulting in increased extracellular fluid volume. A sustained small increase in extracellular fluid volume increases the production of ADH, which regulates osmostat, and also increases thirst with increases of a few mmol per liter (mEq/L). As a result, patients with primary hyperaldosteronism typically have stable plasma sodium concentrations between 143 mmol/L (143 mEq/L) and 147 mmol/L (147 mEq/L) (mild hypernatremia).
Urgent measures
Hypernatremia is usually mild unless there is an abnormal response to thirst or access to water is not limited. However, if this occurs, severe hypernatremia may occur, which can have profound consequences, including cerebrovascular injury and death.
Severe hypernatremia
Defined as plasma sodium concentration >158 mmol/L (158 mEq/L); Severe signs and symptoms such as pyrexia, delirium, seizures and coma may occur and require immediate treatment of the condition. Symptoms in older adults may be nonspecific, but cases of recent changes in consciousness are associated with a poor prognosis. Osmotic demyelination is rare, but sequelae have been reported.
The initial stage of treatment is based on stabilizing patients in a hypovolemic state using an isotonic solution. Once the patient's condition has stabilized, the free water deficit can be corrected with oral and intravenous fluids for 48 hours. The free water deficit can be calculated using the following equation:
- Free water deficiency = body weight (kg) x ratio of total body weight (for men 0.6; for women 0.4), expressed as a percentage x ( - 1).
Patients in a normovolemic state should be treated with hypotonic solutions, such as dextrose aqueous solution 5%, to correct free water deficiency. Patients in a hypervolemic state require correction with diuretics in addition to hypotonic fluids. To avoid cerebral edema, serum sodium should be adjusted no faster than 0.5 mmol/L (0.5 mEq/L) hourly.
Diseases that require immediate correction of hypernatremia
Hypernatremia, which is a consequence of the hyperosmolar hyperglycemic state (HGS) in diabetic patients, requires immediate correction due to the serious nature of the disease. HGS is observed predominantly in elderly patients with type 2 diabetes. Although the condition accounts for <1% of all diabetes-related hospitalizations, the mortality rate is high (5% to 15%).
The main goals of treatment for HGS are to restore blood volume deficiency (patients with hypernatremia are recommended to be given 0.45% saline, which should be replaced with 5% dextrose with 0.45% saline when blood glucose reaches 16.7 mmol/L [300 mg/dL], normalization of hyperglycemia (continuous but gradual reduction in blood glucose and plasma osmolality using low-dose insulin therapy), correction of additional electrolyte imbalances (potassium concentration should be >3.3 mmol/L [3.3 mEq/L ] before starting insulin therapy), treatment of aggravating circumstances, and prevention of consequences. Most patients will require admission to the intensive care unit.
Hypernatremia associated with breastfeeding is an emergency. Infants are predisposed to dehydration due to their low body water reserves and relatively high potential for evaporative water loss. Lack of milk production in the mother without supplementation or difficulty sucking in the infant can quickly lead to severe dehydration and hypovolemic hypernatremia. Replenishment of circulating blood volume should be started immediately with intravenous administration of isotonic saline.
Both central and nephrogenic diabetes insipidus are associated with plasma sodium concentrations >170 mmol/L (170 mEq/L), requiring immediate correction with intravenous hypotonic solutions (eg, 5% dextrose water, quarter to half isotonic saline) due to the high risk of cerebrovascular injury and death.
Exogenous intake of sodium by ingestion or infusion, such as the accidental administration of hypertonic sodium chloride or sodium bicarbonate, accidental or intentional salt poisoning of an infant, or the ingestion of a highly concentrated emetic or gargle, often results in severe hypernatremia (sodium concentration plasma > 190 mmol/L [190 mEq/L]), which requires immediate action. Intravenous administration of a 5% dextrose water solution along with the use of a diuretic to remove excess sodium is the key to therapy.
Diagnostics
Hypernatremia is defined as a plasma sodium concentration greater than 145 mmol/L (145 mEq/L). A thorough physical examination should be performed, including assessment of the degree of dehydration, mental status, and neurological evaluation. Symptoms and signs of hypernatremia are relatively nonspecific. These usually include manifestations from the central nervous system, in particular, irritability, anxiety, lethargy, muscle twitching, spasticity, hyperreflexia, which are a consequence of reduced water content in the brain.
Severe hypernatremia (plasma sodium concentration >158 mmol/L [158 mEq/L]) may present with serious signs and symptoms such as pyrexia, delirium, seizures, and coma. Having established the presence of hypernatremia, the underlying cause should be sought.
Anamnesis
The age and mobility of the patient may help determine the etiology. Limited access to water should be considered for infants, people with disabilities, people with cognitive impairment, postoperative patients, and intubated intensive care unit patients. A complete medical history should be obtained and any chronic diseases should be excluded, especially severe uncontrolled diabetes mellitus (can lead to hyperglycemia and further glycosuria, hypernatremia, and hyperosmolar hyperglycemic state), Cushing's syndrome, primary aldosteronism, underlying kidney disease (eg, sickle cell disease, obstructive uropathy, as well as reflux nephropathy) or Crohn's disease (may be a consequence of intestinal fistula).
If there is a history of diarrhea or loose stools, the patient should be asked about the use of any laxative or cleansing fluid (eg, lactulose or sorbitol). In addition, any conditions (sprue, pancreatitis, and lactose intolerance) that may provoke carbohydrate malabsorption, in particular a history of intestinal surgery, should be excluded. Symptoms of viral gastroenteritis should be established (nausea, vomiting, abdominal pain that accompany diarrhea). These patients may have a history of exposure to infected people, food, or body fluids. A history of prolonged vomiting should be excluded. Regardless of the cause, these patients may lose more water than sodium, resulting in decreased circulating blood volume and hypernatremia.
A complete medical history is important to determine the cause of hypernatremia. Some drugs, in particular colchicine, gentamicin, lithium, rifampin, propoxyphene, can provoke nephrogenic diabetes insipidus. In addition, loop diuretics (eg, furosemide and torsemide) and intravenous mannitol may stimulate osmotic diuresis with subsequent hypernatremia.
Traumatic brain injury or a history of brain damage (vascular syndromes, infections, tumors, or aggressive neurosurgery to treat craniopharyngioma, Rathke's pouch cyst, or other hypothalamic tumor) may be a reason to suspect central diabetes insipidus. In rare cases, the presence of a brain tumor or cerebral vascular occlusion may lead to primary adipsia, which is manifested by a hypernatremic decrease in circulating blood volume.
Increased body temperature and external influences should also be considered. Prolonged exposure to heat, fever, excessive sweating, exercise, and severe skin burns lead to imperceptible water loss due to evaporation from the skin surface and can provoke hypernatremia.
A history of recurrent urinary tract infections and pneumaturia (gas in the urine, usually described as bubbles in the urine) may be a reason to suspect a enterovesical fistula (connecting the intestine to the bladder). If gas or feces enters the vagina, a enterovaginal fistula (connecting the intestine to the vagina) should be suspected. Drainage of intestinal contents onto the surface of the skin gives reason to suspect an external enteric fistula, while vomiting with feces is a symptom of an intestinal fistula.
Iatrogenic causes may be characteristic of patients who are currently being treated in hospital. These include the inadvertent use of hypertonic sodium chloride, sodium bicarbonate, or even the use of isotonic saline in patients with osmotic diuresis. Diet history should be considered. Inappropriate breastfeeding without supplementation can result in severe and potentially life-threatening hypernatremia in the infant. Replacing formula sugar with salt (accidentally or intentionally) can also cause hypernatremia.
A protein-rich diet, particularly high-protein tube feeding, results in increased urea production and subsequent osmotic diuresis, increasing the risk of hypernatremia. In addition, the possible ingestion of a highly concentrated emetic or gargle (eg, Epsom salts) should be considered.
Physical examination
Typically, the easiest way to determine the etiology is to determine the degree of dehydration of the patient. However, it should be noted that extracellular fluid volume is better preserved in hypernatremia at the expense of greater loss of intracellular fluid, and physical examination may thus underestimate total body water loss.
- Hypovolemic hypernatremia Includes renal and extrarenal losses. In patients with signs of dehydration (dry mucous membranes, poor skin turgor, sunken eyes, irritability, tachycardia, hypotension, decreased urine output and weight loss), severe diarrhea, vomiting and significant burns should be excluded. It is important to take your temperature because fever can cause this condition. Diuretic use, post-obstructive diuresis, and underlying renal dysfunction (eg, sickle cell disease, obstructive uropathy, and reflux nephropathy) may also cause hypovolemic hypernatremia. Primary adipsia should be considered, although it is rare. The possibility of developing an intestinal fistula should also be taken into account. Patients with hyperosmolar hyperglycemic state (HHS) usually show signs of severe dehydration and may have neurological deficits (hemianopia or hemiparesis). In many cases, the clinical signs of HGS and hypernatremia overlap and occur simultaneously.
- These patients may exhibit signs of volume overload, including weight gain, peripheral edema, hypertension, irritating cough, dyspnea, jugular venous distension, and crepitus to auscultation. Usually due to exogenous sodium ingestion and excessive mineralocorticoids. Thus, signs of Cushing's syndrome as well as primary aldosteronism should be looked for. Cushing's syndrome: moon face, facial congestion, supraclavicular and/or dorsocervical fat accumulations, trunk obesity, purpuric stretch marks, proximal muscle weakness, hirsutism, growth retardation (in children), hypertension.
- There are no signs of a decrease in the volume of intercellular fluid or overload. The main cause is nephrogenic diabetes insipidus or central diabetes insipidus. A patient with central diabetes insipidus may have signs of recent trauma, pituitary surgery, or hypoxic or ischemic encephalopathy.
Basic laboratory tests
A plasma sodium concentration >145 mmol/L (145 mEq/L) confirms the presence of hypernatremia. A serum sodium concentration of 150-170 mmol/L (150-170 mEq/L) usually indicates dehydration and related causes. Serum sodium concentrations >170 mmol/L (170 mEq/L) are usually associated with diabetes insipidus (nephrogenic or central). Serum sodium concentrations >190 mmol/L (190 mEq/L) usually result from exogenous sodium intake.
Urine osmolality can help determine the underlying etiology. The normal renal response to hypernatremia is for the kidneys to excrete a minimal amount of urine that is maximally concentrated (urine osmolality >800 mmol/kg [800 mOsm/kg]). Hypertonic urine usually occurs in conjunction with extrarenal fluid loss, as is the case with vomiting, diarrhea, burns, and excessive sweating. Isotonic urine may accompany the use of diuretics, osmotic diuresis, and salt loss. Hypotonic urine associated with polyuria is observed in diabetes insipidus (central or nephrogenic).
A metabolic panel, including serum glucose, potassium, chloride, urea, and creatinine concentrations, should also be part of the initial workup to rule out electrolyte abnormalities and renal failure. In addition, a complete blood count should also be performed as a baseline investigation in cases of severe burns to rule out sepsis, and patients with prolonged vomiting or hypernatremia associated with breastfeeding may require an arterial blood gas test to rule out related violation of acid-base balance.
Specific laboratory tests
For patients with severe diarrhea, stool laboratory tests (fecal leukocytes, fecal pH, fecal reducing agent/sugar) can help determine whether the etiology is infectious in origin or due to carbohydrate malabsorption. Ion gap obtained from stool examination helps distinguish between osmotic and secretory diarrhea; Secretory diarrhea does not usually lead to hypernatremia.
Diabetes insipidus can usually be confirmed on the basis of hypertonic hypernatremia and increased hypotonic urine production (>3 L/24 hours), with commensurately increased fluid intake in response to thirst. Partial or mild cases of the disease may require confirmation through additional studies at a specialized center where a water deprivation test is performed. Plasma arginine vasopressin (AVP) levels do not always distinguish central diabetes insipidus from nephrogenic diabetes insipidus; The medical history usually indicates that central diabetes insipidus or nephrogenic diabetes insipidus is more likely, with confirmation coming from the response (or lack thereof) to an arginine-vasopressin (desmopressin) stimulation test.
If primary aldosteronism is suspected based on history or physical examination, the diagnosis is supported by a decreased plasma renin activity (PRA) level and a high plasma aldosterone concentration (PAC), giving a high PCA:PR ratio. Diagnostic specificity can be enhanced by ensuring that the patient has replaced potassium losses first and, where possible, temporarily avoiding the use of medications, in particular diuretics, beta-blockers, ACE inhibitors, and angiotensin II receptor antagonists, several weeks before research. An aldosterone suppression test may be needed to confirm the diagnosis. If plasma renin activity and plasma aldosterone concentrations are normal, or if there is clinical suspicion of occult vomiting or laxative abuse, a 24-hour urine potassium concentration test may be considered.
A serum osmolality >320 mmol/kg (320 mOsm/kg) together with a plasma glucose concentration >33.3 mmol/L (600 mg/dL) in patients with cognitive impairment strongly suggests HGS. Significantly increased serum osmolality is also observed in primary adipsia.
If Cushing's syndrome is suspected, the diagnosis can be confirmed with a 24-hour urine free cortisol test, a low-dose (or overnight) dexamethasone suppression test, and a midnight serum or salivary cortisol concentration test.
Visualization methods
CT or MRI of the head can reveal the central cause of hypernatremia and should be performed to evaluate all patients with severe hypernatremia and no underlying etiology. In addition, brain imaging helps rule out intracranial bleeding caused by tension in the connecting veins of the dura mater and sinuses due to brain recession. Dural sinus thrombosis may develop due to a decrease in plasma volume due to general loss of water and can also be detected using CT or MRI. CT or MRI of the adrenal glands may reveal an adrenal tumor, which may be the underlying cause in patients with suspected primary aldosteronism.
Prognosis and prevention
Hypernatremia is a severe electrolyte disorder with a poor prognosis. Death occurs in 50% of cases in the acute form and in 10-15% in the chronic form. In children, the mortality rate is much higher - about 65-70%. Approximately 2/3 of surviving patients have residual effects in the form of neurological deficit to varying degrees.
Prevention consists of proper treatment of diseases against which hypernatremia may develop (diabetes mellitus, diabetes insipidus, aldosteroma). The occurrence of this disorder in patients receiving hypotonic solutions or osmotic diuretics can be prevented by regular monitoring of sodium levels.