A problem in which the blood does not clot well is called a bleeding disorder. It is caused by the fact that blood vessels do not normally become blocked when they are damaged.
When everything is fine, when there is bleeding at the wound site, the blood begins to thicken, which prevents large losses. But sometimes this complex mechanism fails, resulting in severe or prolonged bleeding.
When blood does not clot well, this does not always lead to external loss. It can also appear as bleeding under the skin or in the brain.
Causes
Bleeding disorders are divided depending on the etiology - acquired, genetically determined and congenital, as well as autoimmune.
Blood clotting disorders can be caused by hereditary pathologies, but not only. Genetic diseases can also influence the development of the disease. For example, a newborn baby may be diagnosed with hemophilia or von Willebrand disease.
Also, the disorder can be caused by a lack of vitamin K. In addition, such problems can be a consequence of cancer of the liver and other organs.
Most often, bleeding disorders occur due to infectious hepatitis or scarring, which usually occurs with cirrhosis.
Long-term use of strong antibiotics or drugs to treat blood clots can also cause bleeding problems.
The role of hemocoagulation
The platelet process is complex and stepwise. Hemocoagulation is a reaction that can protect the body from significant blood loss in case of vascular damage. Otherwise, sudden death will occur. After all, poor coagulation is fraught with bleeding, which is difficult to stop.
If coagulation is normal, then even with severe damage to blood vessels, the blood will clot in 7 minutes. A blood clot will form, which will protect the body from blood loss and stop the bleeding. The mechanism of hemocoagulation is:
- platelet clot formation,
- the production of fibrin, a fibrous thread-like substance that promotes the formation of a blood clot,
- involvement of plasma protein – fibrinogen – in the process.
This is how a chain reaction occurs. Intensive blood clotting factors are activated and react with calcium ions, converting fibrinogen and thrombin into insoluble jelly-like substances. Fibrin is transformed into a long thin network, and when formed blood cells enter it, a blood clot appears.
Symptoms
Main symptoms of the disease:
- Skin rashes. Depending on the cause of poor coagulation, both small pinpoint hemorrhages (petechiae) and extensive hematomas may appear on the skin.
- Nosebleeds.
- Hemorrhages in the mucous membranes of the mouth, nose, and intestines. The latter option can lead to the appearance of blood in the stool.
- Hemorrhages in the brain. Occurs with fragility of blood vessels and low blood clotting.
- In the event of an injury, cut, or bruise, the bleeding does not stop for a long time.
- Hemorrhages in joints, muscles and internal organs can be observed with the hereditary disease hemophilia. Bruises (hematomas) can form even with minor mechanical damage.
How does blood clotting work?
Stopping bleeding is based on the same idea that housewives use to prepare jellied meat - the transformation of liquid into a gel (a colloidal system in which a network of molecules is formed, capable of holding in its cells a thousand times its weight due to hydrogen bonds with water molecules). By the way, the same idea is used in disposable baby diapers, which contain material that swells when wet. From a physical point of view, it is necessary to solve the same problem as in coagulation - combating leaks with minimal effort.
Blood coagulation is the central element of hemostasis (stopping bleeding). The second link of hemostasis is special cells - platelets - that are capable of attaching to each other and to the site of injury to create a plug that stops the blood.
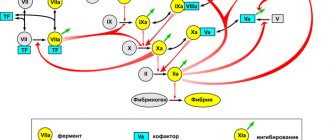
A general idea of the biochemistry of coagulation can be obtained from Figure 1, which shows the reaction that converts the soluble protein fibrinogen into fibrin, which then polymerizes into a network. This reaction is the only part of the cascade that has a direct physical meaning and solves a clear physical problem. The role of the remaining reactions is exclusively regulatory: to ensure the conversion of fibrinogen into fibrin only in the right place and at the right time.
Figure 1. Basic blood clotting reactions. The coagulation system is a cascade - a sequence of reactions, where the product of each reaction acts as a catalyst for the next. The main “entrance” to this cascade is in its middle part, at the level of factors IX and X: the protein tissue factor (indicated in the diagram as TF) binds factor VIIa, and the resulting enzymatic complex activates factors IX and X. The result of the cascade is the protein fibrin , capable of polymerizing and forming a clot (gel). The vast majority of activation reactions are proteolysis reactions, i.e. partial breakdown of the protein, increasing its activity. Almost every coagulation factor is necessarily inhibited in one way or another: feedback is necessary for stable operation of the system.
Legend: Reactions that convert coagulation factors into active forms are shown by one-sided thin black arrows. At the same time, curly red arrows show under the influence of which enzymes activation occurs. Loss of activity reactions due to inhibition are shown by thin green arrows (for simplicity, the arrows are depicted as simply “leaving”, i.e., it is not shown which inhibitors bind to). Reversible complex formation reactions are indicated by double-sided thin black arrows. Coagulation proteins are designated either by names, Roman numerals, or abbreviations ( TF —tissue factor, PC —protein C, APC —activated protein C). To avoid overload, the diagram does not show: binding of thrombin to thrombomodulin, activation and secretion of platelets, contact activation of coagulation.
[1], figure adapted
Fibrinogen resembles a rod 50 nm long and 5 nm thick (Fig. 2a). Activation allows its molecules to stick together into a fibrin thread (Fig. 2b), and then into a fiber capable of branching and forming a three-dimensional network (Fig. 2c).
Figure 2. Fibrin gel. a — Schematic structure of the fibrinogen molecule. Its basis is composed of three pairs of mirror-arranged polypeptide chains α, β, γ. In the center of the molecule you can see binding regions that become accessible when thrombin cuts off fibrinopeptides A and B (FPA and FPB in the figure). b — The assembly mechanism of fibrin fiber: the molecules are attached to each other “overlapping” according to the head-to-middle principle, forming a double-stranded fiber. c — Electron micrograph of the gel: fibrin fibers can stick together and split, forming a complex three-dimensional structure.
[2–4]
Figure 3. Three-dimensional structure of the thrombin molecule. The diagram shows the active site and parts of the molecule responsible for binding thrombin to substrates and cofactors. (The active site is the part of the molecule that directly recognizes the site of cleavage and carries out enzymatic catalysis.) Protruding parts of the molecule (exosites) allow the thrombin molecule to be “switched,” making it a multifunctional protein capable of working in different modes. For example, binding of thrombomodulin to exosite I physically blocks access of procoagulant substrates (fibrinogen, factor V) to thrombin and allosterically stimulates activity towards protein C.
[5]
The fibrinogen activator thrombin (Fig. 3) belongs to the family of serine proteinases - enzymes capable of cleaving peptide bonds in proteins. It is related to the digestive enzymes trypsin and chymotrypsin. Proteinases are synthesized in an inactive form called zymogen. To activate them, it is necessary to cleave the peptide bond holding the part of the protein that closes the active site. Thus, thrombin is synthesized in the form of prothrombin, which can be activated. As can be seen from Fig. 1 (where prothrombin is designated factor II), it is catalyzed by factor Xa.
In general, coagulation proteins are called factors and are numbered with Roman numerals in order of official discovery. The subscript “a” indicates the active form, and its absence indicates an inactive precursor. Proper names are also used for long-discovered proteins, such as fibrin and thrombin. Some numbers (III, IV, VI) are not used for historical reasons.
The coagulation activator is a protein called tissue factor, which is present in the cell membranes of all tissues, with the exception of the endothelium and blood. Thus, the blood remains liquid only due to the fact that it is normally protected by a thin protective membrane of the endothelium. When there is any violation of the integrity of the vessel, tissue factor binds factor VIIa from the plasma, and their complex - called external tenase (tenase, or Xase, from the word ten - ten, i.e. the number of the activated factor) - activates factor X.
Thrombin also activates factors V, VIII, XI, which leads to an acceleration of its own production: factor XIa activates factor IX, and factors VIIIa and Va bind factors IXa and Xa, respectively, increasing their activity by orders of magnitude (the complex of factors IXa and VIIIa is called intrinsic tenase). A deficiency of these proteins leads to severe disorders: for example, the absence of factors VIII, IX or XI causes the most severe disease hemophilia (the famous “royal disease” that Tsarevich Alexei Romanov suffered from); and deficiency of factors X, VII, V or prothrombin is incompatible with life.
This arrangement of the system is called positive feedback: thrombin activates proteins that accelerate its own production. And here an interesting question arises: why are they needed? Why can’t a reaction be made fast right away? Why does nature make it initially slow, and then come up with a way to further speed it up? Why is there duplication in the coagulation system? For example, factor X can be activated by both the VIIa–TF complex (extrinsic tenase) and the IXa–VIIIa complex (intrinsic tenase); it seems completely pointless.
Coagulation proteinase inhibitors are also present in the blood. The main ones are antithrombin III and tissue factor pathway inhibitor. In addition, thrombin is able to activate serine proteinase protein C, which cleaves coagulation factors Va and VIIIa, causing them to completely lose their activity.
Protein C is a precursor to a serine proteinase, very similar to factors IX, X, VII and prothrombin. It is activated by thrombin, like factor XI. However, when activated, the resulting serine proteinase uses its enzymatic activity not to activate other proteins, but to inactivate them. Activated protein C produces several proteolytic cleavages in coagulation factors Va and VIIIa, causing them to completely lose their cofactor activity. Thus, thrombin, a product of the coagulation cascade, inhibits its own production: this is called negative feedback. And again we have a regulatory question: why does thrombin simultaneously accelerate and slow down its own activation?
Evolutionary origins of folding
The formation of protective blood systems began in multicellular organisms over a billion years ago - in fact, precisely in connection with the appearance of blood. The coagulation system itself is the result of overcoming another historical milestone - the emergence of vertebrates about five hundred million years ago. Most likely, this system arose from the immune system. The emergence of yet another immune response system that fought bacteria by enveloping them in fibrin gel led to an accidental side effect: bleeding began to stop faster. This made it possible to increase the pressure and strength of flows in the circulatory system, and the improvement of the vascular system, that is, the improvement of the transport of all substances, opened up new horizons of development. Who knows whether the advent of folding was not the advantage that allowed vertebrates to take their current place in the Earth's biosphere?
In a number of arthropods (such as the horseshoe crab), coagulation also exists, but it arose independently and remained in immunological roles. Insects, like other invertebrates, usually make do with a weaker version of the bleeding control system, based on the aggregation of platelets (more precisely, amoebocytes - distant relatives of platelets). This mechanism is quite functional, but it imposes fundamental limitations on the efficiency of the vascular system, just as the tracheal form of breathing limits the maximum possible size of an insect.
Unfortunately, creatures with intermediate forms of the coagulation system have almost all become extinct. The only exception is jawless fish: genomic analysis of the coagulation system in the lamprey showed that it contains much fewer components (that is, it is much simpler) [6]. From jawed fish to mammals, the coagulation systems are very similar. Cellular hemostasis systems also operate on similar principles, despite the fact that small, anucleate platelets are characteristic only of mammals. In other vertebrates, platelets are large cells with a nucleus.
To summarize, the coagulation system is very well studied. No new proteins or reactions have been discovered in it for fifteen years, which is an eternity for modern biochemistry. Of course, the possibility of such a discovery cannot be completely ruled out, but so far there is not a single phenomenon that we could not explain with the help of existing information. Rather, on the contrary, the system looks much more complicated than it needs to be: we remind you that out of this entire (rather cumbersome!) cascade, only one reaction is actually involved in gelation, and all the others are needed for some kind of incomprehensible regulation.
That is why now coagulology researchers working in a variety of fields - from clinical hemostasiology to mathematical biophysics - are actively moving from the question “How does coagulation work?” to the questions “Why is folding arranged this way?”, “How does it work?” and finally, “How do we need to influence coagulation to achieve the desired effect?” The first thing you need to do to answer is to learn to study coagulation as a whole, and not just individual reactions.
Treatment
For treatment of this condition to be effective, the causes of the disease must be determined. It is very important to promptly identify and treat the main disorders - liver pathologies or oncological lesions.
Additional therapy methods include:
- injection of vitamin K;
- drugs to improve clotting;
- transfusion of frozen blood plasma;
- Other medications include hydroxyurea and oprelvekin, which help eliminate platelet problems.
The patient's diet should include foods high in calcium, folic acid, vikasol, and amino acids.
These include dairy products: cheese, cottage cheese, kefir. Fish and meat will also help eliminate the symptoms of pathology. It is equally important to eat leafy vegetables - green onions, spinach, cabbage.
Diagnosis of pathology
Diagnostic procedures are performed by doctors of several specializations: neonatologist, pediatrician, geneticist and hematologist. For concomitant pathologies, consultation with a gastroenterologist, orthopedist, otolaryngologist and neurologist may be required.
At-risk couples should visit a doctor before conceiving a child. Molecular genetic research of biomaterials from future parents will allow us to take into account the risk of having a child with hemophilia. After conception, prenatal screenings may be performed. Their results will confirm or refute the fact that the child inherited hemophilia.
Neonatal tests performed in the first days of a baby’s life are no less effective. A coagulogram provides the neonatologist with comprehensive information about the production of coagulation factors by the newborn’s body.
In case of hemarthrosis, the child is prescribed an X-ray examination of the joints. Ultrasound diagnostics is performed when signs of internal bleeding and retroperitoneal hematomas are detected.