Blood coagulation is an extremely complex and in many ways still mysterious biochemical process that is triggered when the circulatory system is damaged and leads to the transformation of blood plasma into a gelatinous clot that plugs the wound and stops bleeding. Disturbances in this system are extremely dangerous and can lead to bleeding, thrombosis or other pathologies, which together are responsible for the lion's share of mortality and disability in the modern world. Here we will look at the structure of this system and talk about the most modern achievements in its study.
Anyone who has received a scratch or wound at least once in their life has thus acquired a wonderful opportunity to observe the transformation of blood from a liquid into a viscous non-flowing mass, leading to the cessation of bleeding. This process is called blood coagulation and is controlled by a complex system of biochemical reactions.
Having some kind of system to stop bleeding is absolutely necessary for any multicellular organism that has a liquid internal environment. Blood clotting is also vital for us: mutations in the genes of the main clotting proteins are usually lethal. Alas, among the many systems of our body, disturbances in the functioning of which pose a danger to health, blood clotting also takes absolute first place as the main direct cause of death: people suffer from various diseases, but almost always die from blood clotting disorders. Cancer, sepsis, trauma, atherosclerosis, heart attack, stroke - for a wide range of diseases, the direct cause of death is the inability of the coagulation system to maintain a balance between the liquid and solid states of blood in the body.
If the reason is known, why can’t it be fought? Of course, it is possible and necessary to fight: scientists are constantly creating new methods for diagnosing and treating coagulation disorders. But the problem is that the coagulation system is very complex. And the science of regulating complex systems teaches that such systems need to be managed in a special way. Their reaction to external influences is nonlinear and unpredictable, and in order to achieve the desired result, you need to know where to put the effort. The simplest analogy: to launch a paper airplane into the air, you just need to throw it in the right direction; at the same time, for an airliner to take off, you will need to press the right buttons in the cockpit at the right time and in the right sequence. But if you try to launch an airliner with a throw, like a paper airplane, it will end badly. It’s the same with the coagulation system: in order to successfully treat, you need to know the “control points”.
Until very recently, blood clotting successfully resisted researchers' attempts to understand its workings, and only in recent years has there been a qualitative leap. In this article we will talk about this remarkable system: how it works, why it is so difficult to study, and - most importantly - we will tell you about the latest discoveries in understanding how it works.
How does blood clotting work?
Stopping bleeding is based on the same idea that housewives use to prepare jellied meat - the transformation of liquid into a gel (a colloidal system in which a network of molecules is formed, capable of holding in its cells a thousand times its weight due to hydrogen bonds with water molecules). By the way, the same idea is used in disposable baby diapers, which contain material that swells when wet. From a physical point of view, it is necessary to solve the same problem as in coagulation - combating leaks with minimal effort.
Blood coagulation is the central element of hemostasis (stopping bleeding). The second link of hemostasis is special cells - platelets - that are capable of attaching to each other and to the site of injury to create a plug that stops the blood.
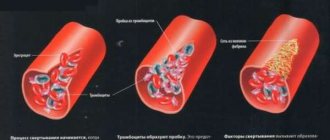
A general idea of the biochemistry of coagulation can be obtained from Figure 1, which shows the reaction that converts the soluble protein fibrinogen into fibrin, which then polymerizes into a network. This reaction is the only part of the cascade that has a direct physical meaning and solves a clear physical problem. The role of the remaining reactions is exclusively regulatory: to ensure the conversion of fibrinogen into fibrin only in the right place and at the right time.
Figure 1. Basic blood clotting reactions. The coagulation system is a cascade - a sequence of reactions, where the product of each reaction acts as a catalyst for the next. The main “entrance” to this cascade is in its middle part, at the level of factors IX and X: the protein tissue factor (indicated in the diagram as TF) binds factor VIIa, and the resulting enzymatic complex activates factors IX and X. The result of the cascade is the protein fibrin , capable of polymerizing and forming a clot (gel). The vast majority of activation reactions are proteolysis reactions, i.e. partial breakdown of the protein, increasing its activity. Almost every coagulation factor is necessarily inhibited in one way or another: feedback is necessary for stable operation of the system.
Legend: Reactions that convert coagulation factors into active forms are shown by one-sided thin black arrows. At the same time, curly red arrows show under the influence of which enzymes activation occurs. Loss of activity reactions due to inhibition are shown by thin green arrows (for simplicity, the arrows are depicted as simply “leaving”, i.e., it is not shown which inhibitors bind to). Reversible complex formation reactions are indicated by double-sided thin black arrows. Coagulation proteins are designated either by names, Roman numerals, or abbreviations ( TF —tissue factor, PC —protein C, APC —activated protein C). To avoid overload, the diagram does not show: binding of thrombin to thrombomodulin, activation and secretion of platelets, contact activation of coagulation.
[1], figure adapted
Fibrinogen resembles a rod 50 nm long and 5 nm thick (Fig. 2a). Activation allows its molecules to stick together into a fibrin thread (Fig. 2b), and then into a fiber capable of branching and forming a three-dimensional network (Fig. 2c).
Figure 2. Fibrin gel. a — Schematic structure of the fibrinogen molecule. Its basis is composed of three pairs of mirror-arranged polypeptide chains α, β, γ. In the center of the molecule you can see binding regions that become accessible when thrombin cuts off fibrinopeptides A and B (FPA and FPB in the figure). b — The assembly mechanism of fibrin fiber: the molecules are attached to each other “overlapping” according to the head-to-middle principle, forming a double-stranded fiber. c — Electron micrograph of the gel: fibrin fibers can stick together and split, forming a complex three-dimensional structure.
[2–4]
Figure 3. Three-dimensional structure of the thrombin molecule. The diagram shows the active site and parts of the molecule responsible for binding thrombin to substrates and cofactors. (The active site is the part of the molecule that directly recognizes the site of cleavage and carries out enzymatic catalysis.) Protruding parts of the molecule (exosites) allow the thrombin molecule to be “switched,” making it a multifunctional protein capable of working in different modes. For example, binding of thrombomodulin to exosite I physically blocks access of procoagulant substrates (fibrinogen, factor V) to thrombin and allosterically stimulates activity towards protein C.
[5]
The fibrinogen activator thrombin (Fig. 3) belongs to the family of serine proteinases - enzymes capable of cleaving peptide bonds in proteins. It is related to the digestive enzymes trypsin and chymotrypsin. Proteinases are synthesized in an inactive form called zymogen. To activate them, it is necessary to cleave the peptide bond holding the part of the protein that closes the active site. Thus, thrombin is synthesized in the form of prothrombin, which can be activated. As can be seen from Fig. 1 (where prothrombin is designated factor II), it is catalyzed by factor Xa.
In general, coagulation proteins are called factors and are numbered with Roman numerals in order of official discovery. The subscript “a” indicates the active form, and its absence indicates an inactive precursor. Proper names are also used for long-discovered proteins, such as fibrin and thrombin. Some numbers (III, IV, VI) are not used for historical reasons.
The coagulation activator is a protein called tissue factor, which is present in the cell membranes of all tissues, with the exception of the endothelium and blood. Thus, the blood remains liquid only due to the fact that it is normally protected by a thin protective membrane of the endothelium. When there is any violation of the integrity of the vessel, tissue factor binds factor VIIa from the plasma, and their complex - called external tenase (tenase, or Xase, from the word ten - ten, i.e. the number of the activated factor) - activates factor X.
Thrombin also activates factors V, VIII, XI, which leads to an acceleration of its own production: factor XIa activates factor IX, and factors VIIIa and Va bind factors IXa and Xa, respectively, increasing their activity by orders of magnitude (the complex of factors IXa and VIIIa is called intrinsic tenase). A deficiency of these proteins leads to severe disorders: for example, the absence of factors VIII, IX or XI causes the most severe disease hemophilia (the famous “royal disease” that Tsarevich Alexei Romanov suffered from); and deficiency of factors X, VII, V or prothrombin is incompatible with life.
This arrangement of the system is called positive feedback: thrombin activates proteins that accelerate its own production. And here an interesting question arises: why are they needed? Why can’t a reaction be made fast right away? Why does nature make it initially slow, and then come up with a way to further speed it up? Why is there duplication in the coagulation system? For example, factor X can be activated by both the VIIa–TF complex (extrinsic tenase) and the IXa–VIIIa complex (intrinsic tenase); it seems completely pointless.
Coagulation proteinase inhibitors are also present in the blood. The main ones are antithrombin III and tissue factor pathway inhibitor. In addition, thrombin is able to activate serine proteinase protein C, which cleaves coagulation factors Va and VIIIa, causing them to completely lose their activity.
Protein C is a precursor to a serine proteinase, very similar to factors IX, X, VII and prothrombin. It is activated by thrombin, like factor XI. However, when activated, the resulting serine proteinase uses its enzymatic activity not to activate other proteins, but to inactivate them. Activated protein C produces several proteolytic cleavages in coagulation factors Va and VIIIa, causing them to completely lose their cofactor activity. Thus, thrombin, a product of the coagulation cascade, inhibits its own production: this is called negative feedback. And again we have a regulatory question: why does thrombin simultaneously accelerate and slow down its own activation?
Evolutionary origins of folding
The formation of protective blood systems began in multicellular organisms over a billion years ago - in fact, precisely in connection with the appearance of blood. The coagulation system itself is the result of overcoming another historical milestone - the emergence of vertebrates about five hundred million years ago. Most likely, this system arose from the immune system. The emergence of yet another immune response system that fought bacteria by enveloping them in fibrin gel led to an accidental side effect: bleeding began to stop faster. This made it possible to increase the pressure and strength of flows in the circulatory system, and the improvement of the vascular system, that is, the improvement of the transport of all substances, opened up new horizons of development. Who knows whether the advent of folding was not the advantage that allowed vertebrates to take their current place in the Earth's biosphere?
In a number of arthropods (such as the horseshoe crab), coagulation also exists, but it arose independently and remained in immunological roles. Insects, like other invertebrates, usually make do with a weaker version of the bleeding control system, based on the aggregation of platelets (more precisely, amoebocytes - distant relatives of platelets). This mechanism is quite functional, but it imposes fundamental limitations on the efficiency of the vascular system, just as the tracheal form of breathing limits the maximum possible size of an insect.
Unfortunately, creatures with intermediate forms of the coagulation system have almost all become extinct. The only exception is jawless fish: genomic analysis of the coagulation system in the lamprey showed that it contains much fewer components (that is, it is much simpler) [6]. From jawed fish to mammals, the coagulation systems are very similar. Cellular hemostasis systems also operate on similar principles, despite the fact that small, anucleate platelets are characteristic only of mammals. In other vertebrates, platelets are large cells with a nucleus.
To summarize, the coagulation system is very well studied. No new proteins or reactions have been discovered in it for fifteen years, which is an eternity for modern biochemistry. Of course, the possibility of such a discovery cannot be completely ruled out, but so far there is not a single phenomenon that we could not explain with the help of existing information. Rather, on the contrary, the system looks much more complicated than it needs to be: we remind you that out of this entire (rather cumbersome!) cascade, only one reaction is actually involved in gelation, and all the others are needed for some kind of incomprehensible regulation.
That is why now coagulology researchers working in a variety of fields - from clinical hemostasiology to mathematical biophysics - are actively moving from the question “How does coagulation work?” to the questions “Why is folding arranged this way?”, “How does it work?” and finally, “How do we need to influence coagulation to achieve the desired effect?” The first thing you need to do to answer is to learn to study coagulation as a whole, and not just individual reactions.
Blood is like an explosive
It was possible to conduct an analytical study for some modified models of the blood coagulation system. A typical state diagram is shown in Fig. 3. The parametric plane is divided by two curves into four regions. Region I corresponds to the absolute stability of the liquid state of blood (any disturbances relax). Region II corresponds to conditions in which the steady-state thrombin concentration should gradually increase as the representing point moves away from the red line. Region III corresponds to completely clotted blood. Region IV is of greatest interest. For values of system parameters belonging to this region, the system must be in a metastable state. This means that with parameter values from region IV, the liquid state of blood is stable only with respect to disturbances not exceeding in amplitude a certain threshold level (an explicit expression has been found for the value of the latter). Transthreshold disturbances can cause explosive production of thrombin.
Clumped red blood cells are shown on the filter fiber
Photo: Science Photo Library/Diomedia
It is within the physiological norm that the state of the blood coagulation system should be metastable. That is, the blood must be liquid, but at the same time ready to clot if special circumstances require it.
This conclusion immediately puts blood not on a par with liquids of different rheology, but forces us to look at it as a substance of a special kind [2], similar in properties to liquid explosives: any supercritical disturbance can provoke a threshold change in the state of aggregation. And since the value of the activation threshold itself tends to zero as the representing point approaches the red border of region IV, the class of external disturbances that can provoke thrombus formation will expand.
Reaching the red limit corresponds to an absolute loss of regulation stability. Similar states in the theory of phase transitions lie on curves called spinodals.
How to study coagulation?
To study coagulation, various models are created - experimental and mathematical. What exactly do they allow you to get?
On the one hand, it seems that the best approximation for studying an object is the object itself. In this case, a person or an animal. This allows you to take into account all factors, including blood flow through the vessels, interactions with the walls of blood vessels and much more. However, in this case the complexity of the problem exceeds reasonable limits. Convolution models make it possible to simplify the object of study without losing its essential features.
Let's try to get an idea of what requirements these models must meet in order to correctly reflect the coagulation process in vivo.
The experimental model must contain the same biochemical reactions as in the body. Not only proteins of the coagulation system must be present, but also other participants in the coagulation process - blood cells, endothelial and subendothelial cells. The system must take into account the spatial heterogeneity of coagulation in vivo: activation from a damaged area of the endothelium, the spread of active factors, the presence of blood flow.
It is natural to begin the consideration of coagulation models with methods for studying coagulation in vivo. The basis of almost all of these approaches used is to inflict controlled injury on the experimental animal in order to induce a hemostatic or thrombotic response. This reaction is studied by various methods:
- monitoring bleeding time;
- analysis of plasma taken from an animal;
- autopsy of a euthanized animal and histological examination;
- Real-time thrombus monitoring using microscopy or nuclear magnetic resonance (Figure 4).
Figure 4. In vivo thrombus formation in a laser-induced thrombosis model. This picture is reproduced from a historical work where scientists were able to observe the development of a blood clot “live” for the first time. To do this, a concentrate of fluorescently labeled antibodies to coagulation proteins and platelets was injected into the mouse’s blood, and, placing the animal under the lens of a confocal microscope (allowing for three-dimensional scanning), they selected an arteriole under the skin accessible for optical observation and damaged the endothelium with a laser. Antibodies began to attach to the growing clot, making it possible to observe it.
[7]
The classic setup of an in vitro coagulation experiment is that blood plasma (or whole blood) is mixed in a container with an activator, after which the coagulation process is observed. According to the observation method, experimental techniques can be divided into the following types:
- monitoring the coagulation process itself;
- monitoring changes in concentrations of coagulation factors over time.
The second approach provides incomparably more information. Theoretically, knowing the concentrations of all factors at an arbitrary point in time, one can obtain complete information about the system. In practice, studying even two proteins simultaneously is expensive and involves great technical difficulties.
Finally, coagulation in the body is heterogeneous. The formation of a clot starts on the damaged wall, spreads with the participation of activated platelets in the plasma volume, and is stopped with the help of the vascular endothelium. It is impossible to adequately study these processes using classical methods. The second important factor is the presence of blood flow in the vessels.
Awareness of these problems has led to the development of a variety of in vitro flow experimental systems since the 1970s. It took a little more time to understand the spatial aspects of the problem. Only in the 1990s did methods begin to appear that take into account spatial heterogeneity and diffusion of coagulation factors, and only in the last decade have they begun to be actively used in scientific laboratories (Fig. 5).
Figure 5. Spatial growth of a fibrin clot in normal and pathological conditions. Coagulation in a thin layer of blood plasma was activated by tissue factor immobilized on the wall. In the photographs the activator is located on the left. The gray expanding stripe is a growing fibrin clot.
Along with experimental approaches, mathematical models are also used to study hemostasis and thrombosis (this research method is often called in silico [8]). Mathematical modeling in biology makes it possible to establish deep and complex relationships between biological theory and experience. Conducting an experiment has certain boundaries and is associated with a number of difficulties. In addition, some theoretically possible experiments are infeasible or prohibitively expensive due to limitations in experimental technology. Modeling simplifies experiments, since it is possible to select in advance the necessary conditions for in vitro and in vivo experiments under which the effect of interest will be observed.
Given the pressure
Red blood cells entangled in a fibrin network
Photo: Science Photo Library/Diomedia
The very idea that something similar to spinodal breakdown may take place in the blood is very tonic. The discovery of this fact, as well as the construction of the diagram (Fig. 3) [1], gave the author reason to think more deeply about what kind of disturbances are capable, in principle, of undermining the blood coagulation system.
Discussion of the problem with leading clinicians, Professor Zinoviy Barkagan (1925-2006) and academician Andrei Vorobyov led to the conviction that kinetic analysis of reaction transformations in the blood coagulation system alone is completely insufficient to understand the causes of sudden intravascular thrombus formation in humans.
Various poisons, drugs and chemical agents can directly affect the blood's ability to clot. But all clinicians unanimously say that a sudden drop in blood pressure can cause both intravascular disseminated coagulation and stroke.
This means that pressure must somehow be involved in the processes of regulating the aggregate state of the blood. And since the distribution of pressure throughout the circulatory system is very whimsical, it was necessary to start with those vessels, organs and tissues where the risk of thrombus formation is greatest.
Regulation of the coagulation system
Figure 6. Contribution of extrinsic and intrinsic tenase to fibrin clot formation in space. We used a mathematical model to investigate how far the influence of a clotting activator (tissue factor) could extend in space. To do this, we calculated the distribution of factor Xa (which determines the distribution of thrombin, which determines the distribution of fibrin). The animation shows the distributions of factor Xa produced by extrinsic tenase (VIIa–TF complex) or intrinsic tenase (IXa–VIIIa complex), as well as the total amount of factor Xa (shaded area). (The inset shows the same thing on a larger concentration scale.) It can be seen that activator-produced factor Xa cannot travel far from the activator due to the high rate of inhibition in the plasma. On the contrary, the IXa–VIIIa complex works far from the activator (since factor IXa is inhibited more slowly and therefore has a greater effective diffusion distance from the activator), and ensures the distribution of factor Xa in space.
[9]
Let's take the next logical step and try to answer the question - how does the system described above work?
Cascade device of the coagulation system
Let's start with the cascade - a chain of enzymes that activate each other. A single enzyme operating at a constant speed produces a linear dependence of product concentration on time. For a cascade of N enzymes, this dependence will have the form tN, where t is time. For the effective operation of the system, it is important that the response is of precisely this “explosive” nature, since this minimizes the period when the fibrin clot is still fragile.
Triggering of coagulation and the role of positive feedbacks
As mentioned in the first part of the article, many clotting reactions are slow. Thus, factors IXa and Xa themselves are very poor enzymes and require cofactors (factors VIIIa and Va, respectively) to function effectively. These cofactors are activated by thrombin, a device where the enzyme activates its own production is called a positive feedback loop.
As we have shown experimentally and theoretically, the positive feedback of factor V activation by thrombin forms the activation threshold - the property of the system not to respond to small activation, but to quickly respond when a large one appears. This ability to switch seems to be very valuable for folding: it helps prevent “false positives” of the system.
The role of the intrinsic pathway in the spatial dynamics of folding
One of the intriguing mysteries that haunted biochemists for many years after the discovery of the essential coagulation proteins was the role of factor XII in hemostasis. Its deficiency was detected in simple clotting tests, increasing the time required for clot formation, but, unlike factor XI deficiency, was not accompanied by coagulation disorders.
One of the most plausible options for unraveling the role of the internal pathway was proposed by us using spatially inhomogeneous experimental systems. Positive feedbacks have been found to be important specifically for the propagation of coagulation. Effective activation of factor X by external tenase on the activator will not help form a clot away from the activator, since factor Xa is rapidly inhibited in the plasma and cannot move far from the activator. But factor IXa, which is inhibited an order of magnitude slower, is quite capable of this (and is helped by factor VIIIa, which is activated by thrombin). And where it is difficult for him to reach, factor XI, also activated by thrombin, begins to work. Thus, the presence of positive feedback loops helps create the three-dimensional structure of the clot.
Protein C pathway as a possible localization mechanism for thrombus formation
Activation of protein C by thrombin itself is slow, but accelerates sharply when thrombin binds to the transmembrane protein thrombomodulin, synthesized by endothelial cells. Activated protein C is capable of destroying factors Va and VIIIa, slowing down the coagulation system by orders of magnitude. The key to understanding the role of this reaction was spatially inhomogeneous experimental approaches. Our experiments suggested that it stops the spatial growth of the thrombus, limiting its size.
Decoding the result
I. Quick's prothrombin, prothrombin time, and international normalized ratio (INR) reflect the activity of the extrinsic coagulation pathway.
- Prothrombin time
is the time of blood clotting after the addition of substances (thromboplastin with calcium) to the plasma that trigger the extrinsic blood coagulation pathway. Time is measured in seconds (sec.). The longer the prothrombin time, the lower the activity of coagulation factors. - Prothrombin according to Quick
is considered a more objective indicator of the external pathway, since during the analysis the clotting time is studied depending on the different concentration of coagulation factors in the blood obtained by diluting the test material in the laboratory. The indicator is measured as a percentage (%). The longer blood clotting occurs, the lower the Quick prothrombin percentage. - The international normalized ratio (INR)
is a prothrombin test standardized in accordance with international recommendations. It is calculated using a certain formula. The higher the INR, the longer it takes for a blood clot to form.
Prothrombin time, prothrombin according to Quick and INR with normal fibrinogen content and activity also reflect the activity of the prothrombin complex (II, V, VII, X blood coagulation factors).
When prescribing therapy with anticoagulants from the group of vitamin K antagonists (warfarin), there is an increase in INR and prothrombin time and a decrease in prothrombin according to Quick. Other causes of these changes may be vitamin K deficiency (for example, with hemorrhagic disease of the newborn), hereditary deficiency of factors II, V, VII, X, liver and intestinal diseases. The opposite picture, i.e., a decrease in INR, a decrease in prothrombin time and an increase in prothrombin according to Quick, occurs in conditions accompanied by increased blood clotting: thrombosis, blood thickening during dehydration, when taking oral contraceptives, barbiturates.
II. Activated partial thromboplastin time (aPTT)
with normal content and activity of fibrinogen, it characterizes the internal pathway of blood coagulation, which includes the activity of factors II, V, VIII, IX, X, XI, XII. APTT is determined by adding reagents that trigger this clotting pathway to a blood sample and measuring the time it takes for the blood to form a clot. Measured in seconds (sec.). The slower the blood clotting, the longer the APTT.
This indicator is used to monitor therapy with heparin (direct anticoagulant), and an increase in the aPTT value is noted. Other causes of increased time include hereditary and acquired deficiencies of intrinsic pathway coagulation factors, for example, in hemophilia, hemorrhagic disease of the newborn, liver and intestinal diseases, some autoimmune diseases, etc. The reasons for increased aPTT are the same as for Quick's prothrombin.
III. Thrombin time and fibrinogen reflect the overall, or final, pathway of blood clotting.
- Fibrinogen
is blood clotting factor I, a protein synthesized in the liver and converted into fibrin, the basis of the clot during blood clotting. It is also an acute phase protein. It is measured in grams per liter (g/l). With increased thrombus formation and various inflammatory diseases, the synthesis of this protein increases. In case of liver diseases, hereditary fibrinogen deficiency, etc., its concentration decreases. - Thrombin time
is the blood clotting time necessary for the formation of a fibrin clot when thrombin is added to the plasma - an enzyme (factor IIa), which appears when blood coagulation factors interact when a vessel is damaged. Thrombin time depends on the level and activity of fibrinogen. Measured in seconds (sec.).
Changes in thrombin time generally correlate with fibrinogen levels. However, with such a hereditary disease as dysfibrinogenemia, i.e., a violation of the functional activity of fibrinogen, an increase in thrombin time and a coagulation disorder occurs, despite the normal amount of fibrinogen in the blood.
Summarizing
In recent years, the complexity of the coagulation system has gradually become less mysterious. The discovery of all essential components of the system, the development of mathematical models and the use of new experimental approaches made it possible to lift the veil of secrecy. The structure of the coagulation cascade is being deciphered, and now, as we saw above, for almost every significant part of the system, the role it plays in the regulation of the entire process has been identified or proposed.
Figure 7 shows the most recent attempt to reconsider the structure of the coagulation system. This is the same diagram as in Fig. 1, where parts of the system responsible for different tasks are highlighted with multi-colored shading, as discussed above. Not everything in this scheme is securely established. For example, our theoretical prediction that activation of factor VII by factor Xa allows clotting to respond in a threshold manner to flow rate remains as yet untested experimentally.
Figure 7. Modular structure of the coagulation system: the role of individual coagulation reactions in the functioning of the system.
[1]
It is quite possible that this picture is not yet completely complete. However, progress in this field in recent years gives hope that in the foreseeable future, the remaining unsolved regions of the coagulation circuitry will gain meaningful physiological function. And then it will be possible to talk about the birth of a new concept of blood coagulation, which replaced the old cascade model, which faithfully served medicine for many decades.
The article was written with the participation of A.N. Balandina and F.I. Ataullakhanova and was originally published in Priroda [10].