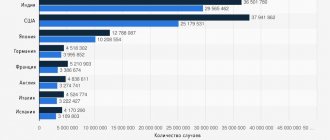
Symptoms of diabetes
If you notice any changes in your health or recurring unpleasant symptoms, consult your doctor immediately. Signs of diabetes mellitus may include:
- a feeling of unquenchable thirst and a metallic taste in the mouth,
- frequent night urination,
- increased appetite, constant feeling of hunger and need for sweets,
- change in body weight, weight loss or weight gain,
- itching of the skin, feet, palms,
- disruption of wound healing processes,
- susceptibility to dermatological diseases and fungal infections,
- hair loss and weakened hair growth,
- constant weakness and drowsiness,
- dental problems, frequent stomatitis and periodontal diseases.
In addition to increased fatigue, the patient may experience a loss of quality of vision. This is how the toxic effect of sugar on all cells of the body manifests itself. The level of acetone in the urine increases, and especially attentive relatives may notice the smell of acetone from the mouth of a person with diabetes.
If you notice the above signs of a dangerous disease, immediately go to a medical facility to measure your blood sugar levels and begin drug therapy.
If the diagnosis is not confirmed
If the examination did not confirm the presence of diabetes, but identified factors for its development, then you need to be attentive to your health and not neglect prevention. Don't forget that prevention is always better than cure. Patients at risk are advised to follow a diet, lead an active lifestyle and have their blood glucose tested once a year.
If prediabetes is detected
Proper diet and lifestyle can help delay or prevent the onset of diabetes. Prediabetes indicates an early disorder of carbohydrate metabolism. Signs of prediabetes include impaired fasting blood glucose and impaired glucose tolerance. Patients are advised to consume less easily digestible carbohydrates and increase physical activity. In addition, it is recommended to be examined by a therapist at least once a year and have your blood glucose tested.
If you have been diagnosed with type 2 diabetes mellitus
Once the diagnosis of diabetes has been confirmed, treatment should not be delayed. First, you need to go to an appointment with an endocrinologist to clarify the concomitant pathology and select individual treatment. A referral to an endocrinologist will be given by a therapist or general practitioner if, as a result of the examination, it turns out that the level of blood glucose in venous plasma exceeds 7.0 mmol/l, and glycated hemoglobin is higher than 6.5%. The endocrinologist determines the individual target level of glycated hemoglobin (HbA1c) for the patient and develops glucose-lowering therapy based on the examination results and the presence of concomitant diseases.
Additional examinations
An endocrinologist may prescribe additional examinations for the patient. This is necessary to anticipate possible complications of T2DM. As an additional examination, the endocrinologist may refer the patient to an ophthalmologist to check the condition of the fundus and evaluate visual acuity at the time of diagnosis of type 2 diabetes.
There are a number of mandatory examinations that a patient with T2DM must undergo every year. These include ECG and chest x-ray. If indicated, the patient may be referred for additional examinations:
- See a cardiologist for arterial hypertension and dyslipidemia;
- See a nephrologist or urologist for kidney pathology;
- See a neurologist if you complain of loss of sensation in the limbs and poor wound healing.
Selection of treatment and free medications
The treatment program for the patient is drawn up by an endocrinologist. There is no standard program, since treatment is selected taking into account the patient’s lifestyle, the presence of concomitant diseases and a tendency to hypoglycemia. While the selection and correction of insulin therapy is underway, the patient should regularly visit an endocrinologist.
It is useful to know that patients with diabetes can receive preferential drug coverage; benefits apply to:
- hypoglycemic drugs and other medications prescribed by your doctor;
- means for administering insulin;
- means for self-monitoring of glycemia;
- consumables for insulin pump.
Medicines can be obtained with a prescription from a doctor. They are issued at the pharmacy of the clinic to which the patient is assigned. Prescriptions are issued for a period of no more than 90 days. If the drug is not available, the prescription is registered in a special journal in the prescribed form, and the drug is delivered to the point within 10 days.
How to prevent diabetes
It is important to understand that the symptoms of the disease clearly manifest themselves during the period when the level of glucose in the blood significantly exceeds the permissible values. At the initial stage of diabetes, symptoms are almost invisible. For this reason, all adult patients over 40 years of age are recommended to have their blood tested annually for sugar in order to timely diagnose the onset of the disease and prevent its further development.
Contact the medical office and you will receive qualified medical assistance from an endocrinologist in the diagnosis and treatment of diabetes mellitus in children and adults.
Diagnosis of diabetes mellitus types 1 and 2
Diabetes mellitus is a group of metabolic (metabolic) diseases characterized by hyperglycemia, which develops as a result of an absolute or relative deficiency of insulin and is also manifested by glucosuria, polyuria, polydipsia, lipid disorders (hyperlipidemia, dyslipidemia), protein (dysproteinemia) and mineral (for example, hypokalemia) exchanges, in addition, provokes the development of complications. Clinical manifestations of the disease can sometimes be associated with a previous infection, mental trauma, pancreatitis, or a pancreatic tumor. Diabetes mellitus often develops with obesity and some other endocrine diseases. Heredity may also play a role. In terms of medical and social significance, diabetes mellitus ranks immediately after heart and cancer diseases.
There are 4 clinical types of diabetes mellitus: type 1 diabetes mellitus, type 2 diabetes mellitus, other types (with genetic defects, endocrinopathies, infections, pancreatic diseases, etc.) and gestational diabetes (pregnant diabetes). The new classification is not yet generally accepted and is advisory in nature. At the same time, the need to revise the old classification is primarily due to the emergence of new data on the heterogeneity of diabetes mellitus, and this, in turn, requires the development of special differentiated approaches to the diagnosis and treatment of the disease. SD
Type 1 is a chronic disease caused by an absolute deficiency of insulin, resulting from insufficient production of it by the pancreas. Type 1 diabetes leads to persistent hyperglycemia and the development of complications. The detection rate is 15:100,000 population. Develops mainly in childhood and adolescence. SD
Type 2 is a chronic disease caused by a relative deficiency of insulin (reduced sensitivity of insulin-dependent tissue receptors to insulin) and manifested by chronic hyperglycemia with the development of characteristic complications. Type 2 diabetes accounts for 80% of all cases of diabetes. The frequency of occurrence is 300:100,000 population. The predominant age is usually over 40 years. More often diagnosed in women. Risk factors are genetic and obesity.
Screening for diabetes
The WHO Expert Committee recommends screening for diabetes in the following categories of citizens:
- all patients over the age of 45 years (if the examination result is negative, repeat every 3 years);
- younger patients with: obesity; hereditary history of diabetes mellitus; high-risk ethnicity/race; history of gestational diabetes; birth of a child weighing more than 4.5 kg; hypertension; hyperlipidemia; previously identified IGT or high fasting blood glucose.
For screening (both centralized and decentralized) for diabetes, WHO recommends measuring both glucose and hemoglobin A1c levels.
Glycosylated hemoglobin is hemoglobin in which a glucose molecule is condensed with the β-terminal valine of the β chain of the hemoglobin molecule. Glycosylated hemoglobin has a direct correlation with blood glucose levels and is an integrated indicator of compensation of carbohydrate metabolism over the last 60–90 days preceding the examination. The rate of HbA1c formation depends on the magnitude of hyperglycemia, and normalization of its level in the blood occurs 4–6 weeks after reaching euglycemia. In this regard, the HbA1c content is determined if it is necessary to control carbohydrate metabolism and confirm its compensation in diabetic patients for a long time. According to WHO recommendations (2002), determination of the content of glycosylated hemoglobin in the blood of patients with diabetes mellitus should be carried out once a quarter. This indicator is widely used both for screening the population and pregnant women, carried out to identify disorders of carbohydrate metabolism, and for monitoring the treatment of patients with diabetes.
The BioChemMac company offers equipment and reagents for the analysis of glycosylated hemoglobin HbA1c from Drew Scientific (England) and Axis-Shield (Norway) - world leaders specializing in clinical systems for monitoring diabetes (see at the end of this section). The products of these companies have the international standardization NGSP for HbA1c measurement.
Prevention of diabetes
Type 1 diabetes is a chronic autoimmune disease accompanied by destruction of β-cells of the islets of Langerhans, therefore early and accurate prognosis of the disease at the preclinical (asymptomatic) stage is very important. This will stop cellular destruction and preserve the cell mass of β-cells as much as possible.
Screening high-risk groups for all three types of antibodies will help prevent or reduce the incidence of diabetes. In individuals at risk who have antibodies to two or more antigens, diabetes develops within 7–14 years.
To identify individuals at high risk of developing type 1 diabetes, it is necessary to conduct a study of genetic, immunological and metabolic markers of the disease. It should be noted that it is advisable to study immunological and hormonal parameters over time - once every 6-12 months. If autoantibodies to the β-cell are detected, with an increase in their titer, a decrease in C-peptide levels, it is necessary to begin to carry out therapeutic preventive measures before the appearance of clinical symptoms.
Markers of type 1 diabetes mellitus
- Genetic - HLA DR3, DR4 and DQ.
- Immunological - antibodies to glutamic acid decarboxylase (GAD), insulin (IAA) and islet cell antibodies (ICA).
- Metabolic - glycohemoglobin A1, loss of the first phase of insulin secretion after an intravenous glucose tolerance test.
HLA typing
According to modern concepts, type 1 diabetes, despite its acute onset, has a long latent period. It is customary to distinguish six stages in the development of the disease. The first of these is the stage of genetic predisposition, characterized by the presence or absence of genes associated with type 1 diabetes mellitus. The presence of HLA antigens, especially class II - DR 3, DR 4 and DQ, is of great importance. At the same time, the risk of developing the disease increases many times over. Today, genetic predisposition to the development of type 1 diabetes mellitus is considered as a combination of different alleles of normal genes.
The most informative genetic markers of type 1 diabetes mellitus are HLA antigens. The study of genetic markers associated with type 1 diabetes mellitus in patients with LADA seems appropriate and necessary for making a differential diagnosis between types of diabetes mellitus when the disease develops after 30 years. “Classical” haplotypes characteristic of type 1 diabetes were identified in 37.5% of patients. At the same time, haplotypes considered protective were found in 6% of patients. Perhaps this is what can explain the slower progression and milder clinical course of diabetes mellitus in these cases.
Islet cell antibodies (ICA)
The production of specific autoantibodies to β-cells of the islets of Langerhans leads to the destruction of the latter through the mechanism of antibody-dependent cytotoxicity, which, in turn, entails a violation of insulin synthesis and the development of clinical signs of type 1 diabetes. Autoimmune mechanisms of cell destruction can be hereditary in nature and/or triggered by a number of external factors, such as viral infections, exposure to toxic substances and various forms of stress. Type 1 diabetes is characterized by the presence of an asymptomatic stage of prediabetes, which can last for several years. Impaired synthesis and secretion of insulin during this period can only be detected using a glucose tolerance test. In most cases, these individuals with asymptomatic type 1 diabetes have autoantibodies to the cells of the islets of Langerhans and/or antibodies to insulin. Cases of ICA detection 8 or more years before the onset of clinical signs of type 1 diabetes have been described. Thus, determination of ICA levels can be used for early diagnosis and identification of predisposition to type 1 diabetes. Patients with ICA experience a progressive decline in β-cell function, which is manifested by an impairment of the early phase of insulin secretion. When this phase of secretion is completely disrupted, clinical signs of type 1 diabetes appear.
Studies have shown that ICAs are detected in 70% of patients with new-onset type 1 diabetes, compared with a nondiabetic control population where ICAs are detected in 0.1–0.5% of cases. ICAs are also detected in close relatives of diabetic patients. These individuals constitute a group at increased risk of developing type 1 diabetes. A number of studies have shown that ICA-positive first-degree relatives of diabetic patients subsequently develop type 1 diabetes. The high prognostic significance of the determination of ICA is also determined by the fact that patients with the presence of ICA, even in the absence of signs of diabetes, ultimately also develop type 1 diabetes. Therefore, determination of ICA facilitates early diagnosis of type 1 diabetes. It has been shown that determination of ICA levels in patients with type 2 diabetes mellitus can help identify diabetes before the onset of relevant clinical symptoms and determine the need for insulin therapy. Therefore, in patients with type 2 diabetes in the presence of ICA, the development of insulin dependence can be highly expected.
Antibodies to insulin
Antibodies to insulin are found in 35–40% of patients with newly diagnosed type 1 diabetes. A correlation has been reported between the appearance of insulin antibodies and islet cell antibodies. Antibodies to insulin can be observed in the stage of prediabetes and symptomatic events of type 1 diabetes. Anti-insulin antibodies in some cases also appear in patients after treatment with insulin.
Glutamic acid decarboxylase (GAD)
Recent studies have identified the main antigen that is the main target for autoantibodies associated with the development of insulin-dependent diabetes - glutamic acid decarboxylase. This membrane enzyme that carries out the biosynthesis of the inhibitory neurotransmitter of the central nervous system of mammals, gamma-aminobutyric acid, was first found in patients with generalized neurological disorders. Antibodies to GAD are a very informative marker for identifying prediabetes, as well as identifying individuals at high risk of developing type 1 diabetes. During the period of asymptomatic development of diabetes, antibodies to GAD can be detected in the patient 7 years before the clinical manifestation of the disease.
According to foreign authors, the frequency of detection of autoantibodies in patients with “classical” type 1 diabetes mellitus is: ICA - 60-90%, IAA - 16-69%, GAD - 22-81%. In recent years, studies have been published whose authors have shown that in patients with LADA, autoantibodies to GAD are the most informative. However, according to the Russian Research Center, only 53% of patients with LADA had antibodies to GAD, compared to 70% of ICA. One does not contradict the other and can serve as confirmation of the need to determine all three immunological markers to achieve a higher level of information content. Determination of these markers allows in 97% of cases to differentiate type 1 diabetes from type 2, when the clinical picture of type 1 diabetes is disguised as type 2.
Clinical value of serological markers of type 1 diabetes
The most informative and reliable is the simultaneous study of 2-3 markers in the blood (absence of all markers - 0%, one marker - 20%, two markers - 44%, three markers - 95%).
Determination of antibodies against the cellular components of β-cells of the islets of Langerhans, against glutamic acid decarboxylase and insulin in peripheral blood is important for identifying in the population individuals predisposed to developing the disease and relatives of diabetic patients who have a genetic predisposition to type 1 diabetes. A recent international study has confirmed the enormous importance of this test for the diagnosis of autoimmune processes directed against islet cells.
Diagnosis and monitoring of diabetes mellitus
The following laboratory tests are used to diagnose and monitor diabetes mellitus (according to WHO recommendations from 2002).
- Routine laboratory tests: glucose (blood, urine); ketones; glucose tolerance test; HbA1c; fructosamine; microalbumin; creatinine in urine; lipid profile.
- Additional laboratory tests to monitor the development of diabetes: determination of antibodies to insulin; determination of C-peptide; determination of antibodies to the islets of Langenhars; determination of antibodies to tyrosine phosphatase (IA2); determination of antibodies to glutamic acid decarboxylase; determination of leptin, ghrelin, resistin, adiponectin; HLA typing.
For a long time, both to identify diabetes and to monitor the degree of its compensation, it was recommended to determine the level of glucose in the blood on an empty stomach and before each meal. Recent studies have established that a clearer association between blood glucose levels, the presence of vascular complications of diabetes and the degree of their progression is revealed not with fasting glucose levels, but with the degree of its increase in the period after meals - postprandial hyperglycemia.
It must be emphasized that the criteria for compensation for diabetes mellitus have undergone significant changes over recent years, which can be traced based on the data presented in the table.
Thus, the criteria for diagnosing diabetes and its compensation, in accordance with the latest WHO recommendations (2002), need to be “tightened.” This is due to recent studies (DCCT, 1993; UKPDS, 1998), which have shown that the frequency, time of development of late vascular complications of diabetes and the rate of their progression have a direct correlation with the degree of compensation of diabetes.
Insulin
Insulin is a hormone produced by the β-cells of the islets of Langerhans in the pancreas and is involved in the regulation of carbohydrate metabolism and maintaining constant blood glucose levels. Insulin is initially synthesized as a preprohormone with a molecular weight of 12 kDa, then processed inside the cell to form a prohormone with a molecular weight of 9 kDa and a length of 86 amino acid residues. This prohormone is deposited in granules. Within these granules, the disulfide bonds between the A and B chains of insulin and the C-peptide are broken, resulting in the formation of an insulin molecule with a molecular weight of 6 kDa and a length of 51 amino acid residues. When stimulated, cells release equimolar amounts of insulin and C-peptide and small amounts of proinsulin, as well as other intermediates (<5% of the normal total amount of insulin secreted). Insulin is one of the important hormones associated with the nutritional process. It is the only physiological hormone that significantly reduces blood glucose levels. In response to changes in the concentration of certain substrates and other stimulating agents, including glucose and amino acids, insulin is recruited into the portal circulation in the liver. 50% of insulin enters the liver, the rest enters the circulation and is sent to target tissues. Insulin then binds to specific receptors located on the cell surface and, through a mechanism that is not yet fully understood, facilitates the uptake of substrates and intracellular utilization of substrates. As a result, the intracellular concentration of lipids, proteins and glycogen increases. In addition, one of the tasks of insulin in peripheral metabolism is to influence the central regulation of energy balance. Insulin is rapidly eliminated through the liver, tissues and kidneys (half-life is 5-10 minutes). Circulating insulin levels are very low during fasting. In contrast, C-peptide is not transported to the liver and kidneys and therefore has a longer half-life in circulation (30 min).
Basal and glucose-stimulated circulating insulin levels are relatively stable in infants and children and increase during puberty as a result of decreased insulin sensitivity. Insulin concentrations are higher in obese individuals: this is partly dependent on the amount of visceral fat. Regulatory hormones that correlate with glucose levels, such as glucagon, glucocorticoids, and growth hormone, reduce insulin sensitivity and its action. Insulin levels may increase due to the exogenous influence of these substrates.
Determining the concentration of insulin in the blood is necessary to differentiate different forms of diabetes mellitus, select a therapeutic drug, select optimal therapy, and determine the degree of β-cell deficiency. Determining insulin makes sense only in patients who have not received insulin preparations, since the formation of antibodies to the exogenous hormone occurs. Determination of the concentration of circulating insulin in some cases is useful in the diagnostic assessment of certain conditions. Elevated insulin levels in the presence of low glucose concentrations may be an indicator of pathological hyperinsulinemia, namely nesidioblastosis and pancreatic islet cell tumors. Elevated insulin levels during fasting in the presence of both normal and elevated glucose concentrations, as well as increases in insulin and glucose concentrations in response to glucose administration, are indicators of the presence of insulin-resistant forms of glucose intolerance and diabetes mellitus, as well as other insulin-resistant conditions. High concentrations of circulating insulin may be associated with the pathogenesis of hypertension and cardiovascular disease. Insulin testing is used to confirm the diagnosis in people with borderline impaired glucose tolerance. Type 1 diabetes is characterized by low, and type 2 diabetes by normal or increased basal insulin levels.
Insulin receptors
Insulin receptors are located on the outer surface of the cell membrane. They interact with insulin and transmit the corresponding information to the intracellular components responsible for the biological action of the hormone. The first stage of action of the insulin receptor complex is a decrease in the activity of adenylate cyclase, and subsequent effects are associated with a decrease in the content of intracellular cAMP. In all tissues studied, insulin receptors have the same binding specificity. In clinical studies, insulin receptors are studied on blood monocytes. Changes in monocyte insulin receptors reflect the state of the insulin apparatus in the most important target tissues, in particular the liver and adipose tissue. Any changes in the number of receptors on monocytes are characteristic of all tissues of the body. In obese individuals, in patients with diabetes mellitus, and resistant to insulin, a decrease in the number of insulin receptors on blood monocytes is detected.
Proinsulin
Measuring serum proinsulin helps diagnose insulinoma. Elevated levels are typical for type 2 diabetes, newly diagnosed type 1 diabetes, and other clinical conditions, including diabetes developing during pregnancy and obesity, functional hypoglycemia and hyperinsulinemia, and age-related changes.
C-peptide
C-peptide is a fragment of the proinsulin molecule, as a result of the cleavage of which insulin is formed. Insulin and C-peptide are secreted into the blood in equimolar quantities. The half-life of C-peptide in the blood is longer than that of insulin. Therefore, the C-peptide/insulin ratio is 5:1. C-peptide is biologically inactive and undergoes relatively less transformation in the liver. The C-peptide level is a more stable indicator of insulin secretion than the rapidly changing level of insulin itself. Another advantage of the C-peptide assay is that it can distinguish endogenous insulin from that which is introduced into the body from the outside by injection, since, unlike insulin, C-peptide does not cross-react with insulin antibodies. Considering the fact that therapeutic insulin preparations do not contain C-peptide, its determination in blood serum makes it possible to assess the function of pancreatic β-cells in patients with diabetes mellitus receiving insulin. In a patient with diabetes mellitus, the value of the basal level of C-peptide and especially its concentration after a glucose load (during a glucose tolerance test) make it possible to determine the presence of insulin resistance or sensitivity, determine the phases of remission and thereby adjust therapeutic measures. With exacerbation of diabetes mellitus, especially type 1 diabetes, the level of C-peptide in the blood decreases, which indicates a deficiency of endogenous insulin. Taking all these factors into account, we can conclude that the study of C-peptide concentration allows us to evaluate insulin secretion in various clinical situations.
Determination of C-peptide also makes it possible to interpret fluctuations in insulin levels when it is retained in the liver. In diabetic patients who have insulin antibodies that bind proinsulin, falsely elevated C-peptide levels are sometimes observed due to antibodies cross-reacting with proinsulin. In patients with insulinoma, the concentration of C-peptide in the blood is significantly increased.
The state of the secretory response to C-peptide has a major prognostic significance in the onset of type 1 diabetes mellitus. Taking into account the incidence of remission in different treatment regimens is used as an objective way to assess their clinical effectiveness. (According to the RF Endoscopy Center, with a preserved but reduced version of the secretory response (basal C-peptide level <0.5 nmol/l), remission was observed in 39% of cases.) With a high secretory response (basal C-peptide level <1 nmol/l) k) spontaneous clinical remission was observed in 81% of patients. In addition, long-term maintenance of residual insulin secretion in patients with type 1 diabetes mellitus is very important, since it is noted that in these cases the disease is more stable, and chronic complications develop more slowly and later.
Monitoring C-peptide levels is especially important after surgical treatment of insulinoma: detection of elevated C-peptide levels in the blood indicates metastases or tumor recurrence.
Glucagon
Glucagon is a peptide hormone synthesized by the α-cells of the islets of Langerhans in the pancreas. Glucagon is one of the insulin antagonists that promotes the formation of glucose in the liver. Normal hormone secretion ensures reliable control over maintaining constant blood glucose levels. A lack of insulin in diabetes mellitus is accompanied by an excess of glucagon, which, in fact, is the cause of hyperglycemia. A significant increase in the concentration of glucagon in the blood is a sign of glucagonoma - a tumor of α-cells. In almost all cases, glucose tolerance is impaired and diabetes mellitus develops. Diagnosis of the disease is based on the detection of very high concentrations of glucagon in the blood plasma. In newborns, if the mother has diabetes, glucagon secretion is impaired, which may play an important role in the development of neonatal hypoglycemia. Hypoglycemic stimulation of glucagon release is absent in patients with type 1 diabetes. Glucagon deficiency may reflect a general decrease in pancreatic tissue mass caused by inflammation, tumor, or pancreatectomy. With glucagon deficiency, a lack of increase in its level is detected in the arginine stimulation test.
Pancreatic peptide
More than 90% of pancreatic peptide is found in the pancreas. The concentration of the peptide in blood plasma increases sharply after food intake and hypoglycemia caused by insulin administration. Metabolism of pancreatic peptide occurs mainly in the liver and kidneys. The main role of pancreatic peptide in the body is to regulate the rate and amount of exocrine secretion of the pancreas and bile. In diabetes mellitus in the stage of decompensation, the level of peptide in the blood increases, and when carbohydrate metabolism is compensated, its concentration in the blood normalizes. Increased levels of pancreatic peptide are detected in benign and malignant tumors arising from the pancreatic islets, as well as in carcinoid syndrome.
Microalbumin
Nephropathy as a complication of diabetes mellitus is the main cause of death in patients. Diagnosis of diabetic nephropathy is based on microalbuminuria, the detection of which depends on the time of onset of the disease and the type of diabetes. In patients with type 1 diabetes, microalbuminuria is determined annually. In patients suffering from type 2 diabetes, microalbuminuria is determined once every 3 months from the moment the disease is diagnosed. When proteinuria appears, monitoring the progression of diabetic nephropathy includes determining once every 5–6 months the glomerular filtration rate (Rehberg test), the level of creatinine and urea in the blood serum and urinary protein excretion, as well as blood pressure.
In patients with type 1 diabetes, the preclinical stage of nephropathy can be detected by monitoring blood pressure and determining the excretion of microalbumin. Usually, already at an early stage of nephropathy, in the presence of only microalbuminuria, moderate but progressively increasing blood pressure is detected. In diabetic patients, microalbumin levels can be 10–100 times higher than normal. This marker also reflects the risk of developing cardiovascular complications in type 1 and type 2 diabetes.
Determination of lipid profile
Numerous studies in recent years have shown that the main role in the pathogenesis of vascular complications of diabetes belongs to hyperglycemia, and in type 2 diabetes, also to lipid metabolism disorders. Lipid metabolism disorders are directly related to excess body weight. As body mass index (BMI) increases, the incidence of hypercholesterolemia increases, and total cholesterol levels are usually higher in individuals with abdominal obesity. In addition, as BMI increases, triglyceride levels increase, HDL cholesterol decreases, and LDL cholesterol increases. This type of lipid profile is characteristic of the precursor to type 2 diabetes mellitus, insulin resistance syndrome.
Thus, the diagnosis of diabetes mellitus should be comprehensive, aimed at examining all body systems: this makes it possible to prevent the development of serious complications and prescribe treatment in a timely manner.
E. E. Petryaykina, Candidate of Medical Sciences N. S. Rytikova, Candidate of Biological Sciences Morozov Children's City Clinical Hospital, Moscow
Causes of diabetes
The causes of diabetes are associated with abnormal metabolic transformation of carbohydrates (sugars) in the human body. This is due to the work of the pancreas, which produces insulin, a hormone necessary to regulate sugar metabolism. Problems arise when the pancreas produces too little or no insulin.
Production of the hormone insulin by the pancreas
There can be many reasons for pancreas dysfunction. They depend primarily on the type of diabetes you have been diagnosed with. Some factors that cause problems with carbohydrate metabolism are genetic and cannot be influenced. However, in addition to genetic determinants, the development of the disease is also influenced by:
- Lifestyle;
- diet;
- physical activity.
Patients most often suffer from type II diabetes, and the majority of diabetics today have this type of disease. It is also important that environmental factors are of great importance for the development of the disease. Lifestyle and diet are the underlying causes, but addiction issues, mainly alcohol and nicotine abuse, are also very important.
Patients are also diagnosed with insulin resistance, that is, a decrease in the body's sensitivity to insulin. Genetic factors play a big role here, although environmental factors can also cause problems.
Insulin resistance is often diagnosed in obese people, which is the result of an unhealthy lifestyle.
Evaluation of treatment effectiveness
The effectiveness of the prescribed treatment must be monitored and, if necessary, the treatment program adjusted. In addition, it is important to identify emerging complications at an early stage. To do this, patients are recommended to check glycated hemoglobin every three months, microalbuminuria (MAU) every six months, and undergo a clinical examination once a year.
Patients with diabetes mellitus of the first group are registered with an endocrinologist, and patients with T2DM, after selecting appropriate therapy in the absence of severe cardiovascular diseases, are observed by a therapist or general practitioner. If the patient's condition worsens, the therapist can re-refer him to an endocrinologist to adjust the therapy. If the complications that arise lead to a person’s loss of ability to work, he is assigned a disability, but this is done on the basis of the conclusion of a medical and social examination.
Why diabetes is dangerous
Diabetes mellitus is called a vascular disease, since it primarily affects the blood vessels. The disease affects the aorta, carotid artery, coronary arteries and smaller vessels (retina). And this can lead to serious complications (Table 1).
| Table 1. Main complications of diabetes mellitus. | |
| Complication | Cause of complication |
| Myocardial infarction, stroke, coronary heart disease | In large vessels, atherosclerosis processes develop and atherosclerotic plaques appear, which narrow their lumen (macroangiopathy). As a result, tissue oxygen starvation occurs. If tissues do not receive enough oxygen, the vessels become unable to deliver blood in the required volume to the organs. |
| Renal dysfunction | Beneficial proteins begin to “leave” in the urine, and harmful substances (creatinine or urea), on the contrary, remain in the blood. They poison the body (uremia). The patient experiences swelling and blood pressure rises. |
| Damage to the blood vessels of the eyes | Due to changes in sugar levels or inadequate effects of insulin, the blood vessels in the eyes become injured and dilate. Balls (microaneurysms) appear on the walls of the arteries, which then burst, resulting in hemorrhage. |
| Retinal damage (diabetic retinopathy) | The disease first affects small arteries, and microhemorrhages may occur. Ultimately, new ones begin to sprout in place of old vessels. This leads to severe vision loss or blindness. |
| Diabetic foot (gangrene of the lower extremities) | The vessels and nerve fibers of the lower extremities are affected. The infection penetrates through the skin ulcer, tissue death occurs due to impaired blood flow and infection (gangrene). As a result, limb amputation is possible. |
| Charcot foot | Inflammatory processes occur in the metatarsal bones. The foot can take on the most bizarre shapes. |
| Hypoglycemic coma | There is a sudden drop in blood sugar. The man becomes covered in cold, sticky sweat and his hands begin to tremble. He ceases to understand what he is doing. Appetite increases sharply, you want to eat or drink something. Ultimately, the person may lose consciousness. |
| Ketoacidotic coma | The patient begins to lose weight quickly, drinks a lot of water and goes to the toilet often. The body stops absorbing glucose. The person sharply weakens, loses energy, and becomes inhibited. Fat breakdown occurs in the body. The breakdown products of fats - ketone bodies - are toxic, they poison the body. The patient is dehydrated, doctors note very high blood sugar levels. The patient emits a strong odor of acetone. The patient begins to lose weight quickly, drinks a lot of water and goes to the toilet often. The body stops absorbing glucose. The person sharply weakens, loses energy, and becomes inhibited. |
In addition, diabetes mellitus is often an aggravating factor for other diseases and increases the risk of their complications.
Types of analyzes
A urine test can detect a variety of substances, including glucose, ketones, protein, bacteria, and bilirubin.
Glucose
A urine test can measure levels of glucose, ketones, and other substances.
Typically, glucose is not present in the urine. However, when a person has diabetes, it can be detected in the urine. Some kidney diseases may also cause sugar to appear in the urine.
Pregnancy:
During pregnancy, almost half of women can detect glucose in their urine, even if they do not have diabetes.
A woman who experiences glucose in her urine during pregnancy should be evaluated for gestational diabetes.
This is a type of diabetes that occurs during pregnancy and usually goes away after delivery, but can have negative effects on the fetus, lead to complications during childbirth and a higher risk of developing type 2 diabetes later in life.