Hypertrophy of the inferior turbinates in adolescents
Features of the treatment of diseases of the ENT organs during puberty are determined by the restructuring of the endocrine system of the developing organism.
One of the critical periods in a child's development is adolescence. It is characterized by a number of pathological features:
- involution of adenoid tissue occurs;
- the severity of atopic diseases in most adolescents decreases;
- the type of immune response is finally established;
- the spectrum of pathogens of chronic inflammatory diseases changes - staphylococci, atypical forms of pathogens, enterobacteria, and opportunistic pathogens begin to prevail in it;
- autoimmune diseases associated with chronic lack of nasal breathing are increasing; Chronic diseases of the digestive and cardiovascular systems are formed.
The course of the disease, as well as the selection of adequate treatment methods in adolescents, can be complicated by drinking alcohol, smoking and taking contraceptives.
One of the most widespread pathologies of the nasal cavity is hypertrophy of the inferior turbinates. Its most typical manifestations most often occur during adolescence.
Hypertrophy of the inferior turbinates in adolescents is one of the signs of a number of nasal diseases:
- vasomotor rhinitis,
- hypertrophic rhinitis,
- allergic rhinitis,
- compensatory hypertrophy (with a deviated nasal septum).
The main symptom of hypertrophy of the inferior turbinates in adolescents is difficulty in nasal breathing, which can be temporary or permanent. It depends on the degree of hypertrophy.
Various therapeutic and surgical methods for treating this pathology are aimed at reducing the size of hypertrophied nasal turbinates while maintaining their functions.
Conventionally, three areas of treatment for hypertrophy of the inferior turbinates in adolescents can be distinguished:
1) conservative methods that use various medications;
2) physiotherapeutic methods;
3) surgical methods.
As a rule, treatment of hypertrophy of the inferior turbinates in adolescents includes a complex of conservative and physiotherapeutic measures aimed at restoring the respiratory function of the nose.
Surgical treatment of enlarged inferior turbinates in adolescence is based on the choice of the most gentle techniques that preserve the mucous membrane of the turbinates as much as possible. When choosing the scope of surgical intervention on altered intranasal structures, a thorough preliminary examination is necessary (endoscopic examination of the nasal cavity and nasopharynx, acoustic rhinometry, rhinopneumometry, etc.). In adolescence, when the manifestation of most diseases is associated with hormonal changes in the developing organism, surgical activity in relation to the structures of the nasal cavity should be carefully justified and be as gentle as possible.
To determine the nature of the disease, make a diagnosis and choose a treatment method for patients with symptoms of difficulty in nasal breathing, an in-person consultation with an otorhinolaryngologist is necessary: Molchanova Ekaterina Borisovna. Sign up by phone. 8(499)-968-69-12 and 8-903-781-56-21
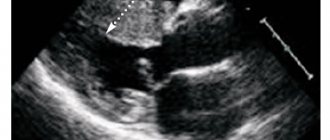
Molecular mechanisms of cardiac hypertrophy: Part 1. Physiological hypertrophy
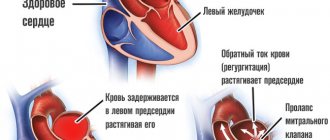
Cardiac hypertrophy is a compensatory reaction that occurs in response to an increase in the load on the myocardium. Physiological hypertrophy leads to increased myocardial function: increased ejection fraction, increased contractile activity.
There are two types of cardiac hypertrophy: physiological and pathological, which differ in molecular mechanisms, cardiac phenotype and prognosis for life. The latter is the most important in clinical practice, since physiological hypertrophy helps to “push” blood through at higher loads, while pathological hypertrophy is associated with unfavorable conditions, including myocardial infarction, arrhythmias, and in the long term can lead to death. It should also be noted that these hypertrophy variants are “competitive antagonists”; There is a constant “struggle” between various signaling systems, the result of which will be the development of either physiological or pathological hypertrophy. [1].
With physiological hypertrophy, there is a slight increase in heart weight (by 10–20%), an increase in cardiomyocytes (CMC) and their proliferation, while the ejection fraction is preserved or increased. There are no sclerotic or necrotic changes in the tissue, and most importantly, physiological hypertrophy is completely reversible (with the exception of postnatal hypertrophy) [1].
Mechanisms of physiological hypertrophy
The initial stimuli leading to hypertrophy do not differ in the case of physiological or pathological variants (for example, physical activity or arterial hypertension). But the reactions triggered in this case are radically different. For the development of physiological hypertrophy, the following is required: an increase in cell size, increased mitochondrial function and energy production, angiogenesis proportional to cell growth, the work of antioxidant systems, regulation of proliferation and regeneration of cardiomyocytes [1].
Physiological hypertrophy of the heart is observed from the birth of a child until he grows up (also called postnatal hypertrophy), with increased physical activity, as well as during pregnancy [2].
Mechanosensors
Mechanotransduction is a fundamental process, the essence of which is the transformation of mechanical signals into biochemical ones. There are sensory systems in the heart that perceive mechanical signals (changes in pressure, volume, etc.) and activate signaling systems responsible for physiological hypertrophy [2].
Ion channels with transient receptor potential (TRP) are one such system. TRPs are a superfamily of transmembrane proteins that function as nonselective ion channels. Among them there are 7 subfamilies; these receptors differ in localization and their role in various cellular processes [3].
In addition to TRP, various integrins exhibit mechanosensitivity. These are transmembrane proteins that can interact with the extracellular matrix (and its various components: fibronectin, laminin, collagen). In response to an extracellular agent, integrins can transmit information into the cell through special integrin-associated protein complexes. This may be focal adhesion kinase (FAK - focal adhesion kinase), integrin-linked kinase (ILK - integrin-linked kinase). The latter, in turn, activate various signaling pathways—standard protein kinase C or PI3K/Act (see below) [2].
We should dwell in somewhat more detail on the integrin-associated protein specific to muscle tissue—melusin. It interacts with β1-integrins, promoting the development of concentric hypertrophy while maintaining cardiac contractility. An increase in melusin synthesis initiates physiological hypertrophy and prevents its development along a pathological path. The opposite is also true: a lack of melusin leads to the development of dilated cardiomyopathy [1].
And, of course, one cannot fail to mention the role of the Z-line as a mechanoreceptor. This part of the cardiomyocyte also responds to stretch by transmitting a signal to many different proteins: teletonin, myopalladin, ankyrins, obscurin... Ultimately, all these processes turn out to be essential for the development of physiological hypertrophy, while defects in some of these proteins lead to cardiomyopathies [2] .
Insulin and insulin-like growth factor 1
These substances regulate many different cellular processes in the heart: proliferation, differentiation, cell growth, metabolism, contractility and apoptosis. Insulin resistance is common in heart failure. IGF1 (insulin-like growth factor 1) is structurally identical to insulin; this agent is synthesized in the liver in response to GH, but can also be formed in other organs (including the heart) [1].
Insulin is known to bind to the insulin receptor, tyrosine kinase, which phosphorylates insulin receptor substrates 1 and 2 (IRS1, 2-insulin receptor substrate). IRSs in turn activate the PI3K/Act signaling pathway. This signaling pathway may be familiar to the reader as a potential target for antitumor therapy. PI3K/Act is involved in the regulation of the cell cycle, and in our case ensures the growth of cardiomyocytes in response to increased load on the myocardium [2], [5].
IGF1, in turn, binds to a specific receptor IGF1R (IGF1 receptor) with subsequent activation of a number of signaling pathways: RAS–RAF–MEK–MAPK, PLC–IP3R3 and others. This also results in physiological hypertrophy of cardiomyocytes.
A little more detail should be taken on the PI3K/Act pathway (it is noteworthy that this signaling pathway is activated during physical activity). PI3K is an enzyme kinase that catalyzes phosphoinositol 3,4,5-trisphosphate. Activation of one of its catalytic subunits—p110α—initiates the processes of physiological hypertrophy and prevents pathological hypertrophy (as we remember, these processes compete with each other). ACT1 is another kinase that is activated by phosphoinositol 3,4,5-phosphate and results in changes in cell metabolism. This is achieved by inhibiting a number of enzymes: glycogen synthase kinase 3β (this kinase inhibits translation initiation factors), fox-O3 (a factor that inhibits the general metabolism of proteins in the cell) and others. As a result, active protein synthesis occurs and the cell increases in size [1].
Triiodothyronine
T3 is triiodothyronine, a thyroid hormone that influences both postnatal cardiac hypertrophy and exercise-induced hypertrophy (although the latter is less reliable) [2].
Immediately after birth, the concentration of T3 in the blood increases significantly. The hormone binds to TRα and TRβ receptors (thyroid hormone receptors), which leads to a “switch” of transcription of the MYH7 gene to MYH6 (these genes encode sequences of myosin heavy chains). MYH7 encodes the β-heavy chains of myosin, MYH6 encodes the α-isoform [1]. This is extremely important, since the α-isoform has greater ATPase activity and, as a result, a cell with active MYH6 is able to contract more strongly [2], [6].
The interaction of T3 with retinoic acid receptors (nuclear receptors for steroid and thyroid hormones, approx.) leads to an increase in the expression of calcium ATPase-2 (SERCA2 - sarcoplasmatic/endoplasmatic reticular calcium ATPase-2), inhibiting MYH7 and the expression of phospholamban (a protein in cardiomyocytes that suppresses SERCA2) [2].
SERCA2 is the evolutionarily most ancient isoform of mammalian calcium ATPases. There are three such isoforms (with subtypes). SERCA2 has three subtypes: a, b and c; for the heart, SERCA2a is most specific (97.5%), there is also a small amount of SERCA2b (2.5%). The function of this protein is to control cytosolic Ca2+ and, as a consequence, to regulate the contractile function of the entire cardiomyocyte [7].
Phospholamban, whose work is inhibited by T3, is a protein regulator of SERCA2 (more precisely, the most studied of these regulators). In its active - dephosphorylated - state, phospholamban suppresses SERCA2 and leads to a decrease in the concentration of Ca2+ in the cytoplasm, which, in turn, reduces the contractility of the cardiomyocyte [2], [7].
In addition, T3 also activates the transcription of β1-adrenergic receptors, sodium and calcium channel proteins, cardiac troponin I, sodium/calcium pump proteins, and adenylate cyclase types V and VI [1], [2]. The hormone also increases the content of TRα1, which activates the already known PI3K [2].
The end result of the work of triiodothyronine in cardiomyocytes is increased myocardial contractile activity and cardioprotection [1].
Nitric oxide (NO)
Physical activity stimulates β3-adrenergic receptors in endothelial cells; in response, endothelial NO synthase (NOS3) is activated, which synthesizes nitric oxide itself, a known vasoactive substance. After this, the following happens:
NO activates soluble guanylate cyclase → cGMP content increases → cGMP-dependent protein kinase G (PKG) is activated → PKG phosphorylates two regulatory proteins (RGS2 and RGS4), which inhibit G-coupled receptors responsible for the development of pathological hypertrophy.
This scheme is needed so that hypertrophy develops according to the physiological variant. A defect in either β3-adrenergic receptors, or NOS3, or the RGS2 and RGS4 proteins leads to a break in the entire chain of cardioprotection, which may well result in myocardial infarction [1].
Angiogenesis
Cells need sufficient blood supply to function normally. If vascular density increases in proportion to the growth of cardiomyocytes, physiological hypertrophy develops. But when cells grow faster than the capillary network can allow, chronic hypoxia occurs.
One of the most important factors determining angiogenesis is vascular endothelial growth factor (VEGF). VEGF may also be familiar to the reader - inhibitors of this factor are used in the treatment of various types of cancer. However, such drugs have significant cardiotoxicity and can lead to various cardiomyopathies (which indirectly indicates the importance of VEGF for myocardial function). Among other things, changes in VEGF levels are also associated with peripartum (postpartum) cardiomyopathy [1], [2].
At the beginning of the article, NFAT signaling molecules were mentioned (in the “mechanosensors” section). VEGF, influencing endothelial cells, acts precisely through the NFAT system, causing enhanced angiogenesis. Thus, these nuclear factors, in addition to their anti-apoptotic role, also affect the blood supply to cardiomyocytes [8].
In addition to VEGF, platelet-derived growth factor (PDGD) also plays an important role. The mechanisms by which PDGD influences angiogenesis are not known for certain; however, a defect in this factor in experiments on mice leads to heart failure and deterioration of blood supply [1].
It is also impossible not to mention another important substance. Hypoxia-inducible factor 1α (HIF1α - hypoxia-inducible factor) is a major transcription factor that ensures a constant supply of oxygen to cells by regulating angiogenesis, vascular modification and regulation of glucose metabolism. In the case of pathological hypertrophy, the p53 antigen (known for its association with tumor development) activates the ubiquitination and proteasomal degradation of HIF1α [1]. Normally, HIF1α activates various signaling molecules (including VEGF) and promotes enhanced angiogenesis.
Neuregulin-1
Neuregulins (1-4) are members of the epidermal growth factor family. In the cardiovascular system, neuregulin-1 is mainly present. The signaling pathway of this factor (and its receptors) plays an important role in the adaptation of the myocardium to the load and the formation of the heart muscle.
Neuregulin-1 acts on the ErbB family kinase (a group of molecules structurally similar to epidermal growth factor; proteins of this family are often considered as potential tumor markers, approx.). Neuregulin-1 activates ErbB2 and ErbB4, which leads to stimulation of the already mentioned PI3K system. The result of this interaction is the proliferation of cardiomyocytes, which prevents ischemic lesions [1].
MicroRNAs and RNA-binding proteins
MicroRNAs (miRNAs) are expressed in the heart during physiological hypertrophy induced by aerobic exercise. For example, miRNA-222 in mice inhibits four targets potentially responsible for decompensation and the development of heart failure: p-27 (encodes a cell cycle inhibitor), Hipk1 and Hipk2 (encodes protein kinases), Hmbox1 (encodes a transcription inhibitor). In other words, activation of miRNA-222 promotes the growth and proliferation of cardiomyocytes [1].
Also, any form of hypertrophy requires de novo protein synthesis. And here, eukaryotic translation factor 4F (elF4F) and the mammalian target of rapamycin mTORC1 are involved - together they trigger increased protein synthesis. In addition, in response to a physiological (and pathological) stimulus in myocardial cells, the poly-A sequence elongates and the expression of specific polyadenylate-binding protein 1 (PABPC1 - polyadenylate-binding protein 1) occurs, which binds to elF4F and thus promotes accumulation in cells various construction (and other) proteins. Increased expression of PABPC1 in cardiomyocytes is necessary for physiological hypertrophy (nothing is known yet about the role of the protein in its pathological version) [1].
Sources:
- M. Nakamura and J. Sadoshima, 'Mechanisms of physiological and pathological cardiac hypertrophy', Nat. Rev. Cardiol., vol. 15, no. 7, pp. 387–407, 2021.
- M. Maillet, J. H. Van Berlo, and J. D. Molkentin, 'Molecular basis of physiological heart growth: Fundamental concepts and new players', Nat. Rev. Mol. Cell Biol., vol. 14, no. 1, pp. 38–48, 2013.
- B. Nilius and G. Owsianik, 'The transient receptor potential family of ion channels', Genome Biol., vol. 12, no. 3, 2011.
- W. T. Pu, Q. Ma, and S. Izumo, 'NFAT transcription factors are critical survival factors that inhibit cardiomyocyte apoptosis during phenylephrine stimulation in vitro', Circ. Res., vol. 92, no. 7, pp. 725–731, 2003.
- M. Osaki, M. Oshimura, and H. Ito, 'PI3K-Akt pathway: Its functions and alterations in human cancer', Apoptosis, vol. 9, no. 6, pp. 667–676, 2004.
- E. M. McNally, R. Kraft, M. Bravo-Zehnder, D. A. Taylor, and L. A. Leinwand, 'Full-length rat alpha and beta cardiac myosin heavy chain sequences', J. Mol. Biol., vol. 210, no. 3, pp. 665–671, 1989.
- P. Vangheluwe, L. Raeymaekers, L. Dode, and F. Wuytack, 'Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: Cell biological implications', Cell Calcium, vol. 38, no. 3-4 SPEC. ISS., pp. 291–302, 2005.
- T. A. Zaichuk, E. H. Shroff, R. Emmanuel, S. Filleur, T. Nelius, and O. V. Volpert, 'Nuclear factor of activated T cells balances angiogenesis activation and inhibition', J. Exp. Med., vol. 199, no. 11, pp. 1513–1522, 2004.
HYPERTROPHY
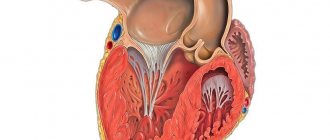
HYPERTROPHY (from hyper... and Greek τροφή - nutrition), an increase in the volume of an organ, tissue, cells, intracellular structures. It occurs both normally and in various diseases, ensuring the stability of homeostasis. G., along with an increase in volume, is often based on an increase in the number of structural units (organ, tissue, cell), that is, hyperplasia. For example, the G. of a parenchymal organ is based on the G. of parenchymal cells, but at the same time the number of stromal elements can increase, and the formation of new vessels (neoangiogenesis) occurs, providing an increased need for nutrition of hypertrophies. organ. Cell growth also occurs due to an increase not only in the volume of its structures, but also in their number. Thus, G. and hyperplasia are two inextricably linked processes.
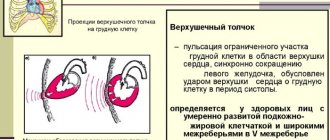
Normally, G. develops with increased functional demand or neurohormonal stimulation (for example, myocardial G. in athletes, an increase in the volume of the uterus and mammary glands during pregnancy).
Pathological G. can be true (an increase in the mass and functional activity of an organ due to the G. of cells providing this activity) and false (due to the growth of connective tissue of the stroma and adipose tissue with possible atrophy of parenchymal cells).
True G. is divided into working (compensatory), vicarious (replacement), regenerative, neurohumoral G. and hypertrophic. overgrowth.
Working G. develops with increased work of an organ and is characterized by an increase in the volume of its functionally active structures. For example, the cause of myocardial gastrointestinal tract may be various. hemodynamic factors - arterial and pulmonary hypertension, congenital or acquired heart defects. The weight of the heart can exceed normal values by 3–4 times. G. of the bladder wall develops in men with prostatic hyperplasia, which narrows the urethra and secretes hormonal and growth factors that stimulate G. of detrusor cells (the muscle that expels urine).
Vicarious G. develops in one of the paired organs (lung, kidney, adrenal gland, etc.) following the death or surgical removal of the other.
Regenerative blood forms in the parenchymal cells of the myocardium, neurons, hepatocytes, and others around scars during incomplete regeneration.
With neurohumoral G. and hypertrophic. growths, in fact, we are talking about an increase in the volume of the organ basically. due to the processes of hyperplasia of cellular structures, which simultaneously increase in number and volume.