Complexes with this research
Coagulogram Study of the functional state of hemostasis 2,020 R Composition
Examination during pregnancy. 3 trimester 9 620 RUR Composition
Miscarriage Identification of the main causes of miscarriage RUB 40,070 Composition
IN OTHER COMPLEXES
- Examination during pregnancy. 1st trimester 16,690 RUR
- Extended coagulogram RUB 4,150
- Female infertility RUB 16,210
- Entry into IVF RUB 23,020
- Pregnancy planning. Clinical indicators 6,630 R
Indications
Antithrombin III testing is most often carried out as part of a coagulogram in the following cases:
- Screening of patients at risk (there were cases of antithrombin deficiency in the family history, thrombosis of veins or arteries was observed before the age of 50);
- Estimation of the amount of functionally useful antithrombin III;
- Thromboembolic pathologies: thrombosis of deep veins or mesenteric vessels;
- myocardial infarction;
- pulmonary embolism;
- stroke;
- thrombophlebitis;
- miscarriage (history);
Detailed description of the study
During bleeding, platelets circulating in the blood plasma, with the participation of many clotting factors, form a clot. Along with this, under the influence of thrombin, the fibrin protein is formed, which stabilizes the formed blood clot, after which the bleeding stops.
Antithrombin III is a protein that regulates blood clotting. This protein prevents the formation of blood clots, which is necessary to maintain balance between the coagulation and anticoagulation systems. To this end, antithrombin III binds to enzymes called serine proteases, as well as thrombin, factor IXa and Xa, and inhibits their activity. Sufficient amounts of clotting factors and antithrombin III allow bleeding to be stopped without excessive clot formation.
Antithrombin III deficiency is associated with an increased risk of thrombosis and thromboembolism. It may be hereditary or acquired. According to various estimates, the prevalence of hereditary antithrombin III deficiency ranges from 1 in 2000 to 1 in 5000 cases.
Hereditary antithrombin deficiency is divided into type I and type II. Type I leads to the complete absence of this protein in the blood due to the absence of genes that ensure normal synthesis of antithrombin III. Type II deficiency is characterized by the production of altered protein, which impairs its function. The overall activity of antithrombin III decreases.
Lack of production or incomplete activity of antithrombin III most often manifests itself in the form of deep vein thrombosis. However, there is a risk of repeated sudden thrombosis of atypical vessels, for example, veins of the brain or intestines. The first occurrence of thrombosis occurs at a relatively young age, with the risk of blood clots peaking between the ages of 15 and 40 years. This is especially important against the background of concomitant factors that increase the risk of thrombosis, for example, during pregnancy.
Acquired deficiency of antithrombin III is usually associated with either a decrease in its production in the liver in the case of liver failure, cirrhosis, malnutrition, or with loss of protein by the body in nephrotic syndrome and some other conditions. A decrease in the concentration of antithrombin III is observed in coagulopathies, including disseminated intravascular coagulation syndrome, microangiopathy with thrombosis. In many malignant neoplasms, its production is also disrupted. The use of the anticoagulant heparin significantly increases the activity of antithrombin III, which makes it possible to prevent thrombosis.
The influence of antithrombin III on the body is extensive, so determining the level of this indicator in the blood allows you to avoid the negative consequences of its deficiency and select treatment.
General information
Antithrombin III (glycoprotein, plasma protein) is the main anticoagulant of the blood system, which blocks coagulation factors and promotes its natural thinning. Its main function is to regulate the formation of blood clots during bleeding, that is, to deactivate excessive thrombus formation.
Analysis for antithrombin III as part of a coagulogram allows you to evaluate its quantity (antigen test) and quality (activity).
As a result of injury, impact, compression or surgery, the integrity of the tissue and, as a result, the vessels located in it are damaged. The body's response to damage is the release of coagulative components, which contribute to the formation of a blood clot that stops bleeding in the vessel. Antithrombin III regulates the process of thrombus formation, since an excessive number of such clots leads to complete blockage of the lumen of the vessel, which prevents blood from entering a certain area.
The glycoprotein antithrombin III is produced by the inner layer of blood vessels - the endothelium - and liver cells with the participation of vitamin K. After synthesis, it is released into the bloodstream, where it inhibits the coagulation factor thrombin, as well as IXA, XA, XIA, XIIA factors. The natural process proceeds quite slowly, but in the presence of heparin it accelerates sharply. If the level of antithrombin decreases critically, then heparin loses its biological activity - there is a risk of vascular blockage (thrombosis).
Plasma protein deficiency can be acquired and hereditary (1:5000 cases). If the patient is at risk, thrombosis can develop after 20 years.
Experts define 2 types of deficiency. In the first case, antithrombin is biologically active, but is produced in insufficient quantities. The second type is characterized by normal secretion of defective antithrombin, which is not capable of performing an anticoagulation function.
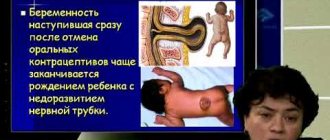
Regardless of the reasons for the decrease in the concentration of this protein, the main manifestation of deficiency is thrombosis of the veins and arteries, which periodically recur. In pregnant women, insufficient production of antithrombin increases the likelihood of spontaneous abortion.
Congenital deficiency manifests itself as strokes or myocardial infarctions in young patients, thrombosis in infants (rarely). The condition is also aggravated by pregnancy and childbirth, surgical interventions, infectious and inflammatory processes, and the use of oral contraceptives.
Normally, antithrombin decreases with age in men, and in women after menopause.
What can distort the result
The following factors influence the results of the study:
- treatment with heparin and estrogens - they reduce the level of antithrombin in the blood;
- in the case of infectious, viral and inflammatory diseases (including influenza, ARVI, sore throat, rhinitis, etc.), the activity of antithrombin decreases, which also reduces its indicator.
Important! The interpretation of the results is always carried out comprehensively. It is impossible to make an accurate diagnosis based on only one analysis.
Antithrombin III human 500 IU No. 1 bottle + solvent + kit for dissolution and administration
Content
Pharmacological action Indications of the drug Antithrombin III human Dosage regimen Rules for the preparation and administration of the solution Preparation of the solution: Administration of the resulting solution: Side effects Contraindications for use Use during pregnancy and lactation Use in children Special instructions Effect on the ability to drive vehicles and operate machinery Drug interactions Storage conditions Antithrombin III human Shelf life Antithrombin III human
pharmachologic effect
Direct acting anticoagulant.
Indications for the drug Antithrombin III human
Congenital or acquired antithrombin deficiency as part of a wide variety of clinical disorders (acquired antithrombin deficiency may result from either increased protein intake or loss, or impaired antithrombin synthesis).
The use of the drug Antithrombin III human is indicated in patients with plasma antithrombin activity less than 70% of normal for the prevention of thrombotic and thromboembolic complications. Administration of antithrombin may be particularly valuable in the following cases:
- surgical procedures in patients with congenital antithrombin III deficiency;
- pregnancy and childbirth in patients with congenital antithrombin III deficiency;
- insufficient or no response to heparin;
- the presence or risk of developing disseminated intravascular coagulation (for example, with concomitant trauma, septic complications, shock, preeclampsia and other disorders associated with acute consumption coagulopathy);
- the presence or risk of thrombosis in patients with nephrotic syndrome or inflammatory diseases of the bladder;
- surgery or bleeding in patients with severe hepatic impairment, especially in patients receiving treatment with clotting factor concentrates.
Dosage regimen
Treatment should be initiated under the supervision of a physician experienced in treating patients with antithrombin deficiency.
For congenital deficiency, the dose should be adjusted individually for each patient, taking into account family history of thromboembolic conditions, existing clinical risk factors and laboratory results.
In cases of acquired deficiency, the dose and duration of replacement therapy depend on the level of antithrombin in plasma, the presence of signs of increased metabolism of antithrombin, as well as the underlying disease and the severity of the clinical condition. The dose and frequency of administration are always determined based on clinical effectiveness and laboratory results in each individual case.
The number of units of antithrombin administered is expressed in ME, which are calculated in relation to the current WHO standard for antithrombin. Antithrombin activity in plasma is expressed either as a percentage (relative to normal human plasma) or as IU (relative to the international standard for antithrombin in plasma).
1 IU of antithrombin activity is equivalent to the antithrombin activity in 1 ml of normal human plasma. Calculation of the required dose of antithrombin is based on empirical data that 1 IU of antithrombin per 1 kg of body weight increases the activity of antithrombin in plasma by approximately 2%.
The initial dose is calculated using the following formula:
Required dose (ME) = body weight (kg) × (target level - baseline activity level [%]) × 0.5
The initial target activity of antithrombin depends on the clinical situation. If replacement therapy is indicated, the dose should be sufficient to achieve target antithrombin activity as well as to maintain effective levels. The dose should be determined and monitored based on laboratory measurements of antithrombin activity, which should be performed at least 2 times a day, preferably immediately before the next administration. The dose should be adjusted if there are laboratory and clinical signs of increased antithrombin metabolism. Antithrombin activity should be maintained above 80% throughout the course of treatment, unless clinical features indicate the need to maintain a different effective level.
Typically, the initial dose for congenital deficiency is 30-50 IU/kg. Subsequently, the dose and interval between administrations, as well as the duration of treatment, should be adapted to the data of biochemical studies and the clinical situation.
The use of antithrombin III in children under 6 years of age has not been sufficiently studied.
Rules for preparing and administering the solution
The drug is administered intravenously. The maximum injection rate is 5 ml/min.
Dissolve the contents of the bottle with the drug Antithrombin III human immediately before administration. For this purpose, only the supplied dissolution and administration kit should be used. The resulting solution must be used immediately after dissolution, because the drug does not contain preservatives. Before administration, the solution must be visually examined for the presence of foreign inclusions or color changes. Solutions that are cloudy or contain sediment should not be used. Unused solution must be disposed of in accordance with established rules.
Preparation of the solution:
- Warm the closed bottle with solvent to room temperature (not higher than 37°C).
- Remove the protective caps from the lyophilisate and solvent bottles and disinfect the rubber stoppers on both bottles.
- Rotate and then remove the protective cap from one end of the supplied adapter needle. Pierce the stopper of the solvent bottle with the free end of the needle.
- Remove the protective cap from the other end of the adapter needle without touching the needle itself.
- Turn the bottle with the solvent over and pierce the stopper of the bottle with the lyophilisate with the free end of the needle. The solvent will flow into the vial with the lyophilisate under the influence of vacuum.
- Separate the bottles by removing the needle from the bottle with the drug. Gently shaking or rotating the bottle will speed up the dissolution of the powder.
- After complete dissolution of the drug, the stopper of the bottle with the drug should be pierced to deposit foam. Then remove the airway needle.
- Twist and then remove the protective cap from the included filter needle and place the needle on a sterile disposable syringe. Draw the solution into a syringe.
- Disconnect the filter needle from the syringe and slowly (maximum injection rate 5 ml/min) inject the solution intravenously using the supplied disposable needle (butterfly needle).
Introduction of the resulting solution:
Side effect
- From the digestive system: nausea, vomiting.
- Immune system: hypersensitivity or allergic reactions, (severe) anaphylaxis (including shock), angioedema, (generalized) urticaria, rash.
- From the nervous system: headache, anxiety, tingling sensation in the body.
- From the respiratory system: wheezing.
- From the cardiovascular system: tachycardia, decreased blood pressure, redness of the skin.
Laboratory and instrumental data: platelet count less than 100,000/μl or a decrease in platelet count by 50%.
General reactions: chills, drowsiness, chest tightness, fever, heparin-induced thrombocytopenia with antibodies (type II).
Local reactions: burning and tingling sensation in the injection area.
Contraindications for use
- history of heparin-induced thrombocytopenia;
- hypersensitivity to the components of the drug.
Use during pregnancy and breastfeeding
Data on the safety of human antithrombin preparations during pregnancy are limited.
The safety of Antithrombin III human when used in pregnant or lactating women has not been established in controlled clinical studies.
Antithrombin III human can be administered to pregnant or lactating women only if strictly indicated, given that pregnancy carries an increased risk of thromboembolic events.
Use in children
The use of antithrombin III in children under 6 years of age has not been sufficiently studied.
special instructions
As with any protein drugs for intravenous administration, the development of allergic-type hypersensitivity reactions is possible. Patients should be closely monitored for any symptoms throughout the infusion period. Patients should be informed of the early signs of hypersensitivity reactions, including rash, generalized urticaria, chest tightness, wheezing, decreased blood pressure, and anaphylaxis. If these symptoms occur after administration of the drug, patients should consult their doctor.
In case of shock, anti-shock therapy should be carried out according to current medical standards.
Standard measures to prevent infections associated with the use of medicinal products derived from human blood or plasma include donor selection, screening of individual plasma donations and plasma pools for specific markers of infection, and the inclusion of efficient manufacturing steps to inactivate/remove viruses. . Despite this, when using medicinal products made from human blood or plasma, the possibility of transmission of infectious agents cannot be completely excluded. This also applies to unknown or new viruses and other pathogenic organisms.
The measures taken are considered effective against enveloped viruses such as HIV, hepatitis B virus and hepatitis C virus. The measures taken may not be sufficient against non-enveloped viruses such as parvovirus B19. Parvovirus B19 infection can pose a serious risk to pregnant women (fetal infection) and to individuals with immunodeficiency or increased erythropoiesis (eg, hemolytic anemia).
For patients receiving regular or repeated doses of human plasma-derived antithrombin preparations, vaccination against hepatitis A and B is recommended.
It is strongly recommended that each time Antithrombin III human is used, the name and batch number of the drug administered to each individual patient are recorded.
Clinical observation and monitoring of biochemical parameters during combined use of antithrombin and heparin:
to adjust the dose of heparin and to avoid an excessive decrease in coagulation, it is necessary to regularly, at frequent intervals, monitor the degree of anticoagulation (aPTT and, if necessary, anti-FXa activity), especially in the first minutes and hours after the start of antithrombin use; Antithrombin levels should be measured daily to adjust the individual dose, as there is a risk of decreased antithrombin levels with long-term treatment with unfractionated heparin.
Impact on the ability to drive vehicles and operate machinery
There were no effects on the ability to drive a car or operate machinery.
Drug interactions
Antithrombin replacement therapy with concomitant use of heparin in therapeutic doses increases the risk of bleeding. The effect of antirhombin is significantly enhanced by heparin. The half-life of antithrombin may be significantly reduced when combined with heparin due to accelerated metabolism of antithrombin. Therefore, the combined use of antithrombin and heparin in a patient with an increased risk of bleeding should be monitored clinically and biochemically.
This medicine should not be mixed with other medicines.
Storage conditions Antithrombin III human
The drug should be stored out of the reach of children at a temperature of 2° to 8°C. Do not freeze.
Shelf life of Antithrombin III human
3 years.
AT III normal
Standard reference ranges
The antithrombin activity of whole donor plasma is taken as 100%.
| Age | Norms |
| Less than 3 days | 58 — 90 % |
| 3 days – 1 month | 60 — 89 % |
| 1 month – year | 72 — 134 % |
| 1-6 years | 101 — 131 % |
| 6-11 years | 95 — 134 % |
| 11-16 years old | 96 — 126 % |
| More than 16 years | 66 — 124 % |
Norms during pregnancy:
| Gestational age in weeks | Antithrombin III, % |
| 13 – 21 | 74 – 115 |
| 21 – 29 | 73 – 114 |
| 29 – 35 | 76 – 112 |
| 35 – 42 | 70 – 116 |
AT III standards in the independent laboratory Invitro
- For women of childbearing age and men – from 83 to 128%.
Norm of antithrombins during pregnancy
Varies depending on trimester. During this period, a woman’s hematopoietic system works differently; the functions responsible for clotting are activated. It is important to monitor the risks of blood clots, which can cause miscarriage problems, as well as complications during childbirth.
In the table of values we present the average data of a healthy pregnant woman, dividing them by week:
| Gestation period, weeks | Normal indicator, percentage |
| from 13th to -21st | from 74 to 115 |
| from 21st to 29th | from 73 to 114 |
| from 29th to 35th | from 76 to 112 |
| from 35th to 42nd | from 70 to 116 |
Preparing for antithrombin III testing
To ensure the reliability of the result, you must follow the following rules for preparing for the study:
- Blood is taken for analysis on an empty stomach - the last meal should be 12 hours before the test. For children this period of time will be shorter and depends on age;
- before the study, you should exclude physical activity and stress (at least 30 minutes);
- It is not recommended to smoke for half an hour before donating blood for analysis.
Venous blood is used for analysis.
Indications for the study
Antithrombin 3 is synthesized mainly in the upper layer of vascular walls and in the liver and, in combination with heparin, is one of the most powerful blood thinning factors.
Analysis for AT III is indicated in the following cases:
- if heparin is insufficiently effective;
- to determine the causes of thrombosis;
- before antigen test;
- for the diagnosis of autoimmune diseases (in particular systemic lupus erythematosus);
- after removal of the blood clot.
Reduced levels of antithrombin III in the blood can be caused by:
- body characteristics characteristic of the third trimester of pregnancy;
- chronic liver diseases (for example, liver failure);
- surgical interventions on the liver;
- nephrotic syndrome;
- malignant tumors;
- thrombotic microangiopathy;
- the patient taking oral contraceptives;
- prescribing certain enzymes (asparaginases) in the course of treatment;
- atherosclerosis;
- development of DIC syndrome (especially acute form);
- development of sepsis;
- development of thromboembolism.
If the antithrombin test result is low
This may indicate the presence of a disease or malfunction of body systems. But not only! This indicator, for example, is typical when taking oral contraceptives, as well as in the middle of the menstrual cycle.
For what diseases will the test result be low?
- atherosclerosis, in which plaques form on the walls of blood vessels;
- inflammation due to infection (sepsis);
- hepatitis and other liver diseases;
- chronic lack of antithrombins in the body;
- thromboembolism (separation of a blood clot and subsequent blockage of the vessel).
Exceeding the norm
- Acute infectious or inflammatory process;
- Diseases of the liver (viral hepatitis, cholestasis) and kidneys, transplantation of these organs;
- Pathologies of the pancreas (acute pancreatitis, oncology);
- Lack of vitamin K in the body;
- Menstrual bleeding (temporary increase, which should be considered normal);
- Therapy with indirect anticoagulants (for example, phenylin, warfarin), anabolic steroids.
Note:
an increase in antithrombin concentration indicates a risk of internal bleeding.