White matter by the brain serves as a vital substance in the human body that it transports impulses to the gray matter of the cell. When a person suffers from periventricular injury to leukomalacia, these functions are impaired. PVL is a strikingly common causative factor among children with cerebral palsy, resulting in mental retardation and spasticity that require therapy and treatment.
Brain injury—damage to white matter brain tissue
Periventricular leukomalacia, or PVL, is a type of brain injury that involves the periventricular white matter of the brain. Damage to white matter results in the death and decay of damaged cells, leaving empty areas in the brain - called lateral ventricles - that fill with fluid (a condition called leukomalacia).
The brain consists mainly of white matter and gray matter. Gray matter has nerve cells in the body that can initiate nerve impulses, while white matter carries impulses between gray matter cells. The periventricular white matter, which surrounds the two horseshoe cavities in the brain, is primarily responsible for transmitting nerve impulses that control motor function. Damage to this area can lead to spasticity and intellectual impairment.
Myelin is an integral component of white matter that coats and essentially insulates the cells of the pathways, facilitating the speedy transmission of nerve impulses. Damage to myelin is slowed and interferes with nerve transmission, possibly impairing brain function.
- Approximately 60-100% of children with periventricular leukomalacia are diagnosed with cerebral palsy.
- Four 26% of premature babies admitted to neonatal intensive care units have cerebral palsy.
- In severe cases, postmortem studies have found that 75% of premature infants who died shortly after birth had periventricular leukomalacia.
Experts believe intrauterine infections are a deterrent for periventricular leukomalacia. The membranes around the fetus are affected by the release of toxins that travel through the amniotic fluid to selectively injure areas of the developing brain. These toxins can also cause premature membrane rupture and premature birth.
When does periventricular leukomalacia occur?
Damage can occur at any time, but researchers have identified stages of fetal development when the unborn child is particularly vulnerable to periventricular leukomalacia.
- Although not all studies agree on an exact range, experts believe the fetus is especially vulnerable to periventricular leukomalacia somewhere between 26 and 34 weeks of pregnancy.
- Premature birth is a high risk factor for periventricular leukomalacia; it is most common among babies born before 32 weeks and with a birth weight below 3.3 pounds.
Brief anatomical information
Foci of the disease are about 2-3 millimeters in diameter and can be found in the parietal and frontal lobes. Moreover, their formation is absolutely asymmetrical.
The main outcome of the disease is the formation of a huge number of cysts, all of them of different sizes. It takes about two weeks for necrosis and the formation of cystic compounds to appear, and then the cavities in the baby’s brain collapse—that is, atrophy of the nerve tissue of the brain.
Article on the topic: Eucalyptus tincture - instructions for use and composition, indications, side effects and price
What are the risk factors and causes of periventricular leukomalacia?
Experts report decreased blood flow or cell damage in periventricular tissue as the main cause of periventricular leukomalacia. Infants born before 32 weeks of gestation and mechanically ventilated are at greatest risk for periventricular leukomalacia. Hypotension, hypoxemia, acidosis, and hypocapnia in ventilated preterm infants can lead to periventricular leukomalacia.
A number of other events also represent a significant increase in the likelihood of developing periventricular leukomalacia. Intrauterine infection, pathological bacteria where the amniotic fluid can infect, is one of the factors; Infection around the delivery time also increases the likelihood. This is more common in preterm births. Other risk factors associated with periventricular leukomalacia include:
- Placental blood vessel conditions known as placental vascular anastomoses
- Twin pregnancy
- Vaginal bleeding during pregnancy, known as antepartum hemorrhage
- Inflammation of the membranes due to a bacterial infection known as chorioamnionitis
- Inflammation of the umbilical cord connective tissue, known as funisitis
- A severe illness in which the blood is overloaded with bacteria, known as sepsis
- Maternal cocaine use
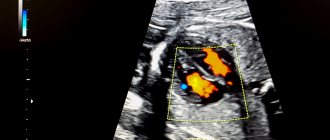
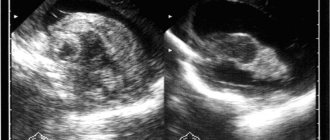
Ultrasound examination of the brain of newborn children (normal anatomy)
Ultrasound machine HM70A
Expert class at an affordable price.
Monocrystal sensors, full-screen display mode, elastography, 3D/4D in a laptop case. Flexible transformation into a stationary scanner with a cart.
Indications for brain echography
- Prematurity.
- Neurological symptoms.
- Multiple stigmas of disembryogenesis.
- Indications of chronic intrauterine hypoxia in the anamnesis.
- Asphyxia during childbirth.
- Respiratory distress syndrome in the neonatal period.
- Infectious diseases in mother and child.
To assess the state of the brain in children with an open anterior fontanel, a sector or microconvex sensor with a frequency of 5-7.5 MHz is used. If the fontanel is closed, then you can use sensors with a lower frequency - 1.75-3.5 MHz, but the resolution will be low, which gives worse quality echograms. When studying premature babies, as well as for assessing superficial structures (furrows and convolutions on the convexital surface of the brain, extracerebral space), sensors with a frequency of 7.5-10 MHz are used.
Any natural opening in the skull can serve as an acoustic window for studying the brain, but in most cases the large fontanel is used, since it is the largest and the last to close. The small size of the fontanel significantly limits the field of view, especially when assessing the peripheral parts of the brain.
To conduct an echoencephalographic study, the sensor is placed over the anterior fontanel, orienting it so as to obtain a series of coronal (frontal) sections, and then turned 90° to perform sagittal and parasagittal scanning. Additional approaches include scanning through the temporal bone above the auricle (axial section), as well as scanning through open sutures, the posterior fontanelle and the atlanto-occipital joint.
Based on their echogenicity, the structures of the brain and skull can be divided into three categories:
- hyperechoic - bone, meninges, fissures, blood vessels, choroid plexuses, cerebellar vermis;
- medium echogenicity - parenchyma of the cerebral hemispheres and cerebellum;
- hypoechoic - corpus callosum, pons, cerebral peduncles, medulla oblongata;
- anechoic - liquor-containing cavities of the ventricles, cisterns, cavities of the transparent septum and Verge.
Normal variants of brain structures
Furrows and convolutions.
The fissures appear as echogenic linear structures separating the gyri. Active differentiation of the gyri begins from the 28th week of gestation; their anatomical appearance precedes echographic visualization by 2-6 weeks. Thus, the number and severity of the furrows can be used to judge the gestational age of the child.
Visualization of the insular complex structures also depends on the maturity of the newborn child. In very premature infants, it remains open and is presented in the form of a triangle, a flag - as a structure of increased echogenicity without identifying grooves in it. The closure of the Sylvian fissure occurs as the frontal, parietal, and occipital lobes form; complete closure of the islet of Reil with a clear Sylvian fissure and vascular formations in it ends by the 40th week of gestation.
Lateral ventricles.
The lateral ventricles, ventriculi lateralis, are cavities filled with cerebrospinal fluid, visible as anechoic zones. Each lateral ventricle consists of the anterior (frontal), posterior (occipital), inferior (temporal) horns, body and atrium (triangle) - Fig. 1. The atrium is located between the body, occipital and parietal horn. The occipital horns are difficult to visualize and their width is variable. The size of the ventricles depends on the degree of maturity of the child; with increasing gestational age, their width decreases; in mature children they are normally slit-like. Mild asymmetry of the lateral ventricles (difference in the sizes of the right and left lateral ventricles on a coronal section at the level of the foramen of Monro up to 2 mm) occurs quite often and is not a sign of pathology. Pathological expansion of the lateral ventricles most often begins with the occipital horns, so the lack of the ability to clearly visualize them is a serious argument against expansion. We can talk about expansion of the lateral ventricles when the diagonal size of the anterior horns on a coronal section through the foramen of Monroe exceeds 5 mm and the concavity of their bottom disappears.
Rice. 1.
Ventricular system of the brain. 1 - interthalamic ligament; 2 - supraoptic recess of the third ventricle; 3 - funnel-shaped pocket of the third ventricle; 4 - anterior horn of the lateral ventricle; 5 - Monroe hole; 6 - body of the lateral ventricle; 7 - III ventricle; 8 - pineal recess of the third ventricle; 9 - glomerulus of the choroid plexus; 10 - posterior horn of the lateral ventricle; 11 - lower horn of the lateral ventricle; 12 - Sylvian aqueduct; 13 - IV ventricle.
Choroid plexuses.
The choroid plexus (plexus chorioideus) is a richly vascularized organ that produces cerebrospinal fluid. Echographically, the plexus tissue appears as a hyperechoic structure. The plexuses pass from the roof of the third ventricle through the foramina of Monro (interventricular foramina) to the bottom of the bodies of the lateral ventricles and continue to the roof of the temporal horns (see Fig. 1); they are also present in the roof of the fourth ventricle, but are not detected echographically in this area. The anterior and occipital horns of the lateral ventricles do not contain choroid plexuses.
The plexuses usually have an even, smooth contour, but there may be irregularities and slight asymmetry. The choroid plexuses reach their greatest width at the level of the body and occipital horn (5-14 mm), forming a local compaction in the atrium area - the choroid glomerulus (glomus), which can have the shape of a finger-like outgrowth, be layered or fragmented. On coronal sections, the plexuses in the occipital horns appear as ellipsoidal densities, almost completely filling the lumen of the ventricles. In infants with a lower gestational age, the size of the plexuses is relatively larger than in full-term infants.
The choroid plexuses can be a source of intraventricular hemorrhages in full-term infants, then their clear asymmetry and local compactions are visible on echograms, in place of which cysts then form.
III ventricle.
The third ventricle (ventriculus tertius) appears as a thin slit-like vertical cavity filled with cerebrospinal fluid, located sagittally between the thalami above the sella turcica. It connects to the lateral ventricles through the foramen of Monroe (foramen interventriculare) and to the IV ventricle through the aqueduct of Sylvius (see Fig. 1). The supraoptic, infundibular and pineal processes give the third ventricle a triangular appearance on a sagittal section. On a coronal section, it is visible as a narrow gap between the echogenic visual nuclei, which are interconnected by the interthalamic commissure (massa intermedia), passing through the cavity of the third ventricle. In the neonatal period, the width of the third ventricle on a coronal section should not exceed 3 mm, in infancy - 3-4 mm. The clear outlines of the third ventricle on the sagittal section indicate its expansion.
Sylvian aqueduct and IV ventricle.
The Sylvian aqueduct (aquaeductus cerebri) is a thin canal connecting the third and fourth ventricles (see Fig. 1), rarely visible during ultrasound examination in standard positions. It can be visualized on an axial section in the form of two echogenic points against the background of hypoechoic cerebral peduncles.
The fourth ventricle (ventriculus quartus) is a small diamond-shaped cavity. On echograms in a strictly sagittal section, it appears as a small anechoic triangle in the middle of the echogenic medial contour of the cerebellar vermis (see Fig. 1). Its anterior border is not clearly visible due to the hypoechogenicity of the dorsal part of the bridge. The anteroposterior size of the IV ventricle in the neonatal period does not exceed 4 mm.
Corpus callosum.
The corpus callosum (corpus callosum) on a sagittal section looks like a thin horizontal arcuate hypoechoic structure (Fig. 2), limited above and below by thin echogenic stripes resulting from reflection from the peri-callosal groove (above) and the lower surface of the corpus callosum. Immediately below it are two leaves of a transparent septum, limiting its cavity. On a frontal section, the corpus callosum appears as a thin, narrow hypoechoic strip forming the roof of the lateral ventricles.
Rice. 2.
Location of the main brain structures on the midsagittal section. 1 - pons; 2 - prepontine cistern; 3 - interpeduncular cistern; 4 - transparent partition; 5 — legs of the arch; 6 - corpus callosum; 7 - III ventricle; 8 - quadrigeminal cistern; 9 - cerebral peduncles; 10 - IV ventricle; 11 - large tank; 12 - medulla oblongata.
Cavity of the septum pellucida and Verge's cavity.
These cavities are located directly under the corpus callosum between the layers of the transparent septum (septum pellucidum) and are limited by glia, not ependyma; they contain fluid but do not connect to either the ventricular system or the subarachnoid space. The cavity of the transparent septum (cavum cepti pellucidi) is located anterior to the fornix of the brain between the anterior horns of the lateral ventricles; Verge's cavity is located under the splenium of the corpus callosum between the bodies of the lateral ventricles. Sometimes, normally, dots and short linear signals originating from the subependymal median veins are visualized in the leaves of the septum pellucidum. On a coronal view, the cavity of the septum pellucidum appears as a square, triangular, or trapezoidal anechoic space with a base beneath the corpus callosum. The width of the cavity of the transparent septum does not exceed 10-12 mm and is wider in premature infants than in full-term infants. Verge's cavity, as a rule, is narrower than the cavity of the transparent septum and is rarely found in full-term children. These cavities begin to obliterate after 6 months of gestation in the dorsoventral direction, but there are no exact dates for their closure, and both of them can be detected in a mature child at the age of 2-3 months.
Basal ganglia, thalami and internal capsule.
The visual nuclei (thalami) are spherical hypoechoic structures located on the sides of the cavity of the septum pellucidum and forming the lateral borders of the third ventricle on coronal sections. The upper surface of the gangliothalamic complex is divided into two parts by the caudothalamic recess - the anterior one belongs to the caudate nucleus, the posterior one to the thalamus (Fig. 3). The optic nuclei are connected to each other by an interthalamic commissure, which becomes clearly visible only with the expansion of the third ventricle both on the frontal (in the form of a double echogenic transverse structure) and on the sagittal sections (in the form of a hyperechoic point structure).
Rice. 3.
The relative position of the structures of the basal-thalamic complex on a parasagittal section. 1 - shell of the lenticular nucleus; 2 - globus pallidus of the lenticular nucleus; 3 - caudate nucleus; 4 - thalamus; 5 - internal capsule.
The basal ganglia are subcortical accumulations of gray matter located between the thalamus and the insula of Reille. They have similar echogenicity, which makes their differentiation difficult. A parasagittal section through the caudothalamic notch is the most optimal approach for detecting the thalami, the lenticular nucleus consisting of the putamen, and the globus pallidus, and the caudate nucleus, as well as the internal capsule - a thin layer of white matter separating the nuclei of the striatum bodies from thalami. Clearer visualization of the basal nuclei is possible when using a 10 MHz sensor, as well as in case of pathology (hemorrhage or ischemia) - as a result of neuronal necrosis, the nuclei acquire increased echogenicity.
Germinal matrix
is an embryonic tissue with high metabolic and fibrinolytic activity that produces glioblasts. This subependymal plate is most active between the 24th and 34th weeks of gestation and is a cluster of fragile vessels, the walls of which are devoid of collagen and elastic fibers, are easily susceptible to rupture and are a source of peri-intraventricular hemorrhages in premature infants. The germinal matrix lies between the caudate nucleus and the lower wall of the lateral ventricle in the caudothalamic recess; on echograms it looks like a hyperechoic strip.
Brain cisterns.
Cisterns are spaces between brain structures containing cerebrospinal fluid (see Fig. 2), which may also contain large vessels and nerves. Normally, they are rarely visible on echograms. When enlarged, the cisterns appear as irregularly defined cavities, which indicates a proximally located obstruction to the flow of cerebrospinal fluid.
The cisterna magna (cisterna magna, c. cerebromedullaris) is located under the cerebellum and medulla oblongata above the occipital bone; normally, its superior-inferior size on a sagittal section does not exceed 10 mm. The pontine cistern is an echogenic zone above the pons in front of the cerebral peduncles, under the anterior recess of the third ventricle. It contains a bifurcation of the basilar artery, which causes its partial echo density and pulsation.
The basal (c. suprasellar) cistern includes the interpeduncular, c. interpeduncularis (between the cerebral peduncles) and chiasmatic, c. chiasmatis (between the optic chiasm and the frontal lobes) cisterns. The chiasm cistern appears as a pentagonal echo-dense zone, the angles of which correspond to the arteries of the circle of Willis.
The quadrigeminal cistern (c. quadrigeminalis) is an echogenic line between the plexus of the third ventricle and the cerebellar vermis. The thickness of this echogenic zone (normally not exceeding 3 mm) may increase with subarachnoid hemorrhage. Arachnoid cysts may also be located in the area of the quadrigeminal cistern.
Bypass (c. ambient) tank - carries out lateral communication between the prepontine and interpeduncular tanks in front and the quadrigeminal cistern behind.
Cerebellum
(cerebellum) can be visualized through both the anterior and posterior fontanel. When scanning through a large fontanel, the image quality is the worst due to the distance. The cerebellum consists of two hemispheres connected by the vermis. The hemispheres are weakly mid-echoic, the vermis is partially hyperechoic. On a sagittal section, the ventral part of the vermis looks like a hypoechoic letter “E” containing cerebrospinal fluid: at the top is the quadrigeminal cistern, in the center is the IV ventricle, at the bottom is the cistern magna. The transverse size of the cerebellum directly correlates with the biparietal diameter of the head, which makes it possible to determine the gestational age of the fetus and newborn based on its measurement.
The cerebral peduncles (pedunculus cerebri), bridge (pons) and medulla oblongata (medulla oblongata) are located longitudinally anterior to the cerebellum and appear as hypoechoic structures.
Parenchyma.
Normally, there is a difference in echogenicity between the cerebral cortex and the underlying white matter. The white matter is slightly more echogenic, possibly due to the relatively greater number of vessels. Normally, the thickness of the cortex does not exceed a few millimeters.
Around the lateral ventricles, predominantly over the occipital and less frequently over the anterior horns, in premature infants and some full-term infants there is a halo of increased echogenicity, the size and visualization of which depends on gestational age. It can persist up to 3-4 weeks of life. Normally, its intensity should be lower than that of the choroid plexus, the edges should be unclear, and the location should be symmetrical. If there is asymmetry or increased echogenicity in the periventricular region, an ultrasound examination of the brain should be performed over time to exclude periventricular leukomalacia.
Standard echoencephalographic sections
Coronal slices
(Fig. 4).
The first slice
passes through the frontal lobes in front of the lateral ventricles (Fig. 5). The interhemispheric fissure is determined in the middle in the form of a vertical echogenic strip separating the hemispheres. When it expands, a signal from the falx cerebri (falx) is visible in the center, which is not normally visualized separately (Fig. 6). The width of the interhemispheric fissure between the gyri does not normally exceed 3-4 mm. On the same section it is convenient to measure the size of the subarachnoid space - between the lateral wall of the superior sagittal sinus and the nearest gyrus (sinocortical width). To do this, it is advisable to use a sensor with a frequency of 7.5-10 MHz, a large amount of gel and very carefully touch the large fontanel without pressing on it. The normal size of the subarachnoid space in full-term babies is up to 3 mm, in premature babies – up to 4 mm.
Rice. 4.
Coronal scanning planes (1-6).
Rice. 5.
Echogram of the newborn's brain, first coronal slice through the frontal lobes. 1 - eye sockets; 2 - interhemispheric fissure (not widened).
Rice. 6.
Measurement of the width of the subarachnoid space and the width of the interhemispheric fissure on one or two coronal sections - diagram (a) and echogram of the brain (b). 1 - superior sagittal sinus; 2 — width of the subarachnoid space; 3 — width of the interhemispheric fissure; 4 - falx cerebri.
Second cut
is performed through the anterior horns of the lateral ventricles anterior to the foramina of Monroe at the level of the cavity of the septum pellucidum (Fig. 7). The frontal horns, which do not contain cerebrospinal fluid, are visualized on both sides of the interhemispheric fissure as echogenic stripes; if they contain cerebrospinal fluid, they look like anechoic structures, similar to boomerangs. The roof of the anterior horns of the lateral ventricles is represented by a hypoechoic strip of the corpus callosum, and between their medial walls there are layers of the transparent septum containing a cavity. On this section, the shape is assessed and the width of the cavity of the transparent septum is measured - the maximum distance between its walls. The lateral walls of the anterior horns form the basal nuclei - directly under the bottom of the horn - the head of the caudate nucleus, and laterally - the lentiform nucleus. Even more lateral on this section, the temporal lobes are identified on both sides of the chiasm cistern.
Rice. 7.
Echogram of the brain, second coronal section through the anterior horns of the lateral ventricles. 1 - temporal lobes; 2 - Sylvian fissure; 3 - cavity of the transparent septum; 4 - anterior horn of the lateral ventricle; 5 - corpus callosum; 6 - interhemispheric fissure; 7 - caudate nucleus; 8 - thalamus.
Third coronal slice
passes through the foramina of Monroe and the third ventricle (Fig. 8). At this level, the lateral ventricles connect to the third ventricle through the interventricular foramina (Monroe). The foramina themselves are not normally visible, but the choroid plexuses passing through them from the roof of the third ventricle to the bottom of the lateral ventricles appear as a hyperechoic Y-shaped structure located in the midline. Normally, the third ventricle may also not be visualized; when it enlarges, its width is measured between the medial surfaces of the thalami, which are its lateral walls. The lateral ventricles in this section are visible as slit-like or boomerang-shaped anechoic structures (Fig. 9), the width of which is measured diagonally (normally up to 5 mm). The cavity of the transparent septum on the third section in some cases still remains visible. Below the third ventricle, the brainstem and pons are visualized. Lateral to the third ventricle are the thalamus, basal ganglia and insula, above which a Y-shaped thin echogenic structure is defined - the Sylvian fissure, containing the pulsating middle cerebral artery.
Rice. 8.
Echogram of the brain, third coronal section through the foramina of Monroe. 1 - III ventricle; 2 - choroid plexuses in the interventricular canals and the roof of the third ventricle and the fornix of the brain; 3 - cavity of the lateral ventricle; 4 - corpus callosum; 5 - caudate nucleus; 6 - thalamus.
Rice. 9.
The relative position of the central brain structures on two to four coronal sections. 1 - III ventricle; 2 - cavity of the transparent septum; 3 - corpus callosum; 4 - lateral ventricle; 5 - caudate nucleus; 6 - peduncle of the cerebral vault; 7 - thalamus.
On the fourth cut
(through the bodies of the lateral ventricles and the posterior part of the third ventricle) the following are visible: interhemispheric fissure, corpus callosum, ventricular cavities with choroid plexuses in their bottom, thalamus, Sylvian fissures, vertically located hypoechoic cerebral peduncles (below the thalami), cerebellum separated from the cerebral peduncles by hyperechoic tentorium (Fig. 10). Inferior to the cerebellar vermis, the cistern magna can be visualized. In the area of the middle cranial fossa, an area of pulsation is visible, originating from the vessels of the circle of Willis.
Rice. 10.
Echogram of the brain, fourth coronal section through the bodies of the lateral ventricles. 1 - cerebellum; 2 - choroid plexuses in the lateral ventricles; 3 - bodies of the lateral ventricles; 4 - Verge's cavity.
Fifth cut
passes through the bodies of the lateral ventricles and the choroid plexuses in the region of the glomus, which on echograms almost completely fill the cavities of the lateral ventricles (Fig. 11). On this section, the density and size of the choroid plexuses on both sides are compared to exclude hemorrhages. If a Verge's cavity is present, it is visualized between the lateral ventricles in the form of a rounded anechoic formation. Inside the posterior cranial fossa, the cerebellum is visualized with medium echogenicity, and above its tentorium is the echogenic quadrigeminal cistern.
Rice. eleven.
Echogram of the brain, fifth coronal section through the glomus of the choroid plexuses - choroid plexuses in the atrium area, completely filling the lumen of the ventricles (1).
Sixth
, the last, coronal section is performed through the occipital lobes above the cavities of the lateral ventricles (Fig. 12). The interhemispheric fissure with grooves and convolutions is visualized in the middle, and on both sides there are cloud-like periventricular densities, which are more pronounced in premature infants. On this section, the symmetry of these seals is assessed.
Rice. 12.
Echogram of the brain, sixth coronal section through the occipital lobes above the lateral ventricles. 1 - normal periventricular seals; 2 - interhemispheric fissure.
Sagittal slices
(Fig. 13).
A midsagittal section
(Fig. 14) allows one to visualize the corpus callosum in the form of a hypoechoic arch, immediately below it is the cavity of the transparent septum (under its anterior sections) and the Verge's cavity connected to it (under the splenium). Near the genu of the corpus callosum there is a pulsating structure - the anterior cerebral artery, which goes around it and runs along the upper edge of the body. The pericallosal groove runs above the corpus callosum. Between the cavities of the transparent septum and Verge, an arcuate hyperechoic strip is determined, originating from the choroid plexus of the third ventricle and the fornix of the brain. Below is a hypoechoic triangular third ventricle, the contours of which are normally not clearly defined. When it expands in the center, you can see the interthalamic commissure in the form of a hyperechoic point. The posterior wall of the third ventricle consists of the pineal gland and the quadrigeminal plate, behind which the quadrigeminal cistern may be visible. Immediately below it, in the posterior cranial fossa, a hyperechoic cerebellar vermis is identified, on the anterior part of which there is a triangular notch - the fourth ventricle. The pons, cerebral peduncles and medulla oblongata are located anterior to the fourth ventricle and are visible as hypoechoic formations. On this section, the cisterna magna is measured - from the lower surface of the vermis to the inner surface of the occipital bone - and the depth of the fourth ventricle is measured.
Rice. 13.
Sagittal scanning planes (1-4).
Rice. 14.
Echograms of the brain, midsagittal section: 1 - cerebellum; 2 - IV ventricle; 3 - III ventricle; 4 - fornix and choroid plexus in the foramina of Monroe and the roof of the third ventricle; 5 - corpus callosum; 6 - cavity of the transparent septum; 7 - cerebral peduncles; 8 - large tank; 9 - Verge's cavity; 10 - corpus callosum; 11 — cavity of the transparent septum; 12 - III ventricle.
With a slight deviation of the sensor to the left and right, a parasagittal slice
through the caudothalamic recess (the location of the germinal matrix in premature infants), where its shape, as well as the structure and echogenicity of the gangliothalamic complex are assessed (Fig. 15).
Rice. 15.
Echogram of the brain, parasagittal section through the caudothalamic notch. 1 - choroid plexus of the lateral ventricle; 2 — cavity of the lateral ventricle; 3 - thalamus; 4 - caudate nucleus.
Next parasagittal slice
is performed through the lateral ventricle on each side so as to obtain its full image - the frontal horn, body, occipital and temporal horns (Fig. 16). In this plane, the height of various parts of the lateral ventricle is measured, and the thickness and shape of the choroid plexus are assessed. Above the body and occipital horn of the lateral ventricle, the homogeneity and density of the periventricular substance of the brain is assessed, comparing it with the density of the choroid plexus.
Rice. 16.
Echogram of the brain, parasagittal section through the lateral ventricle. 1 - lower horn; 2 - posterior horn; 3 - glomus choroid plexus; 4 - body; 5 - anterior horn.
Last parasagittal slice
are obtained by tilting the sensor even more laterally, which makes it possible to visualize the insula, its sulci and gyri and measure the Sylvian fissure in case of its expansion (Fig. 17). Normally, in full-term infants, it appears as an echogenic transverse structure; with subarachnoid hemorrhage or external hydrocephalus, a strip of cerebrospinal fluid is visualized between the insula and the parietal lobe.
Rice. 17.
Echogram of the brain, parasagittal section through the temporal lobe. 1 - temporal lobe of the brain; 2 - Sylvian fissure; 3 - parietal lobe.
If any deviations are determined on the obtained echograms in the coronal section, then they must be confirmed in the sagittal section, and vice versa, since artifacts can often occur.
Axial scanning.
An axial cut is made by placing the transducer horizontally above the ear. In this case, the cerebral peduncles are visualized as a hypoechoic structure shaped like a butterfly (Fig. 18). Between the legs (in contrast to coronal and sagittal sections) an echogenic structure is often visible, consisting of two points - the aqueduct of Sylvius, anterior to the legs - the slit-like third ventricle. On an axial section, the walls of the third ventricle are clearly visible, in contrast to the coronal one, which makes it possible to more accurately measure its size with slight expansion. When the sensor is tilted towards the calvarium, the lateral ventricles are visible, which makes it possible to estimate their size when the large fontanelle is closed. Normally, the parenchyma of the brain is closely adjacent to the bones of the skull in mature children, so the separation of echo signals from them on an axial section suggests the presence of pathological fluid in the subarachnoid or subdural spaces.
Rice. 18.
Echogram of the brain, axial section at the level of the base of the brain. 1 - cerebellum; 2 - Sylvian aqueduct; 3 - cerebral peduncles; 4 - Sylvian fissure; 5 - III ventricle.
Data from an echographic examination of the brain can be supplemented by the results of Doppler ultrasound assessment of cerebral blood flow. This is desirable, since in 40-65% of children, despite severe neurological disorders, echographic examination of the brain remains normal.
The brain is supplied with blood by the branches of the internal carotid and basilar arteries, which form the circle of Willis at the base of the brain. The direct continuation of the internal carotid artery is the middle cerebral artery, and its smaller branch is the anterior cerebral artery. The posterior cerebral arteries branch from the short basilar artery and communicate with the branches of the internal carotid through the posterior communicating arteries. The main cerebral arteries - the anterior, middle and posterior with their branches form an arterial network from which small vessels penetrate into the medulla, feeding the cortex and white matter of the brain.
Doppler examination of blood flow is carried out in the largest arteries and veins of the brain, trying to position the ultrasound sensor so that the angle between the ultrasound beam and the axis of the vessel is minimal.
Anterior cerebral artery
visualized on a sagittal section; To obtain blood flow measurements, a volumetric marker is placed in front of the knee of the corpus callosum or in the proximal part of the artery before it bends around this structure.
To study blood flow in the internal carotid artery
on the parasagittal section, its vertical part is used immediately after exiting the carotid canal above the level of the sella turcica.
Basilar artery
examined in a midsagittal section in the area of the base of the skull immediately in front of the pons, a few millimeters behind the location of the internal carotid artery.
Middle cerebral artery
determined in the Sylvian fissure. The best angle for its insonation is achieved with an axial approach. The vein of Galen is visualized on a coronal section under the corpus callosum along the roof of the third ventricle.
Ultrasound machine HM70A
Expert class at an affordable price.
Monocrystal sensors, full-screen display mode, elastography, 3D/4D in a laptop case. Flexible transformation into a stationary scanner with a cart.
What are the signs of periventricular leukomalacia?
Periventricular leukomalacia can be difficult to detect in newborns, as very young children generally do not show signs of impairment. Periventricular leukomalacia can resemble other conditions, and each case is different. Intellectual impairment, developmental disorders, visual, hearing, and coordination impairments are common with periventricular leukomalacia.
Spastic diplegia (tight muscles and limbs that do not bend easily) is the most common type of cerebral palsy caused by periventricular leukomalacia; quadriplegia is the most severe.
Manifestations of leukomalacia
Signs of leukomalacia are varied and often nonspecific, but severe hypoxic damage to brain tissue cannot be asymptomatic. There are several degrees of PL:
- Mild degree - signs of damage to the nervous system persist up to a week from the moment of birth;
- Moderate severity – from 7 to 10 days, convulsions, intracranial hypertension, autonomic disorders are possible;
- Severe PL – deep damage with brain depression, often coma.
Symptoms of cerebral leukomalacia include:
- Excessive neuro-reflex excitability or, conversely, its inhibition;
- Convulsive syndrome;
- Muscle hypotonia;
- Stem symptoms;
- Paresis and paralysis;
- Visual disturbances in the form of strabismus;
- Delayed psychomotor development, intellectual impairment, hyperactivity, attention deficit.
Experts especially note that neurological symptoms in the acute period and up to 3-5 months of life may not be expressed. In approximately 90% of children, after the acute period, imaginary well-being occurs, lasting up to 5 and even 8-9 months of life. And only after such a long time do signs of a deficit of nervous activity appear against the background of atrophy of brain tissue.
The nerve pathways responsible for the motor function of the limbs are concentrated around the ventricles of the brain, so the main symptom of cerebral leukomalacia in children is cerebral palsy, but its severity depends on the severity of necrosis.
Due to the involvement of the brain stem and cranial nerves, more than half of children suffer from strabismus, more often convergent, swallowing disorders and respiratory disorders are possible. By the age of six months, convulsive syndrome appears. The larger the size of the necrosis fields and, accordingly, the cysts, the more pronounced the brain failure. The nature of the symptoms is determined by the localization of the lesions (motor, visual disturbances, convulsions, mental retardation).
Severe damage to the parietal and frontal lobes leads to cerebral palsy with simultaneous impairment of mental development. If only the pathways responsible for the innervation of the limbs are involved, then paralysis may not be accompanied by impairments in the child’s intelligence and development.
Often, in children who have suffered perinatal hypoxia, symptoms include attention deficit and hyperactivity with intact motor development. This is a relatively favorable variant of the pathology, which can be corrected with special therapeutic measures.
Due to such unpredictability and variety of symptoms, it can be difficult for a mother to figure out what to expect when the baby is born prematurely and under hypoxic conditions. If the damage is moderate or severe, then the child will not develop according to his age - he will not learn to roll over, sit, and, especially, walk in time. Speech development will slow down, the baby will not be able to walk, will not follow toys and will not show such curiosity as is typical for his age.
Particular attention is drawn to hypertension, which can be painful, so the child will be restless, whiny, and sleep will be disturbed. Breastfeeding can be problematic due to a poor sucking reflex, excessive neuromuscular excitability, or atonia.
Poor weight gain, slow growth that is not appropriate for age, and a lack of skills that a growing child needs to master are the main symptoms that parents of a baby with moderate or mild damage to the subcortical structures have to deal with.
By about a year, neurological deficits become noticeable, cerebral palsy develops, and psychomotor development is delayed. After a year, when the recovery period comes to an end, the clinical picture is dominated by such consequences as delayed psycho-speech development, emotional lability, problems with sleep and attention, which can occur along with more severe motor disorders (cerebral palsy).
PL in adults can cause cerebral palsy, hypertonicity, intellectual impairment, and severe mental retardation . With a favorable course of the pathology, adults are not much different from others.
Epidemiology
The mean age was 58 years (range 24–86 years); the majority (69%) were women [2]. Small VR spaces (<2 mm) are detected in all age groups. With age, VR spaces are found with greater frequency and larger size (> 2 mm) [1]. Some studies have found a correlation between expanded VR spaces and neuropsychiatric disorders [1], multiple sclerosis [1], mild traumatic brain injury [1], and diseases associated with microangiopathy [1].
Sources
- "Virchow-Robin Spaces at MR Imaging." Robert M. Kwee, Thomas C. Kwee link
- "Large anterior temporal Virchow-Robin spaces: unique MR imaging features." Lim AT1, Chandra RV, Trost NM, McKelvie PA, Stuckey SL. link
- "Virchow-Robin spaces at MR imaging." Kwee RM1, Kwee TC. link
Author: radiologist, Ph.D. Vlasov Evgeniy Alexandrovich
Full or partial reprint of this article is permitted by installing an active hyperlink to the source
If you still have doubts about the conclusions of your MRI, you can order a review of your study with a detailed transcript here:
How to help your baby?
Cerebral leukomalacia is accompanied by changes in nervous tissue and these changes are irreversible, and, unfortunately, there is simply no clear treatment regimen for the disease at the moment. Typically, doctors look at the symptoms and then prescribe treatment depending on the manifestations of the disorder.
To treat the disease, nootropic medications are used, which improve blood flow in the brain and also improve metabolism. The drugs that are most often prescribed are Piracetam, Stugeron.
All other therapy prescribed by the doctor is used to correct motor movements, as well as disorders of psychomotor development.
In order to avoid the onset of the disease, obstetricians try to maintain pregnancy as long as possible. But if the baby was born prematurely, then they need to do everything possible for his safety. Immediately after childbirth, breathing improves, and it must be controlled. It is important that there is the correct ratio of oxygen and carbon dioxide in the blood, and that blood pressure must be normal.
If the baby has grown...
After the baby reaches the age of one year, treatment becomes more symptomatic. The child’s condition during this period requires constant monitoring and if symptoms of leukomalacia are detected, he is prescribed drugs such as: Pantogam, Phenibut. Thanks to their use, an inhibitory effect occurs in the formation of necrosis zones in the cerebral cortex.
If a child has a sleep disorder, he is prescribed sedatives - Valerian and Melissa. Water procedures also have a good effect.
Article on the topic: Recognan - instructions for use and indications, composition, release form and price
If the baby experiences an increase in blood pressure, then the drugs Diacarb, Furosemide or Glycerin are prescribed. But the last two drugs are not used independently; it is imperative that potassium enters the body.
All convulsive manifestations are removed with the help of the drug Phenobarbital. If its use causes tachycardia and blood pressure, then it can be replaced with beta-blockers - Anaprilin and Obzidan.
Since the disease entails a disruption in the functioning of the musculoskeletal system, massage and exercises are prescribed, as well as physiotherapeutic treatment.
If there is a delay in the development of the speech apparatus, then teachers and speech pathologists come to the rescue and help correct speech.
In particularly difficult cases, respiratory distress syndrome develops. In order to avoid it, surfactant preparations are used. Thanks to its use, the severity of respiratory distress is reduced and the need for a ventilator disappears.
...But here are some rehabilitation methods that can help:
What diseases have symptoms similar to brain leukomalacia in a baby?
Multicystic encephalomalacia
- occurs in full-term children with severe asphyxia during childbirth;
- diffuse generalized brain damage;
— multiple cystic cavities of various sizes;
- cysts may have septa;
- usually occur in the cortex and nearby white matter;
- often in the frontal and occipital lobes.-
Vasculitis
- very rare;
— multiple lesions of the cortex and subcortical region;
- may be combined with hemorrhages;
— perfusion defects in the acute stage;
— sometimes areas of damage to the blood-brain barrier are identified.